Isorhamnetin glycosides (IGs) are a class of essential flavonoids derived from dietary and medicinal plants such as Opuntia ficus-indica, Hippophae rhamnoides, and Ginkgo biloba.
- isorhamnetin glycosides
- phytonutrients
- health-promoting effects
- sources
1. Introduction
2. Structure of IGs
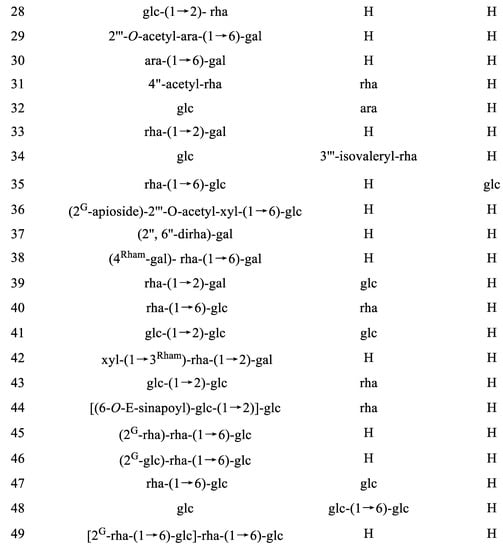
IGs are a type of glycosylated flavonol composed of an isorhamnetin skeleton and sugar groups. Their aglycone isorhamnetin, i.e., 3,4′,5,7-tetrahydroxy-3′-methoxyflavone, is an O-methylated flavonol (Figure 1). Generally, d-glucose, d-galactose, l-rhamnose, d-xylose, l-arabinose, sophorose, and rutinose are the most common sugar groups of IGs. They are linked to the aglycone by an O-glycosidic bond. According to the number of sugar groups, IGs are classified as mono-, di-, tri-, or tetra-glycosides. Position substitutions mostly happen at C-3 and C-7, for example, isorhamnentin-3-O-β-d-glucoside (4) and isorhamnetin-3-O-β-d-glucoside-7-O-α-l-rhamnoside (20) from Hippophae rhamnoids [20]; isorhamnetin-3-O-α-l-rhamnoside (3) from Laportea bulbifera Wedd. [21]; and isorhamnetin-7-O-β-d-glucoside (1) and isorhamnetin-7-O-α-l-rhamnoside (2) from Nitraria tangutorum Bolor [22]. Of course, sometimes, substitution occurs at C-4′, for instance, isorhamnetin-4′-O-β-d glucoside (9) from Allium cepa L. [23]; isorhamnetin-3,4′-O-β-d-diglucoside (17) from Allium ascalonicum [24]; isorhamnetin-3-O-β-d -glucoside-4′-O-β-d-xyloside (21) [25]; and isorhammetin-3-O-α-l-rhamnoside-(1→6)-β-d-glucoside-4′-O-β-d-glucoside (35) [26]. In addition, some sugar group derivatives, such as isorhamnetin-3-O-[2‴-O-acetyl−β-d-xyloside-(1→6)-β-d-glucoside] (10) [27] and isorhamnetin-3-O-β-d (6-acetyl-glucoside) (7) [28], have also been obtained.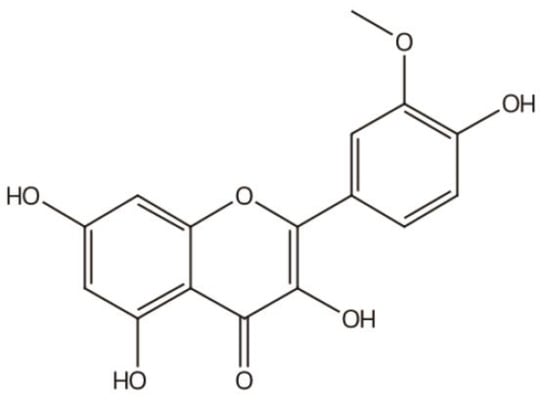

|
No. |
Name |
Trivial Name |
Source |
Ref. |
|---|---|---|---|---|
|
Monoglycosides |
||||
|
1 |
Isorhamnetin-7-O-β-d-glucoside |
Brassicin |
Centaurea cyanus Centaurea kotschyi var. kotschyi Cnicus wallichi Russowia Sogdiana Tagetes lucida (Asteraceae) Sedum sarmentosum Bunge Nitraria tangutorum Bolor |
[29] [30] [31] [32] [33] [34] [22] |
|
2 |
Isorhamnetin-7-O-α-l-rhamnoside |
Carduncellus eriocephalus Nitraria tangutorum Bolor Atriplex centralasiatica Laportea bulbifera Wedd. V. galamensis ssp. galamensis var. petitiana (A. Rich) M. Gilbert Raphanus raphanistrum L. Caragana intermedia |
[35] [22] [36] [21] [37] [38] [39] |
|
|
3 |
Isorhamnetin-3-O-α-l-rhamnoside |
Laportea bulbifera Wedd. |
[21] |
|
|
4 |
Isorhamnentin-3-O-β-d-glucoside |
Astragalus centralpinus Solidago canadensis L. Hippophae rhamnoids Sambucus nigra L. Calendula officinalis |
[40] [28] [20] [41] [42] |
|
|
5 |
Isorhamnetin-3-O-β-d-glucuronide |
Arnica montana Persicaria thunbergii Senecio giganteus Polygonum aviculare L. Senecio argunensis Turcz. |
[43] [44] [45] [46] [47] |
|
|
6 |
Isorhamnetin-3-O-β-d-(2-acetyl-glucuronide) |
Polygonum aviculare L. |
[46] |
|
|
7 |
Isorhamnetin-3-O-β-d (6-acetyl-glucoside) |
Solidago canadensis L. |
[28] |
|
|
8 |
Isorhamnetin-3-O-β-d-galactoside |
Senecio argunensis Turcz. |
[47] |
|
|
9 |
Isorhamnetin-4′-O-β-d glucoside |
Allium cepa L. |
[23] |
|
|
Diglycosides |
||||
|
10 |
Isorhamnetin-3-O-[2‴-O-acetyl−β-d-xyloside-(1→6)-β-d-glucoside] |
Gymnocarpos decander |
[27] |
|
|
11 |
Isorhamnetin-3-O-[2‴,3‴-O-isopropylidene-α-l-rhamnoside]—(1→6)-β-d-glucoside |
Tetraena aegyptia |
[48] |
|
|
12 |
Isorhamnetin-7-O-α-l-rhamnoside-(1→2)-β-d-glucoside |
Isorhamnetin-7-O-β-neohesperidoside |
Cleome droserifolia |
[12] |
|
13 |
Isorhamnetin-7-O-β-d-glucoside-(1→6)-β-d-glucoside |
Astragaloside or Isorhamnetin-7-O-gentiobioside |
Astragalus altaicus |
[49] |
|
14 |
Isorhamnetin-3-O-β-(4‴-p-coumaroyl-α-rhamnosy]—(1→6)-galactoside) |
Aerva javanica |
[50] |
|
|
15 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→2)-β-d-glucoside |
Isorhamnetin-3-O-β-neohesperidoside |
Hippophae rhamnoids Typha augustifolia L. Calendula officinalis |
[20] [51] [42] |
|
16 |
Isorhamnetin-3-O-β-d-xylosidel-(1→2)-β-d-galactoside |
Prunus padus L. |
[52] |
|
|
17 |
Isorhamnetin-3,4′-O-β-d-diglucoside |
