Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Maged Henary.
Hydrogen sulfide (H
2
S) is an essential signaling gas within the cell, and its endogenous levels are correlated with various health diseases such as Alzheimer’s disease, diabetes, Down’s syndrome, and cardiovascular disease. Because it plays such diverse biological functions, being able to detect H
2
S quickly and accurately in vivo is an area of heightened scientific interest.
- H2S sensor
- cyanine dye synthesis
- UV/Vis spectroscopy
1. Introduction
Hydrogen sulfide (H2S) is the third most important endogenous signal gas that cells use [1[1][2],2], after carbon monoxide and nitric oxide. It mediates multiple physiological and pathological processes [3,4][3][4]. While crucial to normal biological function, H2S can prove an extremely toxic gas at higher concentrations. Some basic physicochemical and thermodynamic properties of H2S are listed in Table 1 [5].
Table 1. Basic physicochemical and thermodynamic properties of H2S.
| Properties | Data | |||||||
|---|---|---|---|---|---|---|---|---|
| Dipole moment | 0.97 D | |||||||
| Solubility (in H | 2 | O) | 110 mM/atm, 25 °C | |||||
| 210 mM/atm, 0 °C | ||||||||
| Boiling temperature | −60.2 °C | |||||||
| Density (25 °C, 1 atm) | 1.36 kg/m | 3 | ||||||
| IR | ν | 1 | 2525, 2536 cm | −1 | ||||
| ν | 2 | 1169, 1184, 1189 cm | −1 | |||||
| ν | 3 | 2548 cm | −1 | |||||
| 1 | H-NMR | 0.52 ppm | ||||||
| p | K1 | 6.98 | ||||||
| p | K2 | >17 at 25 °C | ||||||
| λ | max | (HS | − | ) | 230 nm | |||
| ε | 8 × 10 | 3 | M | −1 | ·cm | −1 | ||
| Henry’s law coefficient (298 K) | 0.087135 mol solute/mol water atom | |||||||
| Detection threshold by human nose | 0.02–0.03 ppm | |||||||
| Lethal dose | >500 ppm | |||||||
| Δ | fG | ° (H | 2 | S) | −28 kJ/mol | |||
| Δ | fG | ° (HS | − | ) | +12 kJ/mol | |||
| Δ | fG | ° (S | 2− | ) | +86 kJ/mol | |||
| E | °′ (S | •− | , H | + | /HS | − | ) | +0.91 V |
| E | °′ (HS | 2− | , H | + | /2HS | − | ) | −0.23 V |
A simple molecule, H2S is a marker of some of society’s most prevalent maladies. Cardiovascular disease, diabetes, and Down’s syndrome have all been associated with an increased level of H2S [6]. It is known to regulate myriad biological functions in vivo; oxidative stress, insulin signaling, and cell growth and death are all mediated by this species [7,8][7][8]. Energy available to the cell can also be affected by levels of H2S because it is involved in the inhibition of adenosine triphosphate (ATP) [9,10,11,12,13][9][10][11][12][13].
Biosynthesis of H2S in mammals is carried out by the enzymes cystathionine β-synthase (CBS), 3-mercaptopyruvate sulfotransferase (3-MST), and cystathionine γ-lyase (CSE) [14]. The endogenous pathways are laid out in Scheme 1 [15]. Homocysteine acts as the substrate for CBS, with H2S and cystathionine being liberated. The second mode involves a “desulfurization” reaction, whereby sulfur is cleaved from cysteine directly without its oxidation. Furthermore, it has been reported that the activities of these two enzymes in different cells and tissues are correlated to several health disorders [16,17,18][16][17][18]. For these reasons, H2S has become an important medium in the detection of disease [19,20,21][19][20][21].
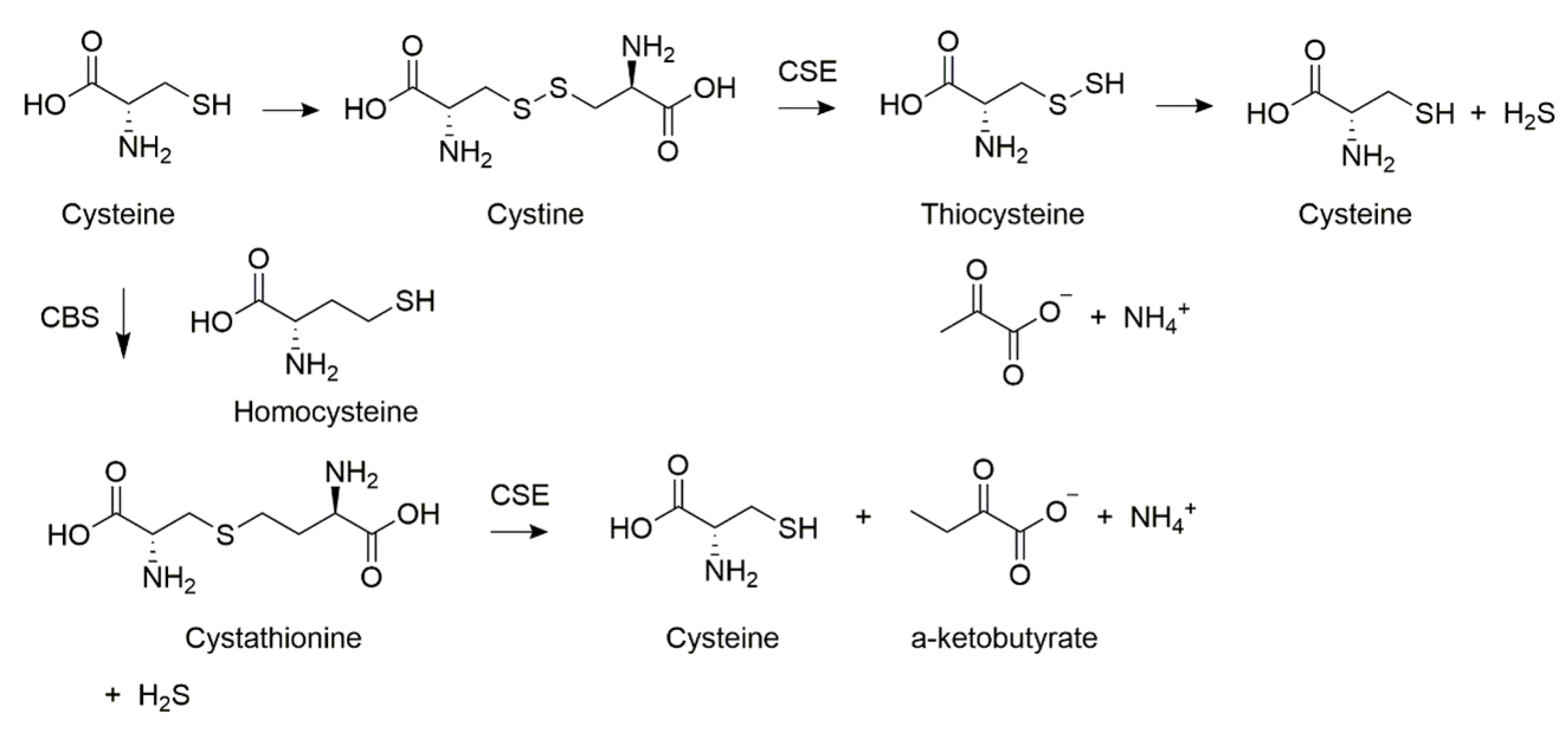
Scheme 1. Endogenous hydrogen sulfide synthesizing pathways.
