Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Tan Lian See.
High-frequency ultrasound (HFU) is an ultrasound technology with a frequency higher than 1000 kHz. It has become increasingly recognized as an emerging process intensification technology in various fields, such as biofuel production, carbon dioxide absorption, and wastewater treatment. HFU is seen as a potential intensifier technology for biofuel production, as its mechanisms, such as cavitational phenomena, microstreaming, and fountain formation, can benefit biofuel production.
- biodiesel
- biofuel
- high-frequency ultrasound
1. Introduction
Ultrasound is a type of mechanically oscillating sound wave that can be sustained by an elastic medium, such as air or water. Its frequency ranges from 20 kHz to 10 MHz, which is higher than the limit of human auditory perception (i.e., between 16 Hz and 20 kHz) [1]. According to Chuah et al. [2], ultrasound can be classified into two categories based on its frequency: high-frequency ultrasound (HFU), which has a frequency range of 1000–10,000 kHz, and low-frequency ultrasound (LFU), which has a frequency range of 20–100 kHz. While HFU is more commonly used in medical applications, its use in non-medical fields has been increasing due to its unique characteristics and mechanisms. The application of ultrasound technology has extended to different fields, such as carbon dioxide (CO2) absorption [3,4,5][3][4][5], chemical synthesis [6], wastewater treatment [6[6][7],7], medical imaging [8], algae biomass disruption [9], biodiesel production [10], and the food industry [11,12][11][12].
Ultrasonic applicators can produce different forms of energy by converting electrical energy into heat and vibrational energy using an ultrasonic probe [13] or discs [14]. Figure 1 illustrates the transformation of electrical energy into various forms of energy using an ultrasonic applicator.
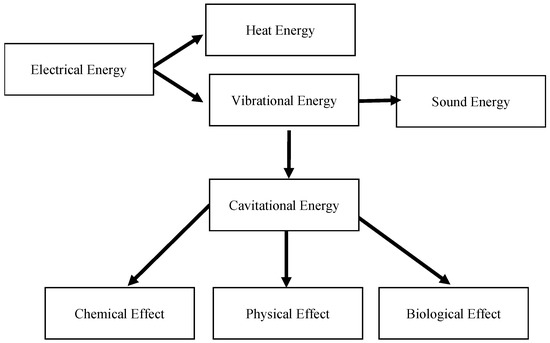
Figure 1.
Energy transformation in ultrasonic applications.
Ultrasound technology can produce different types of effects, including biological effects, such as the break down of algal cell membranes for extraction [15]. Its physical effects, such as ultrasonic cleaning, emulsification, and atomization, have been extensively utilized in commercial products [16]. Atomization emits micro-droplets when the acoustic intensity exceeds a liquid-dependent threshold [17]. On the other hand, the implosion of bubbles during ultrasound irradiation can lead to the formation of highly reactive species, such as OH•, HO2•, and H•, which contributes to the chemical effects of ultrasound technology [18]. The benefits of ultrasound technology extend to various fields, including wastewater treatment, where the vibrational energy from ultrasonic cavitation can help to break down pollutants. The cavitation phenomenon is the formation of bubbles that grow and collapse in a liquid that has been irradiated with ultrasound [19]. Figure 2 illustrates how bubble nuclei in water grow to reach resonance size through a rectified diffusion pathway and a coalescence pathway under the influence of an ultrasonic field. Resonance bubble size is important in ultrasound applications because it affects the effectiveness of the cavitation process. The size of the cavitation bubbles is influenced by the ultrasonic waves, with low-frequency ultrasound producing larger-diameter bubbles, resulting in stronger shear forces, while higher-frequency ultrasound generates smaller and more stable bubbles (stable cavitation) [20,21][20][21]. At lower frequencies, the implosion of cavitation bubbles is more intense compared to that at higher frequencies. At higher ultrasonic frequencies, a larger number of cavitational bubbles are formed, but the collapse intensity is lesser, and higher powers are needed for active bubble formation [22].