Allium ascalonicum Lepidium apetalum willd |
[24] [53] |
|
|
18 |
Isorhamnetin-3,7-O-β-d-diglucoside |
Sedum sarmentosum Bunge Carduncellus eriocephalus |
[34] [35] |
|
|
19 |
Isorhamnetin-3,7-O-α-l-dirhamnoside |
Laportea bulbifera Wedd. |
[21] |
|
|
20 |
Isorhamnetin-3-O-β-d-glucoside-7-O-α-l-rhamnoside |
Brassidine |
Sinapis arvensis Atriplex centralasiatica Hippophae rhamnoids |
[54] [36] [20] |
|
21 |
Isorhamnetin-3-O-β-d-glucoside-4′-O-β-d-xyloside |
Diplotaxis harra (Forssk.) Boiss |
[26] |
|
|
22 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→6)-β-d-galactoside |
Isorhamnetin-3-O-robinobioside |
Nitraria retusa |
[55] |
|
23 |
Isorhamnetin-3-O-α-rhamnoside-(1→2)-rhamnoside |
Laportea bulbifera Wedd. |
[21] |
|
|
24 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→6)-β-d-glucoside |
Narcissin Isorhamnetin-3-O-rutinoside |
V. galamensis ssp. galamensis var. petitiana (A. Rich) M. Gilbert opuntia ficus-indica Hippophae rhamnoids Ginkgo biloba Sambucus nigra L. Calendula officinalis |
[37] [18] [20] [41] [42] |
|
25 |
Isorhamnetin-3-O-β-d-apioide (1→2)-β-d-galactoside |
V. galamensis ssp. galamensis var. petitiana (A. Rich) M. Gilbert |
[37] |
|
|
26 |
Isorhamnetin-3-O-α-l-arabinoside-7-O-β-d-glucoside |
Callianthemum taipaicum Narcissus pseudonarcissus |
[57] [58] |
|
|
27 |
Isorhamnetin-3-O-β-d- (6‴-p-coumaroyl-α-glucoside-(1→2)-rhamnoside) |
Ginkgo biloba |
[56] |
|
|
28 |
Isorhamnetin-3-O-β-d-glucoside-(1→2)-α-l-rhamnoside |
Ginkgo biloba |
[56] |
|
|
29 |
Isorhamnetin-3-O-[2‴-O-acetyl−α-l-arabinoside-(1→6)-β-d-galactoside] |
Trillium tschonoskii Maxim. Trillium apetalon Makino. and T. kamtschaticum Pallas. |
[59] [60] |
|
|
30 |
Isorhamnetin-3-O−α-l-arabinoside-(1→6)-β-d-galactoside |
Trillium apetalon Makino. and T. kamtschaticum Pallas. |
[60] |
|
|
31 |
Isorhamnetin-3-O-α-(4″-acetyl-rhamnoside)-7-O-α-rhamnoside |
Cleome droserifolia |
[12] |
|
|
32 |
Isorhamnetin-3-O-β-d-glucoside-7-O-α-l-arabinoside |
Eschscholtzia mexicana Greene |
[61] |
|
|
33 |
Isorhamnetin-3-O-α-l-rhamnoside(1→2)]-β-d-galactoside |
Glycine max (L.) Merr. |
[62] |
|
|
34 |
Isorhamnetin-3-O-β-glucoside-7-O-α-(3″′-isovaleryl)-rhamnoside |
Lepidium apetalum |
[53] |
|
|
Triglycosides |
||||
|
35 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→6)-β-d-glucoside-4′-O-β-d-glucoside |
Isorhamnetin-3-rutinoside-4′-glucoside |
Mercurialis annua |
[26] |
|
36 |
Isorhamnetin-3-O-(2G-β-d-apiofuranosyl) [2‴-O-acetyl−β-d-xyloside-(1→6)-β-d-glucoside] |
Gymnocarpos decander |
[27] |
|
|
37 |
Isorhamnetin-3-O-(2″,6″-O-α-l-dirhamnoside)-β-d-galactoside |
Alangium premnifolium Lysimachia fortunei |
[63] [64] |
|
|
38 |
Isorhamnetin-3-O-(4Rham-β-d-galactosyl)-α-l-rhamnoside-(1→6)-β-d-galactoside] |
Isorhamnetin-3-O-4Rham-galactosyl-robinobioside |
Nitraria retusa |
|
|
39 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→2)-β-d-galactoside-7-O-β-d-glucoside |
Blackstonia perfoliata |
[66] |
|
|
40 |
Isorhamnetin-3-O-α-l-rhamnoside-(1→6)-β-d-glucoside-7-O-α-l-rhamnoside |
Isorhamnetin-3-rutinoside-7-rhamnoside |
Cassia italica Hippophae rhamnoides |
[67] [68] |
|
41 |
Isorhamnetin-3-O-β-glucoside-(1→2)-β-d-glucoside-7-β-d-glucoside |
Brassicoside or Isorhamnetin-3-O-sophoroside-7-O-β-d-glucoside |
Brassica napus |
[54] |
|
42 |
Isorhamnetin-3-O-β-d-xyloside-(1→3Rham)-α-l-rhamnoside-(1→6)-β-d-galactoside |
Isorhamnetin 3-xylosyl-robinobioside |
Nitraria retusa |
[55] |
|
43 |
Isorhamnetin-3-O-β-glucoside-(1→2)-β-d-glucoside-7-O-α-l-rhamnoside |
Isorhamnetin-3-O-sophoroside-7-O-rhamnoside |
Hippophae rhamnoids |
[20] |
|
44 |
Isorhamnetin-3-O-[(6-O-E-sinapoyl)-β-d-glucoside-(1 → 2)]-β-d-glucoside-7-O-α-l-rhamnoside |
Hippophae rhamnoids |
[20] |
|
|
45 |
Isorhamnetin-3-O-(2G-α-l-rhamnoside)-α-l-rhamnoside-(1→6)-β-d-glucoside |
Typhaneoside |
Typha augustifolia L. Calendula officinalis |
[51] [42] |
|
46 |
Isorhamnetin-3-O-(2G-β-d-glucoside)-α-l-rhamnoside-(1→6)-β-d-glucoside |
Boldo Folium |
[69] |
|
|
47 |
Isorhammetin-3-O-α-l-rhamnoside-(1→6)-β-d-glucoside-7-O-β-d-glucoside |
Isorhammetin-3-rutinoside-7-glucoside |
Hippophae rhamnoids Mercurialis annua |