Traditional methods of H2S detection, such as electrochemical analysis and gas chromatography, require a sample of living cells from target tissues [22,23][22][23]. However, since H2S can exist at levels as low as 30 μM in human blood, such methods are inadequate for detecting the analyte in the blood [24]. With these limitations in mind, the discovery of highly sensitive and selective detection methods for H2S is of peak interest [25,26,27,28,29,30][25][26][27][28][29][30]. Traditional methods also present the inherent drawbacks of trauma from conducting biopsies, inconsistent concentrations of the analyte within the target tissue, and an overall more tedious process. To improve results, various probes have been synthesized for the selective and specific detection of H2S [31,32,33][31][32][33].
Recent studies have shown the visible and NIR probes to be favorable due to their high-yield synthesis, modular structures, low cytotoxicity, and high sensitivity and selectivity [34,35,36,37,38,39,40,41,42,43,44,45,46][34][35][36][37][38][39][40][41][42][43][44][45][46]. Importantly, they work in real time and require nothing but an injection [47]. In the past few years, much work has been put into shortening response times, reducing Stokes shifts, and increasing the fluorescence wavelengths of these compounds [48,49,50,51][48][49][50][51]. Such findings would provide healthcare workers with new tools to prevent, detect, and treat various diseases.
2. Different Mechanisms of H2S Detection
2.1. Nucleophilic Addition to the Conjugated System for H2S Detection
Under the physiological condition of pH 7.6, H2S was deprotonated to HS−. This species was then able to be added to the conjugated system by way of nucleophilic attack onto the electrophilic center of the probe. In this way, cyanine dyes were able to detect H2S. Such a mechanism has four different modes of signal transduction. These include intramolecular charge transfer (ICT) [61[52][53][54],62,63], twisted intramolecular charge transfer [64,65,66][55][56][57] (TICT), fluorescence resonance energy transfer (FRET) [67[58][59][60],68,69], and photoinduced electron transfer (PET) [72,73,74][61][62][63]. Each of these processes involves the transfer of energy within the probe in the presence of the analyte. These mechanisms are discussed individually under the banner of H2S detection by way of nucleophilic addition to a conjugated system.2.1.1. Intramolecular Charge Transfer (ICT)
This is the process of converting light energy into chemical energy. An example is photosynthesis, where plants use sunlight to carry out key charge separation processes in the production of food. The ICT process depends on the relationship between intensities of the donor and acceptor in the system. The donor resides at the HOMO energy level, and the acceptor resides at the LUMO. Moreover, the energy gap between the two can serve to insulate or conduct depending on the circumstances, which determines the electronic coupling degree between the two orbitals [61,62,63][52][53][54].Synthesis and Mechanism of H2S Probes Based on ICT
An example of a H2S probe performing on the basis of ICT is the morpholinostyryl probe 3, as illustrated in Scheme 2. It was synthesized by Li et al. following the reaction of indolium salt 1 with aldehyde 2. Under reflux in ethanol, a yield of 70% was achieved. The probe behaves as a switch, with fluorescence turning on and off in the absence and presence of H2S, respectively. This occurs because H2S, behaving as the nucleophile, attacks the indolium moiety to break conjugation. The result was a loss of fluorescence in the presence of the H2S at levels as low as 30 μM [61][52].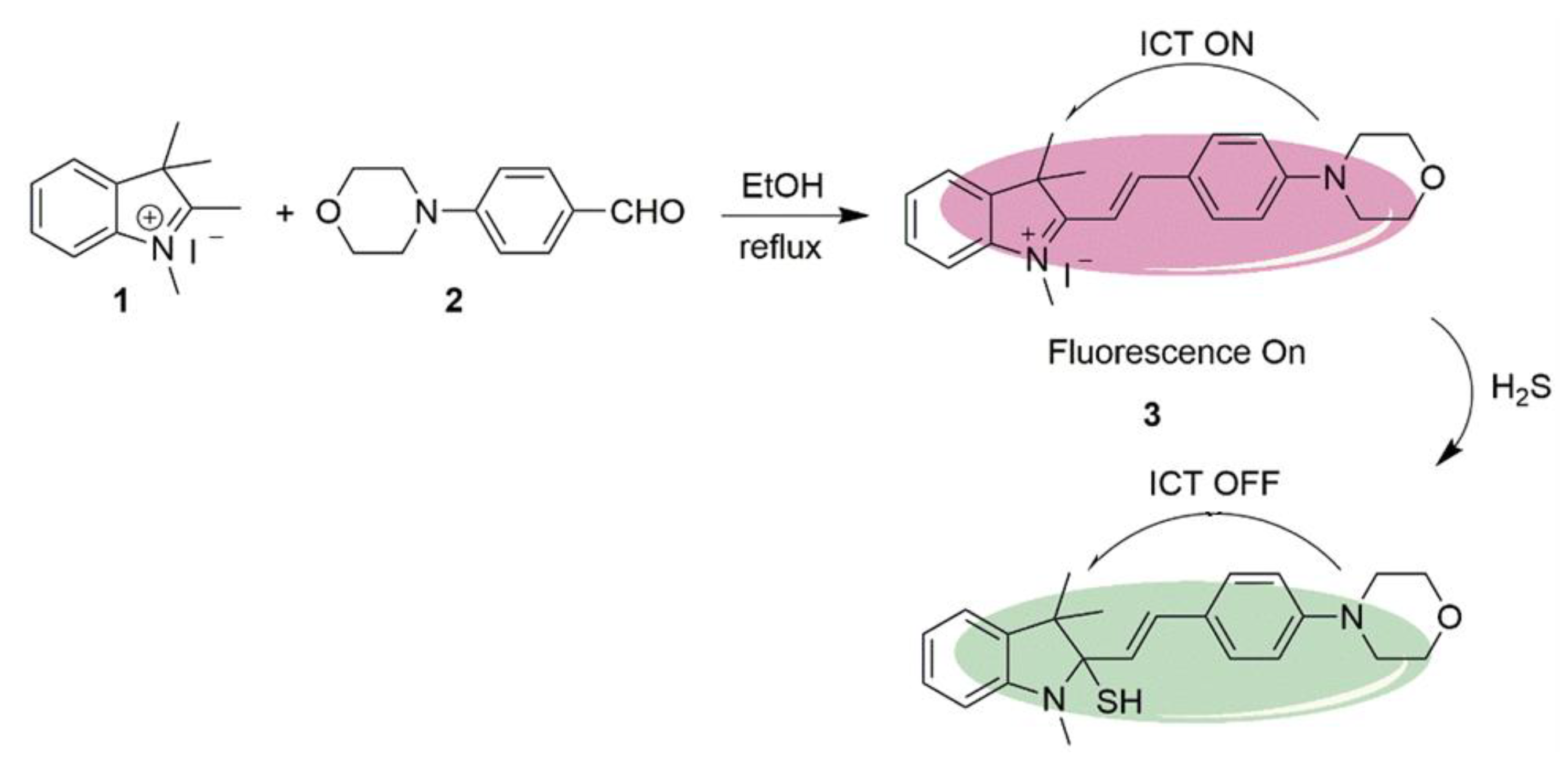
Scheme 2. Synthetic route and detection mechanism of H2S probe 3.