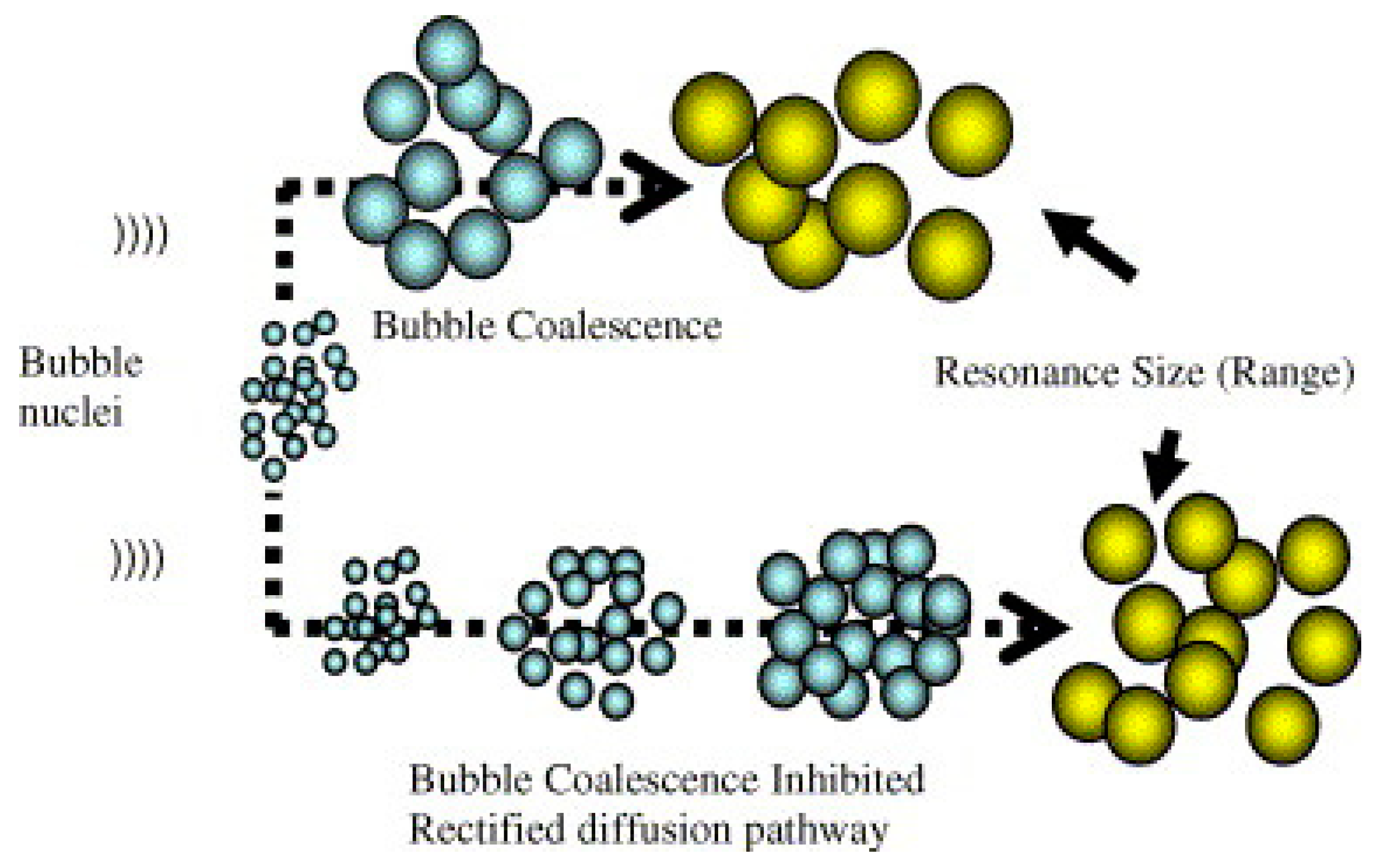
Figure 2. Schematic illustration of bubble nuclei growth in water through a pathway of rectified diffusion and coalescence under the influence of an ultrasonic field (adapted from Ashokkumar et al. [23] with permission from the publisher).
The frequency and cavitation intensity of ultrasound are inversely proportional to each other, as the formation of cavitation bubbles becomes more difficult with shorter rarefaction phases at higher frequencies [1]. At higher ultrasonic frequencies (>1 MHz), jet-like fountain formation and ultrasonic streaming force are enhanced [24]. Shokrollahi et al. [5] found that the fountain formation created under high-frequency ultrasound contributes to the enhancement of mass transfer process. Table 1 summarizes the differences between low-frequency ultrasound and high-frequency ultrasound, while Figure 3 shows the schematic drawing of the behavior of liquid under high-frequency ultrasound conditions.
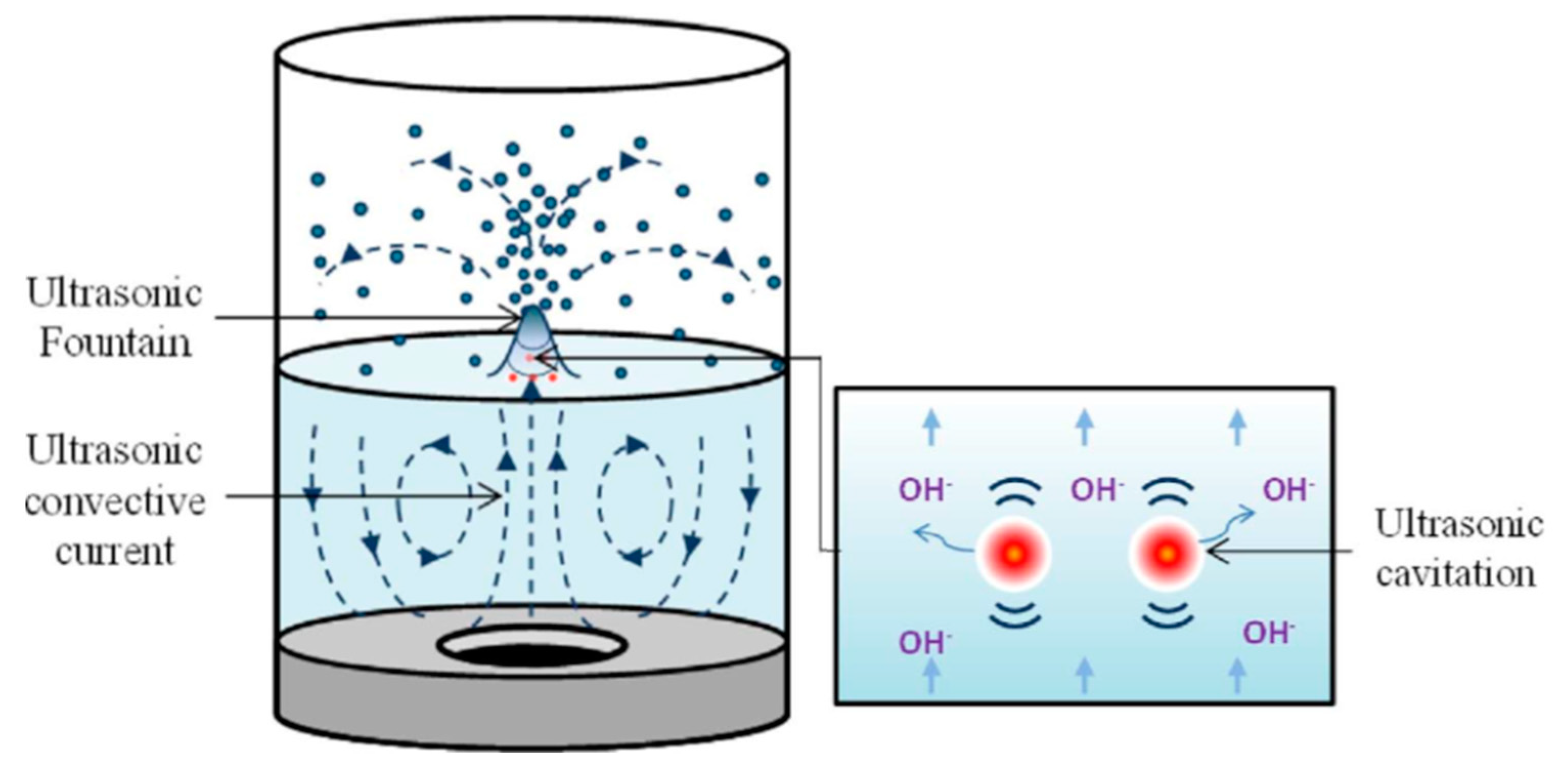
Figure 3. Schematic drawing of the formation of an acoustic fountain (adapted from Tay et al. [3] with permission from the publisher).