[20] [26] |
|
48 |
Isorhamnetin-3-O-β-d-glucoside-7-O-β-d-glucoside-(1→6)-β-d-glucoside |
Isorhamnetin-3-O-glucoside-7-O-gentiobioside |
Lepidium apetalum willd |
[53] |
|
Tetraglycosides |
||||
|
49 |
Isorhamnetin-3-O-[2G-α-l-rhamnoside-(1→6)-β-d-glucoside]-α-l-rhamnoside-(1→6)-β-d-glucoside |
Boldo Folium |
[69] |
|
3. Sources of IGs

4. IG Identification and Quantification Methods
4.1. Spectral Techniques and Mass Spectrometry
4.2. Chromatographic Techniques
IGs can be distinguished from each other on the basis of chromatographic techniques. Therefore, the analysis, characterization, and quantification of IGs are usually performed using the following chromatographic techniques: TLC, HPLC, UPLC, and HSCCC.5. The Health-Promoting Effects of IGs
IGs possess a variety of biological properties, including antioxidant, anti-inflammatory, and anti-cancer properties. Research has recently been undertaken to investigate their pharmacological benefits for the treatment of various diseases, such as diabetes, obesity, hepatic diseases, and thrombosis.5.1. Antioxidant Activity
Oxidative damage induced by free radicals results in detrimental outcomes, such as a loss of cellular function and the dysfunction of organic systems [70][121]. It is worth mentioning that numerous in vitro and in vivo studies have demonstrated the strong antioxidant and radical-scavenging properties of IGs. β-carotene-linoleic acid, 2,2-diphenyl-1-picrylhydrazil (DPPH) scavenging, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS), oxygen radical absorbance capacity (ORAC), peroxyl radical-scavenging capacity (PSC), superoxide scavenging, peroxynitrite (ONOO(-)) assays, and CUPric reducing antioxidant capacity (CUPRAC) are commonly used indirect assays for identifying antioxidant activity. IGs isolated from the stamens of Nelumbo nucifera showed significant antioxidant activity, as determined via DPPH and ONOO(-) assays [11]. Brassicin (1) exhibited stronger free radical-scavenging ability than vitamin C [13] and exhibited DPPH radical- and ONOO(-)-scavenging activity [71][122]. Isorhamnetin 3-O-robinobioside (22), isorhamnetin 3-O-(2″,6″-O-α-dirhamnosyl)-β-galactoside (37) [72][123], typhaneoside (45), and isorhamnetin 3-O-neohesperidoside (15) [73][124] have been demonstrated to exhibit antioxidant activity using a DPPH radical-scavenging activity assay. Astragaloside (13) and narcissin (24) possessed antioxidant capacity, which was evaluated using ABST [74][118]. Narcissin (24) and isorhamnetin 3-O-rutinoside-7-O-glucoside (47) exhibited obvious antioxidant activity, which was detected using DPPH, β-carotene-linoleic acid, and ABST [65][75][65,125]. Isorhamnetin 3-O-neohesperidoside (15) was a potent inhibitor of xanthine oxidase and superoxide anion scavengers [76][126]. Furthermore, researchers have revealed the antioxidant properties of isorhamnetin 3-O-glucoside (4) and isorhamnetin 3-O-galactoside (8) in all the antioxidant activity tests employed [77][78][79][80][127,128,129,130]. Evaluation of the antioxidant properties of IGs were also carried out using various cell type experiments and animal models. The oral administration of isorhamnetin-3,7-diglucoside (18) to streptozotocin-induced diabetic rats significantly reduced their levels of 5-(hydroxymethyl) furfural (5-HMF), which is an indicator of the glycosylation of hemoglobin, and of stress [81][95]. Similarly, isorhamnetin 3-O-robinobioside (22) exhibited significant antioxidant effects on the human chronic myelogenous leukemia cell line K562 [82][131]. IGs had the ability to inhibit the formation of H2O2-induced radicals in the surrounding environment of intestinal epithelial cells [83][132]. Moreover, the transcriptional genes of the antioxidant system and the DNA repair pathway were upregulated after incubation with isorhamnetin 3-O-neohesperidoside (15) in pKS plasmid DNA [84][133]. Narcissin (24) and isorhamnetin 3-O-glucoside (4) demonstrated strong inhibition of reactive oxygen species (ROS) production in the oxidative burst activity of whole blood, neutrophils, and mononuclear cells [85][134]. Plant extracts rich in IGs also exhibited antioxidant activity. IG-rich concentrate from Opuntia ficus-indica juice had the ability to inhibit the formation of H2O2-induced radicals in the surrounding environment of intestinal epithelial cells [86][135]. The total antioxidant activity of Hippophae rhamnoides berry extracts, evaluated via ORAC and PSC, was significantly associated with total phenolics, including isorhamnetin-3-rutinoside (24) and isorhamnetin-3-glucoside (4) [87][136].5.2. Anti-Inflammatory Activity