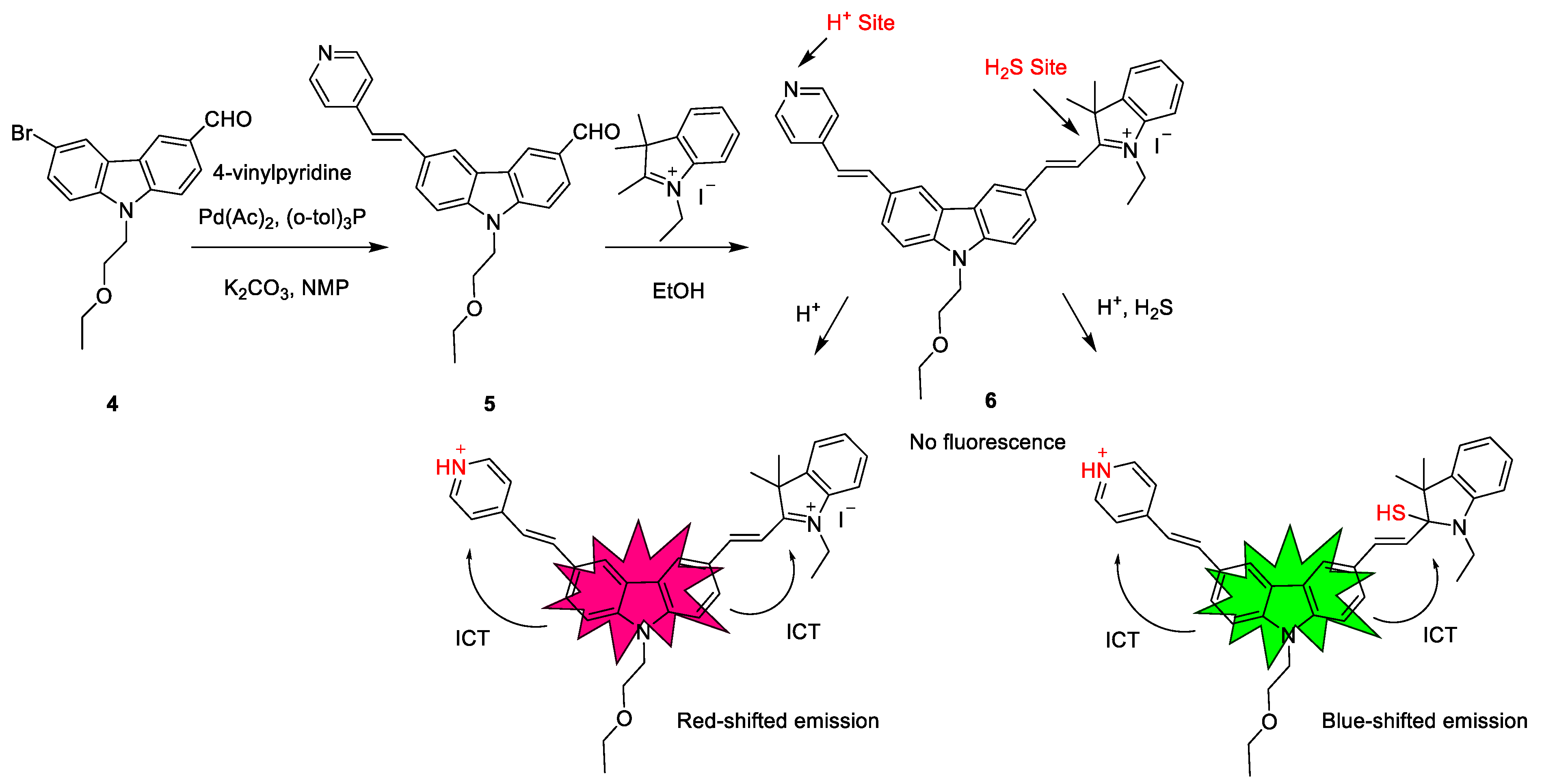
Scheme 3. Synthetic route and detection mechanism of probe 6.
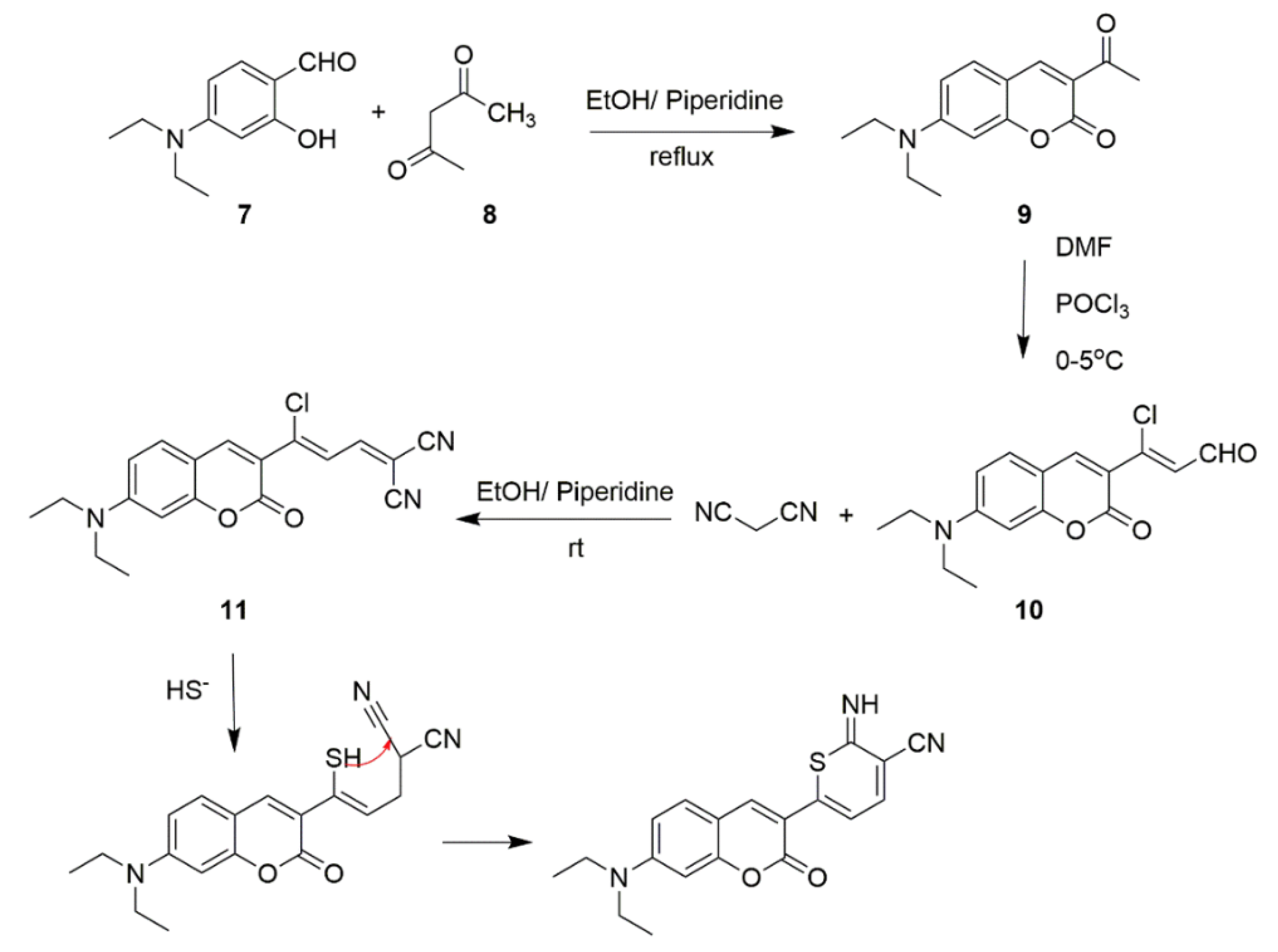
Scheme 4. Synthetic route and detection mechanism of H2S probe 11.