Various parameters, such as power, frequency, reaction condition, operation mode, and mechanical vibrations, can affect the occurrence and intensity of ultrasonic effects [25]. Therefore, understanding the interaction between these parameters is crucial in enhancing the use of high-frequency ultrasound for different purposes.
Table 1.
Comparison between high- and low-frequency ultrasound.
| Low-Frequency Ultrasound | Characteristics | High-Frequency Ultrasound |
|---|---|---|
| 20–100 kHz [2] | Frequency Range | 1000–10,000 kHz [2] |
| Production of larger bubbles [20] | Cavitation Bubbles Size | Production of smaller bubbles [21] |
| Lesser [22] | Quantity of Bubbles | Higher [22] |
| Higher intensity [20] | Bubble Collapse Intensity | Lower intensity [20] |
| Lesser free radical formed [26] | Amount of Free Radical Formation | Higher free radical formed [26] |
| None or weak fountain formation [27] | Formation of Fountain (Figure 3) |
Yes [28] |
Schueller and Yang [29] observed that cavitation phenomena formed under lower frequencies aid in the desorption process more than absorption and adsorption processes. At higher frequencies, the cavitation phenomena are less intense, and fountain formation increases mass transfer coefficients for CO2 absorption The role of high-frequency ultrasound is slowly gaining attention across different fields, particularly in biofuel production. The mechanism of HFU (e.g., cavitation phenomena, jet-like fountain formation, and ultrasonic streaming force) plays a major role in the formation of eddies, turbulence, and shear forces that enhance the transesterification process [30[30][31],31], directly influencing the production of biodiesel. Meanwhile, cavitation phenomena aid in the breakdown of algal cell walls, improving the extraction process of products such as biofuel.
According to Hasan et al. [32], research conducted on biofuel production increased from the year 2005 onwards, with an increase in the number of published articles on the subject being evident over the last 16 years, and 2019 marked the highest rate of publications. Climate change and increasing energy demands are major contributors to the expansion of biofuel economics, and biofuel plays a vital role in addressing the increasing energy demand as a potential energy source [33]. However, the production of biofuel is still not economically viable, and more research and development for technological enhancement is required [34].
Various intensifying technologies have been applied to the production of green biofuels. Still, each technology possesses different advantages and disadvantages. LFU is well-established for biodiesel production [35], but there is still a lack of reports on HFU as an intensification technology for this application [31]. Therefore, a great deal of uncertainty regarding the relationship between HFU technology and biofuel production exists.