5.2. Anti-Inflammatory Activity
IGs have anti-inflammatory properties due to different mechanisms. As an important inflammatory mediator, high-mobility-group protein 1 (HMGB1) contributes to organ damage and inflammation [88][138]. Isorhamnetin 3-O-galactoside (8) (5 μM) has been demonstrated to significantly inhibit the release of HMGB1 and reduce HMGB1-dependent inflammatory responses in human endothelial cells. It was found that 8 (4.8 mg/mouse) could also inhibit HMGB1 receptor expression, the HMGB1-mediated activation of NF-kB, and the production of tumor necrosis factor (TNF-α) in mice [89][139]. Mitogen-activated protein kinase (MAPK) signaling pathways, including p38, c-Jun N-terminal kinase (JNK), and extracellular regulated kinases (ERK), play crucial roles in inflammatory responses [90][140]. Isorhamnetin 3-O-galactoside (8) (50 μM) reduced cecal ligation and endothelin C receptor perforation-mediated shedding and down-regulated the phosphorylation of p38 MAPK, ERK 1/2, and JNK [14]. Similarly, isorhamnetin 3-O-glucuronide (5) exhibited anti-inflammatory activity by increasing heme oxygenase-1 (HO-1) expression and suppressing the JNK and p38 signaling pathways in LPS-induced RAW264.7 macrophage cells [91][141]. Moreover, isorhamnetin 3-O-glucuronide (5) inhibited the production of ROS (10 μM), as well as the release of elastase, in a human neutrophil model (1 μM) and suppressed the upregulation of inducible nitric oxide synthase (iNOS) expression (5 μM), and could be considered to display anti-inflammatory activity [46][92][46,142]. Many studies have shown the anti-inflammatory properties of IGs by inhibiting inflammatory cytokines. The inflammatory activity of narcissin (24) (100 μM) and isorhamnetin 3-O-glucoside (4) (100 μM) was mediated via the inhibition of nuclear factor kappa-B (NFκB) and inflammatory mediators such as TNF-α, interleukin-1β (IL-1β), and interleukin-6 (IL-6) in phytohaemagglutinin-stimulated human peripheral blood mononuclear cells (PBMC) [83][132]. Likewise, narcissin (24) (40 μM) achieved the inhibition of inflammatory cytokines (TNF-α, IL-1β, and IL-6) in advanced glycation end product (AGE)-induced RAW264.7 cells [93][143]. Isorhamnetin-3-O-[2,3-O-isopropylidene-α-l-rhamnopyranosyl]-(1→6)-O-β-d-glucopyranoside (11) (25 μM) showed a significant inhibitory effect on NO release and the secretion of the cytokines IL-6 and TNF-α [48]. Isorhamnetin-3,4′-diglucoside (17) (100 μg/mL) and isorhamnetin 3-O-glucoside (4) (100 μg/mL) have shown the inhibitory effect of IL-6 production on TNF-α-stimulated human osteosarcoma MG-63 cells [94][144]. Isorhamnetin 3-O-glucoside (4) (100 μg/mL) showed distinct anti-inflammatory activity with no toxicity on RAW 264.7 macrophage cells as compared to dexamethasone [95][145]. Seddik Ameur et al. studied the anti-inflammatory activity of IGs extracted from Opuntia ficus-indica flowers, and their results showed that isorhamnetin-3-O-robinobioside (22) is the product responsible for the anti-inflammatory activity [96][146]. Both Opuntia ficus-indica extract (OFI-E) and isorhamnetin-3-O-rhamnosylglucoside (24) (125 ng/mL) significantly inhibited cyclooxygenase-2 (COX-2), TNF-α, and IL-6 production, of which 24 compounds have been suggested to be suitable natural compounds for the development of a new anti-inflammatory ingredient [97][147]. The total flavonoid-rich IGs from sea buckthorn exhibited a protective effect against LPS/CS-induced airway inflammation by inhibiting the ERK, PI3K/Akt, and PKCα pathways and diminishing the expression of IL-1β, IL-6, and COX2 in mice [98][148].5.3. Anti-Cancer Activity