Optical Properties of ICT H2S Probes
Referring to the on/off H2S probe synthesized by Li et al. in Scheme Scheme 22, probe 3 was shown to absorb light at a wavelength of 520 nm and fluoresce at 596 nm, as shown in Figure 1A. Its fluorescence intensity was then measured at 600 nm in the presence of H2S at varying concentrations. Figure 1B reveals that the signal of probe 3 was strongest with the analyte absent, but its signal was unobservable at a concentration of 100 μM of H2S. These results prove that probe 3 is a useful H2S sensor [61][52].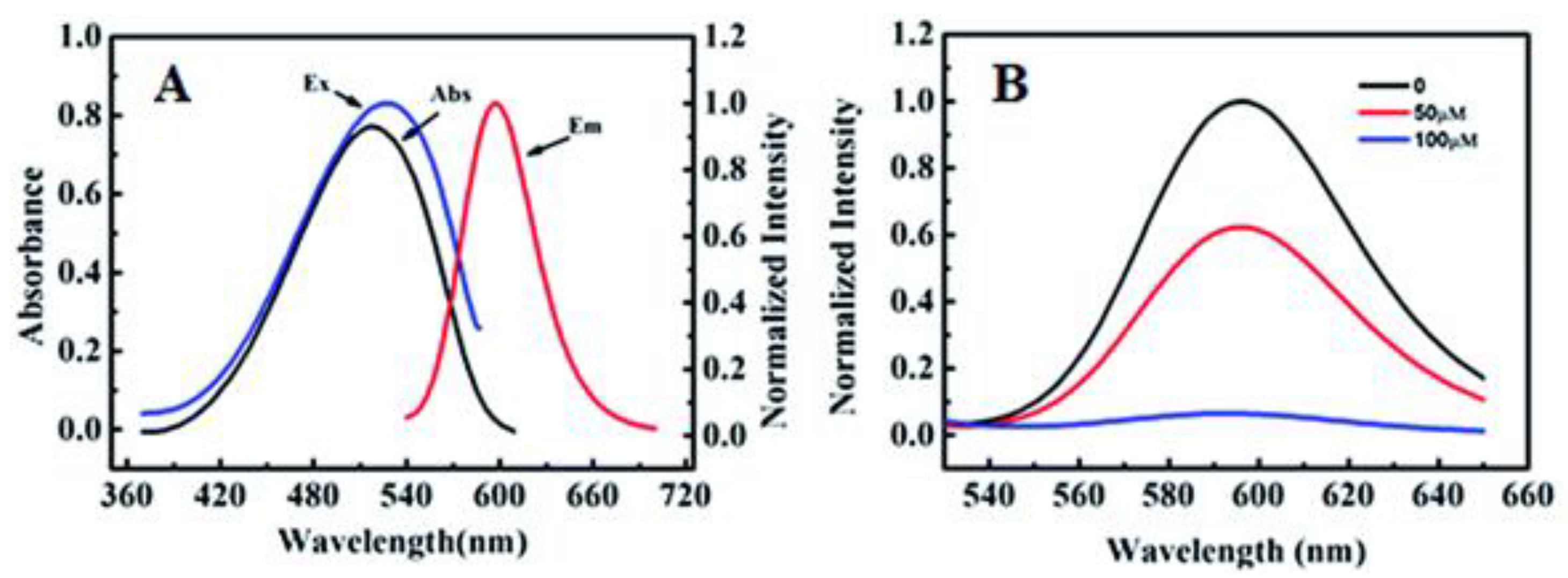
Figure 1.
(
A
) Absorption and fluorescence spectra of probe
3
. (
B
) Fluorescence spectra of probe
3
(10 μM) in H
2
S at varying concentrations.
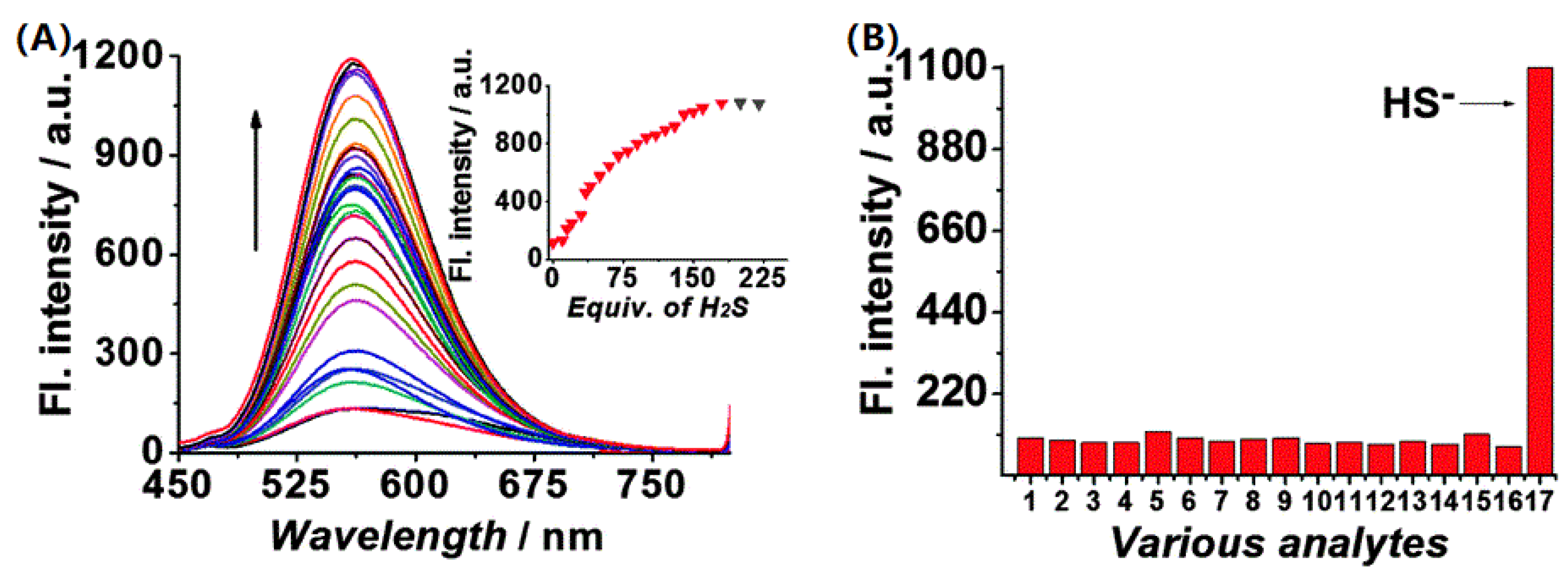
Figure 2. (A) Fluorescence spectra of probe 6 (10 μM) in pH 4.4 PBS buffer solution (containing 5% DMSO) with the addition of H2S. (B) Fluorescence responses of probe 6 at 405 nm in the presence of: 1. Probe 6; 2, Cl−; 3, K+; 4, Ca2+; 5, Mg2+; 6, GSH; 7, H2O2; 8, Hcy; 9, HSO3−; 10, I−; 11; Cys; 12, NO2−; 13, S2O32−; 14, SO32−; 15, SO42−; 16, Zn2+; 17, HS−.