2. High-Frequency Ultrasound Technology for Biofuel Production
Reliance on fossil fuels for energy and transportation is having detrimental effects on the environment. The combustion of fossil fuels releases greenhouse gases, such as CO2, sulfur dioxide (SO2), and nitrogen oxide (NOx), which contribute to global warming. This situation is alarming as society and industries continue to rely heavily on the use of fossil fuels in their power plants and as a primary source of energy for transportation fuel. Therefore, clean, sustainable, and renewable energy sources are critically needed to reduce the negative environmental impact and ensure a continuous supply of energy for the future. Biofuel, a new potential energy source derived from different renewable feedstocks, may reduce the reliance on the usage of non-renewable fossil fuels. Demirbas [36] denoted biofuel as liquid, gas, and solid fuels produced from biomass. Meanwhile, according to Raboni et al. [37], biofuel comprises any product obtained from biomass and this includes biodiesel, biofuels, biogas, bioethanol, and bio-methanol. Biofuel production is highly supported by various countries, including Brazil, Germany, Switzerland, and Sweden, as efforts have been intensified in order to reduce the reliance on fossil fuels as an energy source. Biodiesel, or fatty acid methyl esters (FAME), is regarded as a potential source to replace the use of fossil fuels and has been studied globally [38]. Biodiesel can be potentially utilized as an energy resource in the coming future, as it can be produced from numerous potential feedstocks, such as microalgae [38[38][39][40][41],39,40,41], waste cooking oil [42[42][43],43], animal fat [44[44][45],45], by products such as rice bran [46], and various vegetable seeds oil [47,48,49,50][47][48][49][50]. According to Mahbub et al. [51], the usage of biodiesel has several benefits, including a reduction in CO2 and carbon monoxide (CO) emissions by 8–41%, based on life cycle assessment studies. Previous research has proposed the use of biodiesel as a complete or partially mixed alternative for diesel engines, since biodiesel usage reduces exhaust emissions as the composition of biodiesel contains less carbon, water, and sulfur with a higher amount of oxygen than conventional petroleum [52]. Biodiesel can be introduced into diesel engines without modifications because the current diesel engines are compatible with biodiesel [53].2.1. High-Frequency Ultrasound-Assisted Transesterification Process for Biodiesel Production
Transesterification is a more popular method for producing biodiesel compared to other methods, such as the direct use of blended oils, micro-emulsion of oils, and thermal cracking (pyrolysis) of oils [30,54][30][54]. This process converts feedstock (i.e., oil) into methyl or ethyl esters using an alcohol source, such as methanol or ethanol, and a catalyst. Glycerol is produced as a side product during the reaction [55]. Transesterification is carried out at a mild temperature [56] and requires simpler conditions in comparison to other methods. For example, micro-emulsification requires different alcohol solvents with colloidal microstructures [57], while pyrolysis requires high temperatures ranging from 300 °C to 1300 °C, which may lead to changes in the chemical structure of the compounds [58]. Therefore, transesterification is more economically feasible and simpler, making it the preferred method for producing biodiesel compared to other methods [59]. However, the production of biodiesel via the transesterification process still faces some challenges. Firstly, the heterogenous nature of the reactants (alcohol and vegetable oil) does not form a homogenous mixture [43,60][43][60], which requires intensive mixing processes to increase the mass transfer rate, resulting in a higher power consumption. Additionally, the viscosity of the different feedstocks used may differ, leading to higher or lower agitation requirements. Moreover, the two-way reaction of the transesterification process requires a higher alcohol-to-oil molar ratio. An excess of alcohol aids in product formation instead of reactant formation, resulting in higher conversion rates that directly lead to increasing biodiesel expenditure [61]. Ultrasound-assisted transesterification is considered an approach to enhance biodiesel production. The characteristics of ultrasound technology, such as cavitation bubbles, microstreaming, and fountain formation, enhance mixing between heterogenous mixtures, reducing the reliance on additional mixing processes. The application of ultrasound technology allows for higher reaction rates and lower alcohol-to-oil molar ratios [62,63][62][63]. LFU has been widely researched for transesterification processes [15,30,64,65][15][30][64][65] due to the high-intensity collapse of cavitation bubbles, which aids in overcoming the mass transfer limitation. However, there is still a lack of reports on the use of HFU in the transesterification process [31], which requires further exploration. Recent studies have shown that high-frequency ultrasound technology is beneficial for biofuel production. The phenomena of cavitation, jet-like fountain formation, and ultrasonic streaming force are prominent characteristics that facilitate the mixing of immiscible liquids, such as alcohol and oil. However, it remains unclear which of these characteristics is more advantageous for mixing immiscible liquids. Oliveira et al. [31] studied the influence of low power (3–9 W) and high frequency (1 MHz and 3 MHz) without an external heating source on the transesterification of soybean oil. The results showed that ultrasound-assisted transesterification increased soybean oil conversion from 48.7% to 79.5% when the alcohol-to-oil ratio was 6:1 at 1 MHz and 3 MHz, respectively, while a molar ratio of 8:1 at 1 MHz and 3 MHz achieved a conversion of 59.5% to 84.6%, kept at the same reaction time of 40 min. Within 10 min, the HFU of 3 MHz achieved a conversion of 79.8% compared to the HFU of 1 MHz, at a conversion rate of 53.6% after 20 min at a 8:1 M ratio. This shows that HFU along with excess methanol can result in a higher reaction rate within a shorter time period, as the excess methanol shifts. This is because the diameter of the cavitation bubbles produced by ultrasonic frequency is dependent on the frequency, causing variations in bubble size [31]. Since the size of the cavitation bubbles is inversely proportional to ultrasonic frequency [66[66][67],67], this may, to a certain extent, facilitate higher collision rates between reactants, leading to a greater conversion rate (Figure 4).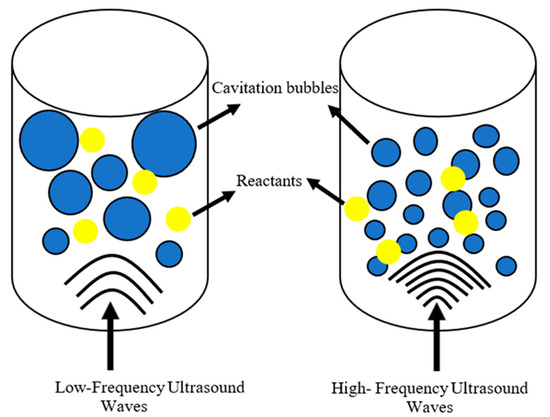
Figure 4.
Differences between the amount of cavitation bubbles between LFU and HFU.
Table 2.