5.3. Anti-Cancer Activity
Flavonoids have great potential for anticancer prevention [99][149]. IGs have also been proven to possess anticancer effects. Brassicin (1) (22.8 µg/mL) showed in vitro cytotoxicity against human colon cancer cells in the HCT116 cell line [100][150]. Isorhamnetin 3-O-neohesperidoside (15) (2.47 μg/mL) showed potent cytotoxicity against breast ductal carcinoma and colorectal adenocarcinoma (Caco-2) cells [101][151]. Narcissin (24) showed cytotoxic effects in Hela cells and the hormone dependent prostate carcinoma LNCaP cell line (IC50 = 20.5 μg/mL) [102][103][152,153]. Mechanically, IGs have been involved in the induction of apoptosis and the inhibition of cancer cell proliferation (Figure 4A). Apoptosis, the most vital cell death mechanism, ultimately contributes to tumor progression [104][154]. Mitochondria play an essential role in cell death signaling and ROS generation [105][155]. The production of ROS above a threshold level can trigger apoptosis in cancer cells, thereby limiting further cancer progression [106][156]. After the excessive production of ROS, the expression of genes related to the mitochondrial apoptosis pathway (Bax, Caspase9, and Caspase3) was aggravated, and the expression of the anti-apoptotic gene Bcl-2 was reduced [107][157]. Emerging evidence suggests that IGs promote ROS generation and the activation of mitochondria-dependent apoptosis in cancer cells (Figure 4B).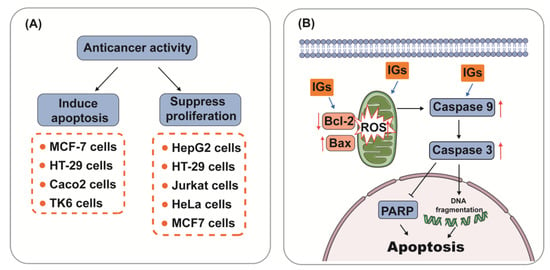
5.4. Hepatoprotective Ability
5.4. Hepatoprotective Ability
The liver is the most essential and functional organ in the body, and it is where primary detox and metabolic events occur [108][167]. Liver injury can be caused by various factors, including alcohol, microbial infection, drugs, biological toxins, and chemical agents [109][168]. Flavonoids in many different foods and medicinal plants have therapeutic potential in liver disease [110][169]. Studies have confirmed that IGs play an important role in liver injury by modulating multiple pathways (Figure 5). The hepatoprotective effects of IGs are closely linked with their antioxidant and anti-inflammatory effects. Isorhamnetin 3-O-galactoside (8) (100 mg/kg) reduced serum TNF-α levels, aminotransferase activities, and the hepatic level of malondialdehyde (MDA); attenuated increases in iNOS and COX-2 protein and mRNA expression levels; attenuated increases in nuclear factor kappa-B (NF-κB) and c-Jun nuclear translocation; and augmented the levels of HO-1 and mRNA expression and the nuclear level of nuclear factor E2-related factor 2 (Nrf2) in a carbon tetrachloride (CCl4)-induced hepatic damage model (Figure 5A). This suggests that IGs exhibit hepatoprotective effects by enhancing the antioxidative defense system and reducing the inflammatory signaling pathways [16]. A similar result was obtained for the hepatoprotective effects of isorhamnetin 3-O-glucoside (4) (20 μg/mL/mouse). It suppressed the increase in plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities in CCl4-induced liver injury mice [111][170]. Opuntia ficus-indica fruit juice (3 mL/rat) administration exerted protective and curative effects against the CCl4-induced degenerative process in rat liver [112][171]. The oral administration of a phenolic-rich fraction of sea buckthorn leaves (25–75 mg/kg) significantly protected against CCl4-induced elevation in AST, ALT, c-glutamyl transpeptidase, and bilirubin in the serum, and also protected against histopathological changes produced by CCl4, such as hepatocytic necrosis, fatty changes, and vacuolation [113][172]. In another study, typhaneoside (45) exhibited hepatoprotective effects on D-GalN-induced cytotoxicity in primary cultured mouse hepatocytes [114][173]. The phytochemical constituents of cactus branch extract (92 mg/kg), which were found to possess excellent antioxidant properties, had protective effects against lithium-induced hepatotoxicity and oxidative stress in rats [115][174].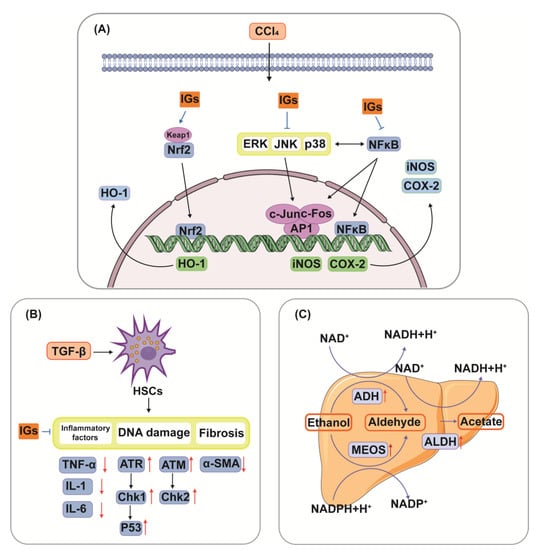
5.5. Antidiabetic Activity
5.5. Antidiabetic Activity