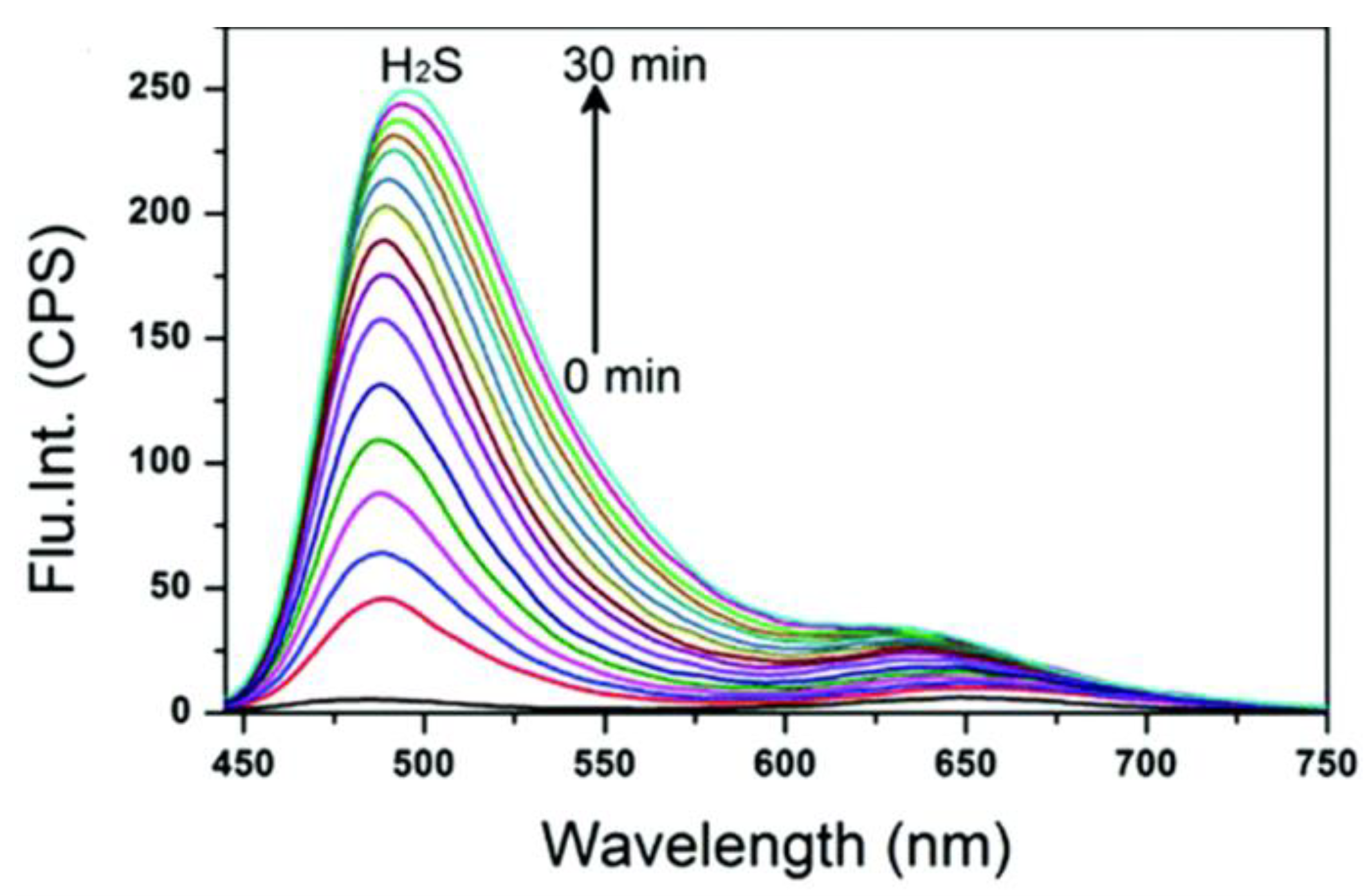
Figure 3. Time-dependent fluorescence spectral changes of probe 11.