Reaction conditions for high-frequency ultrasound-assisted transesterification.
| Authors | |||
|---|---|---|---|
| Oliveira et al. [31] | Mahamuni and Adewuyi [18] | Aghbashlo et al. [35] | |
| Feed | Soybean oil | Waste cooking oil (WCO) | |
| Ultrasonic Reactor Type | Transducer | Piezoelectric-based ultrasonic reactor | |
| Catalyst | Potassium hydroxide (KOH) | ||
| Alcohol | Methanol | ||
| Conditions | Study was conducted at the frequencies of 1 MHz and 3 MHz with alcohol-to-oil molar ratios of 6:1 and 8:1, respectively, at a reaction time of 40 min (without external heating or mechanical stirring). | Study was conducted at the frequencies of 323 kHz, 581 kHz, 611 kHz, and 1.3 MHz with an alcohol-to-oil molar ratio of 6:1 and a power between 12 and 223 W, with a reaction time ranging from 60 min to 180 min. | Study was conducted at the frequency of 1.7 MHz with alcohol-to-oil molar ratios of 4:1, 6:1, and 8:1 and a power of 31 W, with the reaction times of 6, 8, and 10 min. |
2.2. High-Frequency Ultrasound on Microalgal Cell Disruption for Biofuel Production
Microalgae, which are photosynthetic microorganisms that require minimal growth requirements, are considered a promising source of lipids, proteins, and carbohydrates. They can produce large quantities of bioproducts in a short time and, thus, are an ideal feedstock for biodiesel production. Microalgae are environmentally and economically advantageous due to their high growth rate and biomass productivity, and their ability to accumulate bioproducts, such as carbohydrates and lipids, under nutrient-limited conditions [71]. Unlike crops, which require growing cycles ranging from three months to three years [72], microalgae have a short growth cycle, and bioproducts such as lipids can be harvested in just 3–5 days. Once the microalgae biomass is cultivated, harvesting and dewatering are carried out, followed by lipid extraction from microalgae [73]. The use of ultrasound pre-treatment is known to increase the lipid yield from the microalgal biomass, as it aids in the disruption of biomass for lipid extraction [74]. Ultrasonic irradiation breaks down the microalgal cell wall and reduces microalgal particle size, leading to a better release of chemical content, thus enhancing extraction efficiency [75]. Figure 5 illustrates the use of HFU technology to aid microalgal cell lysis.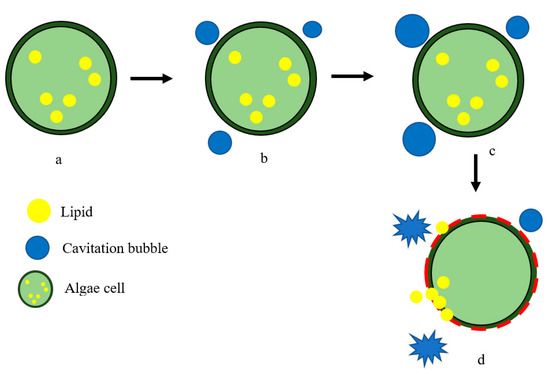
Figure 5. Disruption of algal cell using ultrasound: (a) condition of algal cell prior to disruption; (b) formation of cavitation bubbles; (c) growth of cavitation bubbles; and (d) breakdown of algal cell wall and release of lipids.
References
- Vernès, L.; Vian, M.; Chemat, F. Chapter 12—Ultrasound and Microwave as Green Tools for Solid-Liquid Extraction. In Liquid-Phase Extraction; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 355–374.
- Chuah, L.F.; Klemeš, J.J.; Yusup, S.; Bokhari, A.; Akbar, M.M. A review of cleaner intensification technologies in biodiesel production. J. Clean. Prod. 2017, 146, 181–193.
- Tay, W.H.; Lau, K.K.; Shariff, A.M. High performance promoter-free CO2 absorption using potassium carbonate solution in an ultrasonic irradiation system. J. CO2 Util. 2017, 21, 383–394.
- Yusof, S.M.M.; Shariff, A.M.; Tay, W.H.; Lau, K.K.; Mustafa, N.F.A. Mass transfer intensification of CO2 absorption in monoethanolamine using high frequency ultrasonic technology in continuous system. Int. J. Greenh. Gas Control 2020, 102, 103157.
- Shokrollahi, F.; Lau, K.K.; Tay, W.H. Performance comparison of ultrasonic-assisted and magnetic stirred absorption methods for CO2 separation. SN Appl. Sci. 2020, 2, 1217.
- Tan, S.X.; Lim, S.; Ong, H.C.; Pang, Y.L. State of the art review on development of ultrasound-assisted catalytic transesterification process for biodiesel production. Fuel 2019, 235, 886–907.
- Mohammadi, P.; Karami, N.; Zinatizadeh, A.A.; Falahi, F.; Aghamohammadi, N.; Almasi, A. Using high frequency and low-intensity ultrasound to enhance activated sludge characteristics. Ultrason. Sonochem. 2019, 54, 274–280.
- Mason, T.J. Sonochemistry and sonoprocessing: The link, the trends and (probably) the future. Ultrason. Sonochem. 2003, 10, 175–179.
- Wang, M.; Yuan, W.; Jiang, X.; Jing, Y.; Wang, Z. Disruption of microalgal cells using high-frequency focused ultrasound. Bioresour. Technol. 2014, 153, 315–321.
- Somnuk, K.; Phanyusoh, D.; Thawornprasert, J.; Oo, Y.M.; Prateepchaikul, G. Continuous Ultrasound-Assisted Esterification and Transesterification of Palm Fatty Acid Distillate for Ethyl Ester Production. Processes 2021, 9, 449.
- Yang, F.; Shi, C.; Yan, L.; Xu, Y.; Dai, Y.; Bi, S.; Liu, Y. Low-frequency ultrasonic treatment: A potential strategy to improve the flavor of fresh watermelon juice. Ultrason. Sonochem. 2022, 91, 106238.