The antidiabetic properties of IGs may appear through different functions. IGs inhibit various pathways associated with the progression of diabetes, including the regulation of glucose metabolism and enhancing insulin secretion [121][179]. IGs exert inhibitory activity on several enzymes involved in diabetes management. In the small intestine, IGs inhibit the activity of α-amylase and α-glucosidase, thereby reducing the conversion of dietary saccharides into easily absorbed monosaccharide, and thus, reducing the postprandial enhancement of blood glucose levels (Figure 6). Isorhamnetin-3-O-glucoside (4) showed a strong ability to bind to α-amylase and α-glucosidase (the IC50 values were 0.16 ± 0.06 and 0.09 ± 0.01 µM) [122][180]. Narcissin (24) (IC50 = 0.129 mM) could be useful in lowering postprandial blood glucose by inhibiting α-amylase activity [123][181]. Meanwhile, 24 was a good 15-lipoxygenase (IC50 = 45 ± 2 µM) inhibitor [124][125][182,183]. Isorhamnetin glucosyl-rhamnosyl-pentoside (50 μg/mL) was reported to exhibit antihyperglycemic activity by inhibiting α-amylase activity [126][184]. Sea buckthorn aqueous extracts were correlated with lipase/α-amylase inhibitory activity in all phases of a digestion model in vitro, with gastric and intestinal fractions largely inhibiting enzyme activity [127][185].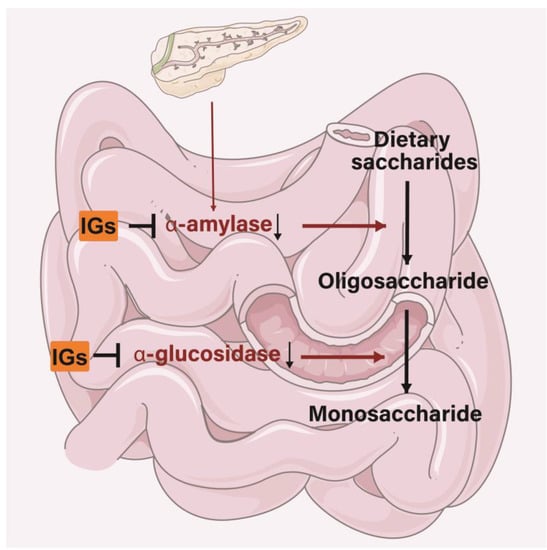
5.6. Anti-Obesity Activity
Flavonoids could protect against obesity-related pathology by inhibiting adipogenesis and exerting anti-inflammatory activity [128][192]. Sea buckthorn leaf extract contains a high content of flavonoid glycosides, especially isorhamnine-3-glucoside (4) and quercetin-3-glucoside [129][78]. Flavonoid glycosides extracted from sea buckthorn leaves (SLGs) could suppress diet-induced obesity in C57BL/6J mice [130][98]. In this researchtudy, the researcheauthors mentioned that 12 weeks of oral administration with a high-fat diet (HFD, 60 kcal% fat) + 0.04% (w/w) SLGs significantly prevented adiposity and dyslipidemia by suppressing lipogenesis and the absorption of dietary fat. This anti-obesity effect was explained by the improvement of inflammation and a decrease in gluconeogenesis. Narcissin (24) and 4 (30 μM) showed moderate inhibitory effects on triglyceride and glycerol-3-phosphate dehydrogenase activity in a 3T3-L1 preadipocyte [131][193]. Furthermore, it was demonstrated by Chang-Suk Kong et al. that 4 (20 μM) potently suppressed adipogenic differentiation by downregulating peroxisome proliferator-activated receptor-γ, CCAAT/enhancer-binding proteins, sterol regulatory element-binding protein 1, and the adipocyte-specific proteins in 3T3-L1 preadipocytes. Furthermore, the specific mechanism mediating its action occurred through the activation of AMPK [132][194].5.7. Antithrombotic Activity
Thrombosis is a critical event in diseases correlated with atherosclerosis, myocardial infarction, and stroke [133][198]. The aggregation of platelets at the site of injury, as well as thrombin generation and fibrin formation triggered by the activation of tissue factors, are involved in thrombosis formation [134][199]. Therefore, the therapeutic mechanism includes the inhibition of platelet activation, adhesion, and aggregation, the improvement of fibrinolytic system function, and the regulation of coagulation system function [135][200].5.8. Toxic Effects
5.8. Toxic Effects
Flavonoids are natural components of fruits, vegetables, tea, wine, traditional medicines (such as ginkgo biloba), and a considerable number of herbal dietary supplements. With growing interest in alternative medicine, the general population is consuming more flavonoids [136][203]. Since flavonoids are common edible ingredients in our daily diets, research on their potential cytotoxicity is warranted. Currently, there are no systematic toxicological studies on IGs, and further studies are needed. Bee bread (BB) is a fermented mixture of plant pollen, honey, and bee saliva, and is rich in flavonoid glycoside derivatives [137]. Filipa Sobral et al. collected a variety of BB samples, and the most abundant compounds in BB1 (>400 µg/mL) were isrohamnetin-O-hexosyl-O-rutinoside and isorhamnetin-O-pentosyl-hexoside. They found that the BB1 sample showed no toxicity to non-tumor porcine liver primary cells [138]. Isorhamnetin-3-rutinoside-4′-glucoside (Currently, there are no systematic toxicological studies on IGs, and further studies are needed. Bee bread (BB) is a fermented mixture of plant pollen, honey, and bee saliva, and is rich in flavonoid glycoside derivatives [204]. Filipa Sobral et al. collected a variety of BB samples, and the most abundant compounds in BB1 (>400 µg/mL) were isrohamnetin-O-hexosyl-O-rutinoside and isorhamnetin-O-pentosyl-hexoside. They found that the BB1 sample showed no toxicity to non-tumor porcine liver primary cells [205]. Isorhamnetin-3-rutinoside-4′ -glucoside (
35), isolated from P. lanceolata inflorescences, showed significantly less cytotoxicity towards the nontumorigenic cell line MCF-12A at a concentration of 400 µM [139]. Isorhamnetin-3-O-β-d-galactopyranoside (), isolated from P. lanceolata inflorescences, showed signifificantlyless cytotoxicity towards the nontumorigenic cell line MCF-12A at a concentration of400 µM [206]. Isorhamnetin-3-O-β-D-galactopyranoside (
8) and isorhamnetin-3-O-β-d-glucopyranoside () and isorhamnetin-3-O-β-D-glucopyranoside (
4) (100 µg/mL) isolated from Salsola imbricata Forssk. exhibited no cytotoxicity in RAW 264.7 macrophage cells [140]. Furthermore, it was demonstrated that the viability of PBMCs was slightly decreased after 48 h of incubation with isoretin-3-O-rutin () (100 µg/mL) isolated from Salsola imbricata Forssk. exhibited no cytotoxicity in RAW 264.7 macrophage cells [158]. Furthermore, it was demonstrated that the viability of PBMCs was slightly decreased after 48 h of incubation with isoretin-3-O-rutin(
24) (0–180 µM) from Cyrtosperma johnstonii. However, the decrease in cell viability was no greater than 30% [141]. A brine shrimp toxicity assay of extracts and isolated compounds from Terminalia macroptera leaves showed that narcissin () (0–180 µM) from Cyrtosperma johnstonii. However, the decrease in cell viability was no greater than 30% [207]. A brine shrimp toxicity assay of extracts and isolated compounds from Terminalia macroptera leaves showed that narcissin (
24) was not toxic against brine shrimp larvae at the tested concentrations (200 µM) [124].) was not toxic against brine shrimp larvae at the tested concentrations (200 µM) [182].
6. Bioaccessibility of IGs
The bioaccessibility of bioactive compounds refers to the maximum fraction of the compound released from the food matrix into the lumen of the gastrointestinal tract to be absorbed [142]. Most flavonoids exist in nature as glycosides, in which sugar residues modify the absorption mechanism and their ability to enter cells or interact with transporters and cellular lipoproteins [143][144]. Flavonoid glycosides exhibit better bioavailability both in vitro and in vivo, which is probably due to their higher aqueous solubility and stability during digestion [8]. At the same time, the gut microbiota plays an important role in improving the bioavailability and enhancing the absorption of flavonoids [145]. The deglycosylation of flavonoid glycosides by the gut microbiota enhances the bioavailability of flavonoids [146].The bioaccessibility of bioactive compounds refers to the maximum fraction of the compound released from the food matrix into the lumen of the gastrointestinal tract to be absorbed [208]. Most flavonoids exist in nature as glycosides, in which sugar residues modify the absorption mechanism and their ability to enter cells or interact with transporters and cellular lipoproteins [209,210]. Flavonoid glycosides exhibit better bioavailability both in vitro and in vivo, which is probably due to their higher aqueous solubility and stability during digestion [8]. At the same time, the gut microbiota plays an important role in improving the bioavailability and enhancing the absorption of flavonoids [211]. The deglycosylation of flavonoid glycosides by the gut microbiota enhances the bioavailability of flavonoids [212].