The Application of H2S Probes Based on ICT
The immortalized cells of Henrietta Lacks, who died of advanced cancer in 1951, were used to further bolster the ability of probe 3 (Scheme 2) to detect H2S in living cells. This sensor penetrated the cell membrane as shown by the contrast between bright-field and fluorescence images of cells incubated with probe 3 (Figure 4A,B). Overlaying the bright-field and fluorescence images of cells treated with probe 3 verified this claim. Conversely, there was no visible fluorescence upon addition of H2S to the culture (Figure 4C,D). These images indicate that probe 3 could effectively penetrate the membrane and was selective toward H2S in living cells [61][52].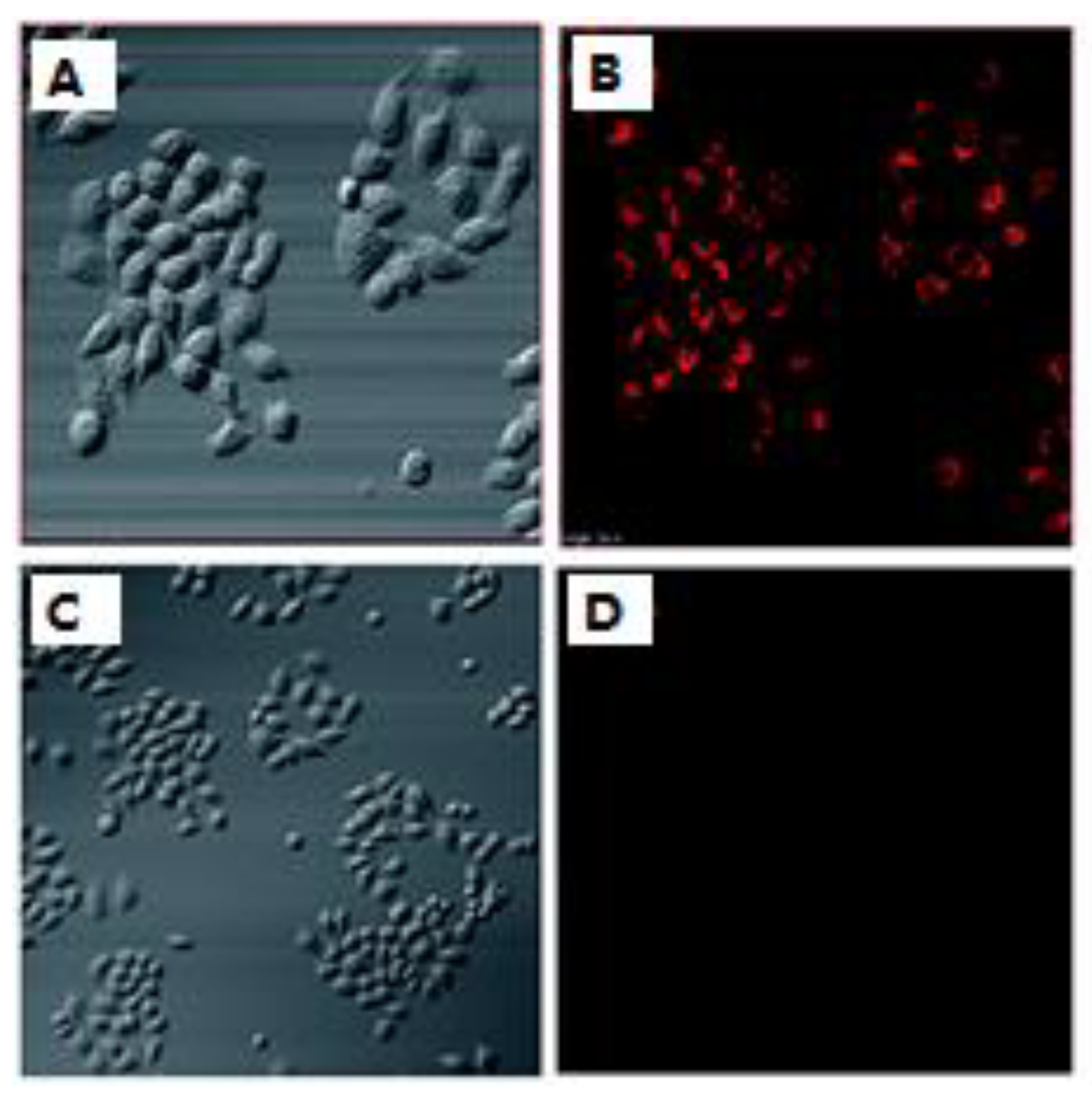
Figure 4. (A) Bright-field images of cells treated with probe 3. (B) Bright-field images of cells preincubated with probe 3 and NaHS. (B,D) are fluorescence images of (A,C), respectively.
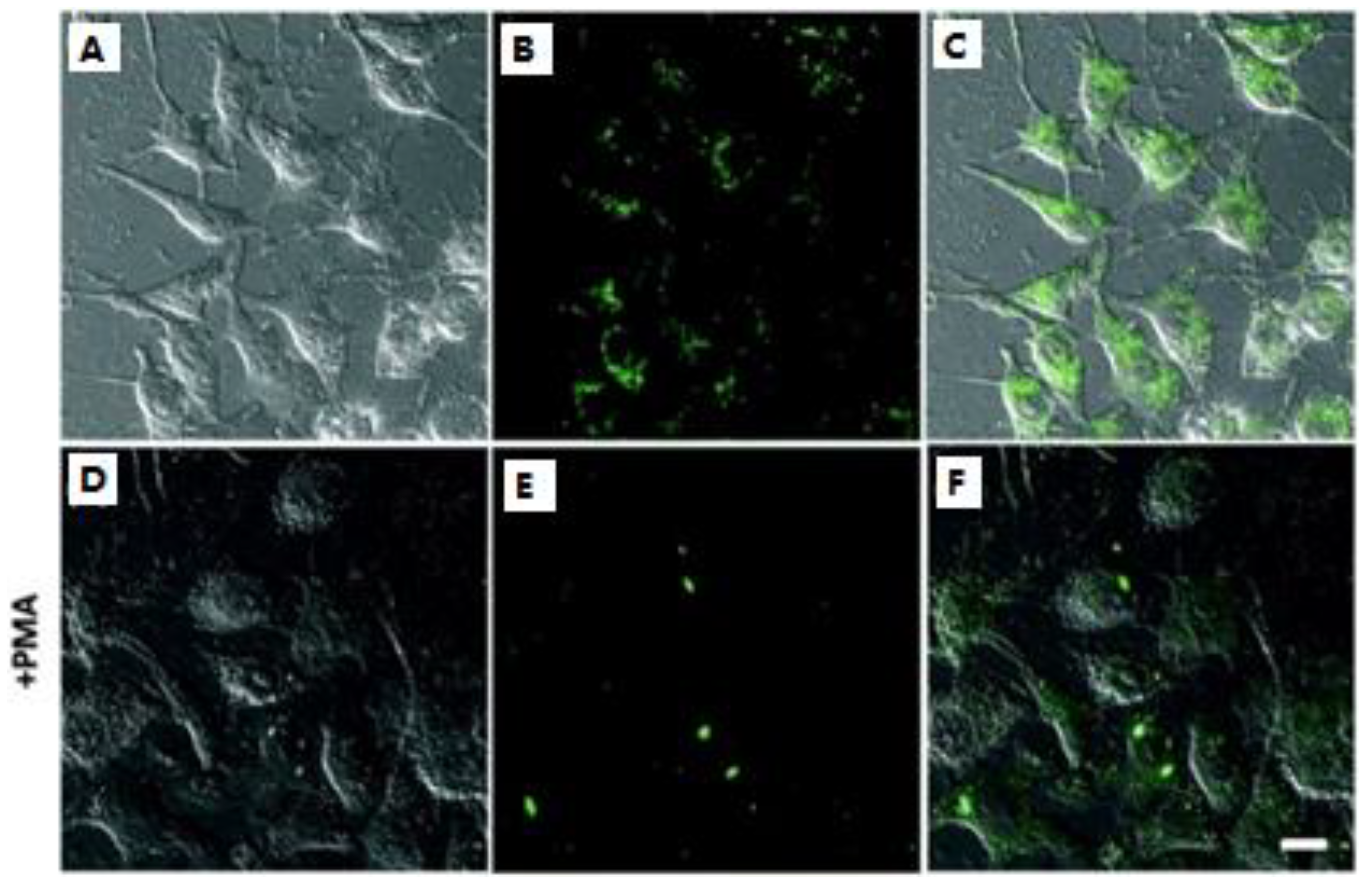
Figure 5. Fluorescence imaging of the endogenous H2S in the lysosomes: (A–C) the cells incubated with 5.0 μM probe 6 only; (D–F) the cells pretreated with PMA and then incubated with probe 6. (A,D) bright-field images; (B,E) fluorescence; (C,F) merged.