- Miladi, M.; Martins, A.A.; Mata, T.M.; Vegara, M.; Pérez-Infantes, M.; Remmani, R.; Ruiz-Canales, A.; Núñez-Gómez, D. Optimization of Ultrasound-Assisted Extraction of Spent Coffee Grounds Oil Using Response Surface Methodology. Processes 2021, 9, 2085.
- Kasaai, M.R. Input power-mechanism relationship for ultrasonic irradiation: Food and polymer applications. Nat. Sci. 2013, 5, 14–22.
- Thanh Nguyen, T.; Asakura, Y.; Koda, S.; Yasuda, K. Dependence of cavitation, chemical effect, and mechanical effect thresholds on ultrasonic frequency. Ultrason. Sonochem. 2017, 39, 301–306.
- Martinez-Guerra, E.; Gude, V.G. Continuous and pulse sonication effects on transesterification of used vegetable oil. Energy Convers. Manag. 2015, 96, 268–276.
- Asakura, Y.; Yasuda, K. Frequency and power dependence of the sonochemical reaction. Ultrason. Sonochem. 2021, 81, 105858.
- Simon, J.C.; Sapozhnikov, O.A.; Khokhlova, V.A.; Crum, L.A.; Bailey, M.R. Ultrasonic atomization of liquids in drop-chain acoustic fountains. J. Fluid Mech. 2015, 766, 129–146.
- Mahamuni, N.N.; Adewuyi, Y.G. Optimization of the Synthesis of Biodiesel via Ultrasound-Enhanced Base-Catalyzed Transesterification of Soybean Oil Using a Multifrequency Ultrasonic Reactor. Energy Fuels 2009, 23, 2757–2766.
- Reuter, F.; Lesnik, S.; Ayaz-Bustami, K.; Brenner, G.; Mettin, R. Bubble size measurements in different acoustic cavitation structures: Filaments, clusters, and the acoustically cavitated jet. Ultrason. Sonochem. 2019, 55, 383–394.
- Tiehm, A.; Nickel, K.; Zellhorn, M.; Neis, U. Ultrasonic waste activated sludge disintegration for improving anaerobic stabilization. Water Res. 2001, 35, 2003–2009.
- Wayment, D.G.; Casadonte, D.J. Frequency effect on the sonochemical remediation of alachlor. Ultrason. Sonochem. 2002, 9, 251–257.
- Bhangu, S.K.; Gupta, S.; Ashokkumar, M. Ultrasonic enhancement of lipase-catalysed transesterification for biodiesel synthesis. Ultrason. Sonochem. 2017, 34, 305–309.
- Ashokkumar, M.; Lee, J.; Kentish, S.; Grieser, F. Bubbles in an acoustic field: An overview. Ultrason. Sonochem. 2007, 14, 470–475.
- Loh, B.G.; Hyun, S.; Ro, P.I.; Kleinstreuer, C. Acoustic streaming induced by ultrasonic flexural vibrations and associated enhancement of convective heat transfer. J. Acoust. Soc. Am. 2002, 111, 875–883.
- Naji, O.; Al-juboori, R.A.; Khan, A.; Yadav, S.; Altaee, A.; Alpatova, A.; Soukane, S.; Ghaffour, N. Ultrasound-assisted membrane technologies for fouling control and performance improvement: A review. J. Water Process Eng. 2021, 43, 102268.
- Crum, L.A. Comments on the evolving field of sonochemistry by a cavitation physicist. Ultrason. Sonochem. 1995, 2, S147–S152.
- Kim, G.; Cheng, S.; Hong, L.; Kim, J.T.; Li, K.C.; Chamorro, L.P. On the acoustic fountain types and flow induced with focused ultrasound. J. Fluid Mech. 2021, 909, R2.
- Gondrexon, N.; Renaudin, V.; Petrier, C.; Clement, M.; Boldo, P.; Gonthier, Y.; Bernis, A. Experimental study of the hydrodynamic behaviour of a high frequency ultrasonic reactor. Ultrason. Sonochem. 1998, 5, 1–6.
- Schueller, B.S.; Yang, R.T. Ultrasound enhanced adsorption and desorption of phenol on activated carbon and polymeric resin. Ind. Eng. Chem. Res. 2001, 40, 4912–4918.
- Degfie, T.A.; Mamo, T.T.; Mekonnen, Y.S. Optimized Biodiesel Production from Waste Cooking Oil (WCO) using Calcium Oxide (CaO) Nano-catalyst. Sci. Rep. 2019, 9, 18982.
- Oliveira, P.A.; Baesso, R.M.; Morais, G.C.; Alvarenga, A.V.; Costa-Félix, R.P.B. Ultrasound-assisted transesterification of soybean oil using low power and high frequency and no external heating source. Ultrason. Sonochem. 2021, 78, 105709.
- Hasan, M.; Abedin, M.Z.; Amin, M.B.; Nekmahmud, M.; Oláh, J. Sustainable biofuel economy: A mapping through bibliometric research. J. Environ. Manag. 2023, 336, 117644.
- Callegari, A.; Bolognesi, S.; Cecconet, D.; Capodaglio, A.G. Production technologies, current role, and future prospects of biofuels feedstocks: A state-of-the-art review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 384–436.
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093.
- Aghbashlo, M.; Tabatabaei, M.; Hosseinpour, S.; Hosseini, S.S.; Ghaffari, A.; Khounani, Z.; Mohammadi, P. Development and evaluation of a novel low power, high frequency piezoelectric-based ultrasonic reactor for intensifying the transesterification reaction. Biofuel Res. J. 2016, 3, 528–535.
- Demirbas, A. Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers. Manag. 2008, 49, 2106–2116.