Compared with isorhamnetin aglycone, IGs have higher accessibility. Antunes-Ricardo et al. found that glycosylation protected isorhamnetin from degradation during simulated digestion, and IGs were better retained in the circulatory system than aglycone [8]. Isorhamnetin-3-O-rutinoside (Compared with isorhamnetin aglycone, IGs have higher accessibility. Antunes Ricardo et al. found that glycosylation protected isorhamnetin from degradation during simulated digestion, and IGs were better retained in the circulatory system than aglycone [8]. Isorhamnetin-3-O-rutinoside (
24) (93.2 ± 0.2%) and isorhamnetin 3-O-glucoside (
4) (66.8 ± 1.7%) from almond skins showed higher bioaccessibility than isorhamnetin (25.1 ± 7.0%) after simulated digestion [147]. Isorhamnetin glucosyl-rhamnosyl-rhamnoside, isorhamnetin glucosyl-rhamnosyl-pentoside, isorhamnetin hexosyl-hexosyl-pentoside, and isorhamnetin glucosyl-pentoside showed high bioaccessibility in the peels of four prickly pear varieties during in vitro simulated gastrointestinal digestion [148]. Isorhamnetin glucosyl-rhamnosyl-rhamnoside and isorhamnetin glucosyl-pentoside in Opuntia ficus-indica cladodes showed bioaccessibility values of 58% and 38% [149].) (66.8 ± 1.7%) from almond skins showed higher bioaccessibility than isorhamnetin (25.1 ± 7.0%) after simulated digestion [213]. Isorhamnetin glucosyl-rhamnosylrhamnoside, isorhamnetin glucosyl-rhamnosyl-pentoside, isorhamnetin hexosyl-hexosyl-pentoside, and isorhamnetin glucosyl-pentoside showed high bioaccessibility in the peels of four prickly pear varieties during in vitro simulated gastrointestinal digestion [58]. Isorhamnetin glucosyl-rhamnosyl-rhamnoside and isorhamnetin glucosyl-pentoside in Opuntia fificus-indica cladodes showed bioaccessibility values of 58% and 38%[215].
It was also reported that the antidiabetic, anti-inflammatory, and antiallergic activities of flavonoid glycosides were similar or even higher than those of aglycones when provided orally [150][151][152][153]. The effect of flavonoid glycosides is beneficial, probably due to the fact that flavonoid glycosides maintain higher plasma concentrations and have a longer mean residence time in the blood than aglycones [154]. Typhaneoside (It was also reported that the antidiabetic, anti-inflammatory, and antiallergic activities of flavonoid glycosides were similar or even higher than those of aglycones when provided orally [216,217,218,219]. The effect of flavonoid glycosides is benefificial, probably due to the fact that flavonoid glycosides maintain higher plasma concentrations and have a longer mean residence time in the blood than aglycones [220]. Typhaneoside (
45) and isorhamnetin-3-O-neohesperidoside (
15) were detected immediately after the oral administrations of pollen typhae extract in rats, indicating that they were rapidly absorbed after oral administration [155][156]. IGs in sea buckthorn berries were monoglucuronidated in humans and were readily bioavailable [157]. Following the ingestion of lightly fried onions, flavonols were absorbed into the plasma of humans as glycosides, with a higher accumulation of isorhamnetin-4′-glucoside () were detected immediately after the oral administrations of pollen typhae extract in rats, indicating that they were rapidly absorbed after oral administration [86,221]. IGs in sea buckthorn berries were monoglucuronidated in humans and were readily bioavailable [222]. Following the ingestion of lightly fried onions, flavonols were absorbed into the plasma of humans as glycosides, with a higher accumulation of isorhamnetin-4′ -glucoside (9) in the plasma and urine than quercetin conjugates, which indicated that 9 may be preferentially absorbed [223]. Similarly, the results of a randomized crossover supplementation trial in female volunteers showed that
9) in the plasma and urine than quercetin conjugates, which indicated that 9 may be preferentially absorbed [158]. Similarly, the results of a randomized crossover supplementation trial in female volunteers showed that 9 underwent significant elevation in the plasma after the ingestion of onion powder [159]. Antunes-Ricardo et al. reported that IGs found naturally in O. ficus-indica have a longer elimination half-life than isorhamnetin, suggesting that they can maintain constant plasma concentrations, and thus, prolong their biological effects [8].underwent significant elevation in the plasma after the ingestion of onion powder [224]. Antunes-Ricardo et al.reported that IGs found naturally in O. ficus-indica have a longer elimination half-life than isorhamnetin, suggesting that they can maintain constant plasma concentrations, and thus, prolong their biological effects [8].
Planar lipophilic polyphenols, such as curcumin, epigallocatechin gallate, quercetin, and genistein, are known as Pan-Assay Interference Compounds (PAINS) or Invalid Metabolic Panaceas (IMPS) because of their ability to interfere with membrane dipole potential [225]. Ana Marta de Matos et al. demonstrated that compounds produced via C-glycosylation are no longer able to alter the membrane dipole potential [226]. However, O-glycosylated compounds are easily hydrolyzed in the gut, so they are not suitable for this strategy. There are no more studies on the interference of isorhamnetin glycosides on membrane dipole potential, so further research in this field is warranted.
Planar lipophilic polyphenols, such as curcumin, epigallocatechin gallate, quercetin, and genistein, are known as Pan-Assay Interference Compounds (PAINS) or Invalid Metabolic Panaceas (IMPS) because of their ability to interfere with membrane dipole potential [160]. Ana Marta de Matos et al. demonstrated that compounds produced via C-glycosylation are no longer able to alter the membrane dipole potential [161]. However, O-glycosylated compounds are easily hydrolyzed in the gut, so they are not suitable for this strategy. There are no more studies on the interference of isorhamnetin glycosides on membrane dipole potential, so further research in this field is warranted.