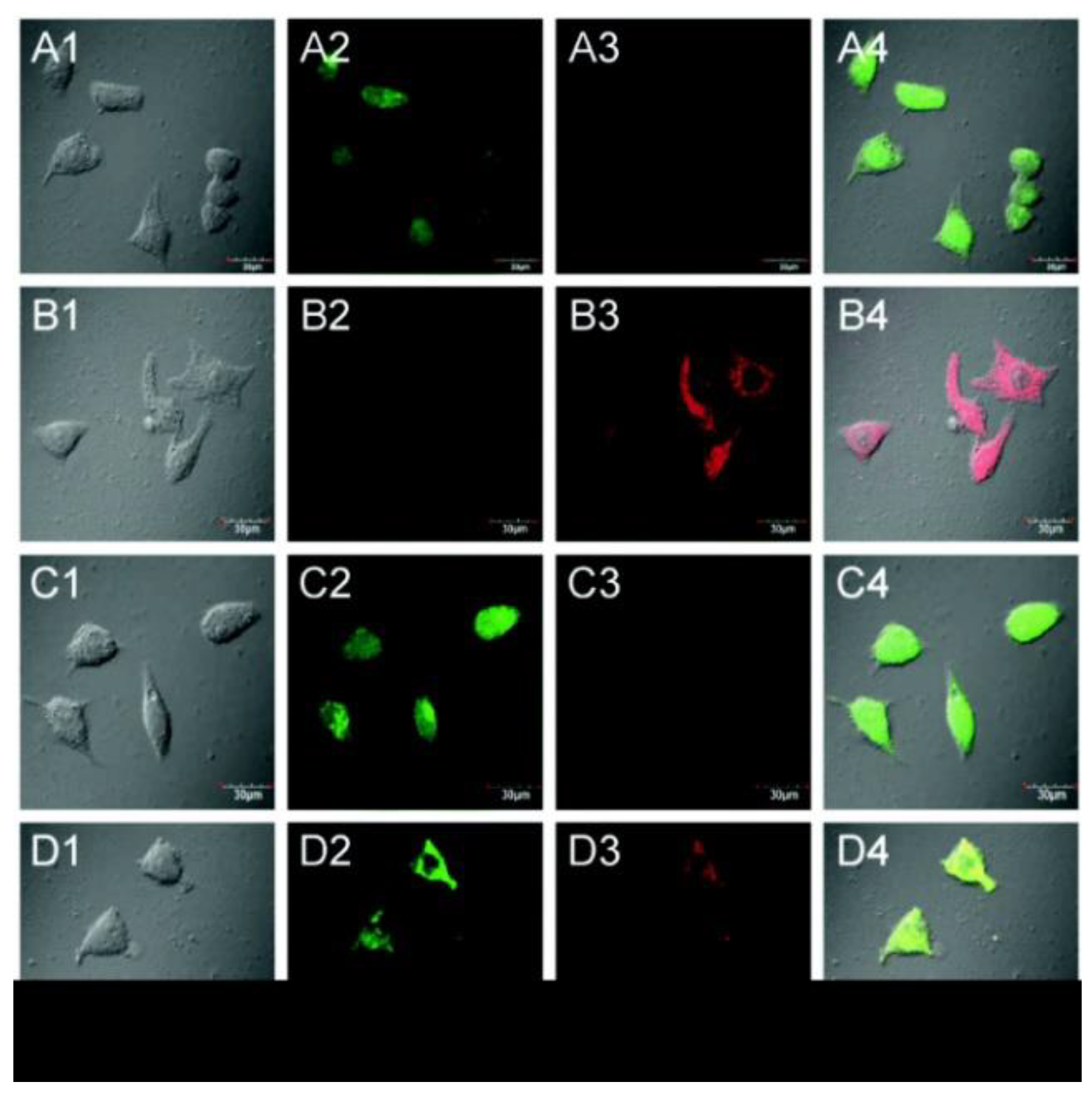
Figure 6. (A) Cells incubated with only probe 11; (B) cells were preincubated with NEM and subsequently treated with probe 11; (C) cells pretreated with NEM followed by treatment with probe 11 and GSH; (D) cells pretreated with 0.6 mM NEM followed by probe 11 and then with H2S. Column 1, bright field; column 2, green channel; column 3, red channel; column 4, merged images of columns 1, 2, and 3.
References
- Wu, D.; Si, W.; Wang, M.; Lv, S.; Ji, A.; Li, Y. Hydrogen Sulfide in Cancer: Friend or Foe? Nitric Oxide-Chem. Biol. 2015, 50, 38–45.
- Wang, B.; Wang, X.; Guo, Z.; Gai, S.; Li, Y.; Wu, Y. A Highly Sensitive Ppb-Level H2S Gas Sensor Based on Fluorophenoxy-Substituted Phthalocyanine Cobalt/RGO Hybrids at Room Temperature. RSC Adv. 2021, 11, 5993–6001.
- Yuvaraja, S.; Bhyranalyar, V.N.; Bhat, S.A.; Surya, S.G.; Yelamaggad, C.V.; Salama, K.N. A Highly Selective Electron Affinity Facilitated H2S Sensor: The Marriage of Tris (Keto-Hydrazone) and an Organic Field-Effect Transistor. Mater. Horiz. 2021, 8, 525–537.
- Abe, K.; Kimura, H. The Possible Role of Hydrogen Sulfide as an Endogenous Neuromodulator. J. Neurosci. 1996, 16, 1066–1071.
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical Biology of H2S Signaling through Persulfidation. Chem. Rev. 2018, 118, 1253–1337.
- Chen, W.; Zhang, Y.; Li, X.; Chen, H.; Sun, J.; Feng, F. H2S Activated Drug Release from Protein Cages. ACS Appl. Mater. Interfaces 2017, 9, 33571–33575.
- Zanardo, R.C.O.; Brancaleone, V.; Distrutti, E.; Fiorucci, S.; Cirino, G.; Wallace, J.L. Hydrogen Sulfide was an Endogenous Modulator of Leukocyte-Mediated Inflammation. Faseb J. 2006, 20, 2118–2120.
- Jiao, X.; Li, Y.; Niu, J.; Xie, X.; Wang, X.; Tang, B. Small-Molecule Fluorescent Probes for Imaging and Detection of Reactive Oxygen, Nitrogen, and Sulfur Species in Biological Systems. Anal. Chem. 2018, 90, 533–555.
- Kimura, H. Hydrogen Sulfide: Its Production and Functions. Exp. Physiol 2011, 96, 833–835.
- Zhang, H.; Kong, X.; Tang, Y.; Lin, W. Hydrogen Sulfide Triggered Charge-Reversal Micelles for Cancer-Targeted Drug Delivery and Imaging. ACS Appl. Mater. Interfaces 2016, 8, 16227–16239.
- Fu, M.; Zhang, W.; Wu, L.; Yang, G.; Li, H.; Wang, R. Hydrogen Sulfide (H2S) Metabolism in Mitochondria and Its Regulatory Role in Energy Production. Proc. Natl. Acad. Sci. USA 2012, 109, 2943–2948.
- Blackstone, E.; Morrison, M.; Roth, M.B. H2S Induces a Suspended Animation-like State in Mice. Science 2005, 308, 518.