- Raboni, M.; Viotti, P.; Capodaglio, A.G. A comprehensive analysis of the current and future role of biofuels for transport in the European union (EU). Rev. Ambiente Agua 2015, 10, 9–21.
- Saengsawang, B.; Bhuyar, P.; Manmai, N.; Ponnusamy, V.K.; Ramaraj, R.; Unpaprom, Y. The optimization of oil extraction from macroalgae, Rhizoclonium sp. by chemical methods for efficient conversion into biodiesel. Fuel 2020, 274, 117841.
- Velasquez-Orta, S.B.; Lee, J.G.M.; Harvey, A. Alkaline in situ transesterification of Chlorella vulgaris. Fuel 2012, 94, 544–550.
- Ma, Y.; Liu, S.; Wang, Y.; Adhikari, S.; Dempster, T.A.; Wang, Y. Direct biodiesel production from wet microalgae assisted by radio frequency heating. Fuel 2019, 256, 115994.
- Lee, J.-Y.; Yoo, C.; Jun, S.-Y.; Ahn, C.-Y.; Oh, H.-M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77.
- Bargole, S.S.; Singh, P.K.; George, S.; Saharan, V.K. Valorisation of low fatty acid content waste cooking oil into biodiesel through transesterification using a basic heterogeneous calcium-based catalyst. Biomass Bioenergy 2021, 146, 105984.
- Gupta, A.R.; Yadav, S.V.; Rathod, V.K. Enhancement in biodiesel production using waste cooking oil and calcium diglyceroxide as a heterogeneous catalyst in presence of ultrasound. Fuel 2015, 158, 800–806.
- Shobhana, G.; Asikin-Mijan, N.; AbdulKareem-Alsultan, G.; Sivasangar, S.; Izham, S.M.; Taufiq-Yap, Y.H. Biodiesel production via simultaneous esterification and transesterification of chicken fat oil by mesoporous sulfated Ce supported activated carbon. Biomass Bioenergy 2020, 141, 105714.
- El-Shafay, A.S.; Ağbulut, Ü.; Attia, E.-A.; Touileb, K.L.; Gad, M.S. Waste to energy: Production of poultry-based fat biodiesel and experimental assessment of its usability on engine behaviors. Energy 2023, 262, 125457.
- Costa, E.; Almeida, M.F.; Alvim-Ferraz, M.C.; Dias, J.M. Exploiting the Complementary Potential of Rice Bran Oil as a Low-Cost Raw Material for Bioenergy Production. Processes 2022, 10, 2460.
- Rao, T.; Rao, G.; Reddy, K. Experimental Investigation of Pongamia, Jatropha and Neem Methyl Esters as Biodiesel on C.I. Engine. Jordan J. Mech. Ind. Eng. 2008, 2, 117–122.
- Li, J.; Fu, Y.-J.; Qu, X.-J.; Wang, W.; Luo, M.; Zhao, C.-J.; Zu, Y.-G. Biodiesel production from yellow horn (Xanthoceras sorbifolia Bunge.) seed oil using ion exchange resin as heterogeneous catalyst. Bioresour. Technol. 2012, 108, 112–118.
- Georgogianni, K.G.; Kontominas, M.G.; Pomonis, P.J.; Avlonitis, D.; Gergis, V. Conventional and in situ transesterification of sunflower seed oil for the production of biodiesel. Fuel Process. Technol. 2008, 89, 503–509.
- Vital-López, L.; Mercader-Trejo, F.; Rodríguez-Reséndiz, J.; Zamora-Antuñano, M.A.; Rodríguez-López, A.; Esquerre-Verastegui, J.E.; Farrera Vázquez, N.; García-García, R. Electrochemical Characterization of Biodiesel from Sunflower Oil Produced by Homogeneous Catalysis and Ultrasound. Processes 2023, 11, 94.
- Mahbub, N.; Gemechu, E.; Zhang, H.; Kumar, A. The life cycle greenhouse gas emission benefits from alternative uses of biofuel coproducts. Sustain. Energy Technol. Assess. 2019, 34, 173–186.
- Xue, J.; Grift, T.E.; Hansen, A.C. Effect of biodiesel on engine performances and emissions. Renew. Sustain. Energy Rev. 2011, 15, 1098–1116.
- Musthafa, M.M.; Kumar, T.A.; Mohanraj, T.; Chandramouli, R. A comparative study on performance, combustion and emission characteristics of diesel engine fuelled by biodiesel blends with and without an additive. Fuel 2018, 225, 343–348.
- Silitonga, A.S.; Shamsuddin, A.H.; Mahlia, T.M.I.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.H.; Masjuki, H.H.; Ong, H.C. Biodiesel synthesis from Ceiba pentandra oil by microwave irradiation-assisted transesterification: ELM modeling and optimization. Renew. Energy 2020, 146, 1278–1291.
- Demirbaş, A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: A survey. Energy Convers. Manag. 2003, 44, 2093–2109.
- Babadi, A.A.; Rahmati, S.; Fakhlaei, R.; Barati, B.; Wang, S.; Doherty, W.; Ostrikov, K. Emerging technologies for biodiesel production: Processes, challenges, and opportunities. Biomass Bioenergy 2022, 163, 106521.
- Mishra, V.K.; Goswami, R. A review of production, properties and advantages of biodiesel. Biofuels 2018, 9, 273–289.
- Karmakar, B.; Halder, G. Progress and future of biodiesel synthesis: Advancements in oil extraction and conversion technologies. Energy Convers. Manag. 2019, 182, 307–339.
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Adam, A.A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509.
- Badday, A.S.; Abdullah, A.Z.; Lee, K.T.; Khayoon, M.S. Intensification of biodiesel production via ultrasonic-assisted process: A critical review on fundamentals and recent development. Renew. Sustain. Energy Rev. 2012, 16, 4574–4587.
- Qiu, Z.; Zhao, L.; Weatherley, L. Process intensification technologies in continuous biodiesel production. Chem. Eng. Process. Process Intensif. 2010, 49, 323–330.
- Hingu, S.M.; Gogate, P.R.; Rathod, V.K. Synthesis of biodiesel from waste cooking oil using sonochemical reactors. Ultrason. Sonochem. 2010, 17, 827–832.
- Badday, A.S.; Abdullah, A.Z.; Lee, K.-T. Ultrasound-assisted transesterification of crude Jatropha oil using cesium doped heteropolyacid catalyst: Interactions between process variables. Energy 2013, 60, 283–291.
- Kumar, D.; Kumar, G.; Poonam; Singh, C.P. Ultrasonic-assisted transesterification of Jatropha curcus oil using solid catalyst, Na/SiO2. Ultrason. Sonochem. 2010, 17, 839–844.
- Mootabadi, H.; Salamatinia, B.; Bhatia, S.; Abdullah, A.Z. Ultrasonic-assisted biodiesel production process from palm oil using alkaline earth metal oxides as the heterogeneous catalysts. Fuel 2010, 89, 1818–1825.
- Gallego-Juarez, J.; Graff, K.F. Power Ultrasonics: Applications of High-Intensity Ultrasound; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–1142.
- Ashokkumar, M.; Hodnett, M.; Zeqiri, B.; Grieser, F.; Price, G.J. Acoustic Emission Spectra from 515 kHz Cavitation in Aqueous Solutions Containing Surface-Active Solutes. J. Am. Chem. Soc. 2007, 129, 2250–2258.
- Ho, W.W.S.; Ng, H.K.; Gan, S. Advances in ultrasound-assisted transesterification for biodiesel production. Appl. Therm. Eng. 2016, 100, 553–563.
- Veljković, V.B.; Avramović, J.M.; Stamenković, O.S. Biodiesel production by ultrasound-assisted transesterification: State of the art and the perspectives. Renew. Sustain. Energy Rev. 2012, 16, 1193–1209.
- Bendicho, C.; Lavilla, I. EXTRACTION | Ultrasound Extractions. In Encyclopedia of Separation Science; Wilson, I.D., Ed.; Academic Press: Oxford, UK, 2000; pp. 1448–1454.
- Yin, Z.; Zhu, L.; Li, S.; Hu, T.; Chu, R.; Mo, F.; Hu, D.; Liu, C.; Li, B. A comprehensive review on cultivation and harvesting of microalgae for biodiesel production: Environmental pollution control and future directions. Bioresour. Technol. 2020, 301, 122804.
- Demirbas, M.F. Biofuels from algae for sustainable development. Appl. Energy 2011, 88, 3473–3480.
- Ajala, E.O.; Ajala, M.A.; Akinpelu, G.S.; Akubude, V.C. Cultivation and Processing of Microalgae for Its Sustainability as a Feedstock for Biodiesel Production. Niger. J. Technol. Dev. 2021, 18, 322–343.
- Naveena, B.; Armshaw, P.; Tony Pembroke, J. Ultrasonic intensification as a tool for enhanced microbial biofuel yields. Biotechnol. Biofuels 2015, 8, 140.
- Luo, J.; Fang, Z.; Smith, R.L. Ultrasound-enhanced conversion of biomass to biofuels. Prog. Energy Combust. Sci. 2014, 41, 56–93.
- Singh, N.; Kumar, K.; Goyal, A.; Moholkar, V.S. Ultrasound-assisted biodiesel synthesis by in–situ transesterification of microalgal biomass: Optimization and kinetic analysis. Algal Res. 2022, 61, 102582.
- Keris-Sen, U.D.; Sen, U.; Soydemir, G.; Gurol, M.D. An investigation of ultrasound effect on microalgal cell integrity and lipid extraction efficiency. Bioresour. Technol. 2014, 152, 407–413.
- Yamamoto, K.; King, P.M.; Wu, X.; Mason, T.J.; Joyce, E.M. Effect of ultrasonic frequency and power on the disruption of algal cells. Ultrason. Sonochem. 2015, 24, 165–171.
- Adam, F.; Abert-Vian, M.; Peltier, G.; Chemat, F. “Solvent-free” ultrasound-assisted extraction of lipids from fresh microalgae cells: A green, clean and scalable process. Bioresour. Technol. 2012, 114, 457–465.
- Ferreira, A.F.; Dias, A.P.S.; Silva, C.M.; Costa, M. Effect of low frequency ultrasound on microalgae solvent extraction: Analysis of products, energy consumption and emissions. Algal Res. 2016, 14, 9–16.
- Zhang, X.; Yan, S.; Tyagi, R.D.; Drogui, P.; Surampalli, R.Y. Ultrasonication assisted lipid extraction from oleaginous microorganisms. Bioresour. Technol. 2014, 158, 253–261.
- Natarajan, R.; Ang, W.M.R.; Chen, X.; Voigtmann, M.; Lau, R. Lipid releasing characteristics of microalgae species through continuous ultrasonication. Bioresour. Technol. 2014, 158, 7–11.
- Kurokawa, M.; King, P.M.; Wu, X.; Joyce, E.M.; Mason, T.J.; Yamamoto, K. Effect of sonication frequency on the disruption of algae. Ultrason. Sonochem. 2016, 31, 157–162.
More