- Zhao, X.; Ning, L.; Zhou, X.; Song, Z.; Zhang, J.; Guan, F.; Yang, X.-F. An Activatable Near-Infrared Fluorescence Hydrogen Sulfide (H2S) Donor for Imaging H2S Release and Inhibiting Inflammation in Cells. Anal. Chem. 2021, 93, 4894–4901.
- Huang, C.W.; Moore, P.K. H2S Synthesizing Enzymes: Biochemistry and Molecular Aspects. In Chemistry, Biochemistry and Pharmacology of Hydrogen Sulfide; Moore, P.K., Whiteman, M., Eds.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2015; pp. 3–25.
- Caliendo, G.; Cirino, G.; Santagada, V.; Wallace, J.L. Synthesis and Biological Effects of Hydrogen Sulfide (H2S): Development of H2S-Releasing Drugs as Pharmaceuticals. J. Med. Chem. 2010, 53, 6275–6286.
- Cai, W.-J.; Wang, M.-J.; Ju, L.-H.; Wang, C.; Zhu, Y.-C. Hydrogen Sulfide Induces Human Colon Cancer Cell Proliferation: Role of Akt, ERK and P21. Cell Bio. Int. 2010, 34, 565–572.
- Liu, X.; Gong, X.; Yuan, J.; Fan, X.; Zhang, X.; Ren, T.; Yang, S.; Yang, R.; Yuan, L.; Zhang, X.-B. Dual-Stimulus Responsive Near-Infrared Reversible Ratiometric Fluorescent and Photoacoustic Probe for In Vivo Tumor Imaging. Anal. Chem. 2021, 93, 5420–5429.
- Bailey, T.S.; Pluth, M.D. Chemiluminescent Detection of Enzymatically Produced Hydrogen Sulfide: Substrate Hydrogen Bonding Influences Selectivity for H2S over Biological Thiols. J. Am. Chem. Soc. 2013, 135, 16697–16704.
- Tian, X.; Li, Z.; Lau, C.; Lu, J. Visualization of in Vivo Hydrogen Sulfide Production by a Bioluminescence Probe in Cancer Cells and Nude Mice. Anal. Chem. 2015, 87, 11325–11331.
- Shi, B.; Yan, Q.; Tang, J.; Cin, K.; Zhang, J.; Zhu, Y.; Xu, G.; Wang, R.; Chen, J.; Gao, W.; et al. Hydrogen Sulfide-Activatable Second Near-Infrared Fluorescent Nanoassemblies for Targeted Photothermal Cancer Therapy. Nano Lett. 2018, 18, 6411–6416.
- Hosoki, R.; Matsuki, N.; Kimura, H. The Possible Role of Hydrogen Sulfide as an Endogenous Smooth Muscle Relaxant in Synergy with Nitric Oxide. Biochem. Biophys. Res. Commun. 1997, 237, 527–531.
- Jimenez, D.; Martinez-Manez, R.; Sancenon, F.; Ros-Lis, J.V.; Benito, A.; Soto, J. A New Chromo-Chemodosimeter Selective for Sulfide Anion. J. Am. Chem. Soc. 2003, 125, 9000–9001.
- Furne, J.; Saeed, A.; Levitt, M.D. Whole Tissue Hydrogen Sulfide Concentrations were Orders of Magnitude Lower than Presently Accepted Values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1479–R1485.
- Wallace, J.L. Hydrogen Sulfide-Releasing Anti-Inflammatory Drugs. Trends Pharmacol. Sci. 2007, 28, 501–505.
- Kaushik, R.; Ghosh, A.; Jose, D.A. Simple Terpyridine Based Cu(II)/Zn(II) Complexes for the Selective Fluorescent Detection of H2S in Aqueous Medium. J. Lumin. 2016, 171, 112–117.
- Wang, S.; Zhang, L.; Zhao, J.; Hou, L.; Wen, C.; Liang, H.; Zhao, S. Hydrogen Sulfide Dual-Activated NIR-II Photoacoustic Probes for Accurate Imaging and Efficient Photothermal Therapy of Colon Cancer. ACS Appl. Bio. Mater. 2021, 4, 974–983.
- Chen, Y.; Zhu, C.; Yang, Z.; Chen, J.; He, Y.; Jiao, Y.; He, W.; Qiu, L.; Cen, J.; Guo, Z. A Ratiometric Fluorescent Probe for Rapid Detection of Hydrogen Sulfide in Mitochondria. Angew. Chem. Int. Ed. 2013, 52, 1688–1691.
- Hammers, M.D.; Taormina, M.J.; Cerda, M.M.; Montoya, L.A.; Seidenkranz, D.T.; Parthasarathy, R.; Pluth, M.D. A Bright Fluorescent Probe for H2S Enables Analyte-Responsive, 3D Imaging in Live Zebrafish Using Light Sheet Fluorescence Microscopy. J. Am. Chem. Soc. 2015, 137, 10216–10223.
- Yang, X.; Lu, X.; Wang, J.; Zhang, Z.; Du, X.; Zhang, J.; Wang, J. Near-Infrared Fluorescent Probe with a Large Stokes Shift for Detection of Hydrogen Sulfide in Food Spoilage, Living Cells, and Zebrafish. J. Agric. Food Chem. 2022, 9, 3047–3055.
- Peng, H.; Cheng, Y.; Dai, C.; King, A.L.; Predmore, B.L.; Lefer, D.J.; Wang, B. A Fluorescent Probe for Fast and Quantitative Detection of Hydrogen Sulfide in Blood. Angew. Chem. Int. Ed. 2011, 50, 9672–9675.
- Deng, Z.; Bi, S.; Jiang, M.; Zeng, S. Endogenous H2S-Activated Orthogonal Second Near-Infrared Emissive Nanoprobe for In Situ Ratiometric Fluorescence Imaging of Metformin-Induced Liver Injury. ACS Nano 2021, 15, 3201–3211.
- Zhang, L.; Wang, J.-L.; Ba, X.-X.; Hua, S.-Y.; Jiang, P.; Jiang, F.-L.; Liu, Y. Multifunction in One Molecule: Mitochondrial Imaging and Photothermal & Photodynamic Cytotoxicity of Fast-Response Near-Infrared Fluorescent Probes with Aggregation-Induced Emission Characteristics. ACS Appl. Mater. Interfaces 2021, 13, 7945–7954.
- Chuang, C.-H.; Chen, W.-Y.; Tseng, W.-B.; Lin, A.; Lu, C.-Y.; Tseng, W.-L. Microwave-Mediated Synthesis of Near-Infrared-Emitting Silver Ion-Modified Gold Nanoclusters for Ratiometric Sensing of Hydrosulfide in Environmental Water and Hydrogen Sulfide in Live Cells. ACS Sustain. Chem. Eng. 2022, 10, 2461–2472.
- Liu, T.; Xu, Z.; Spring, D.R.; Cui, J. A Lysosome-Targetable Fluorescent Probe for Imaging Hydrogen Sulfide in Living Cells. Org. Lett. 2013, 15, 2310–2313.
- Cao, X.; Lin, W.; Zheng, K.; He, L. A Near-Infrared Fluorescent Turn-on Probe for Fluorescence Imaging of Hydrogen Sulfide in Living Cells Based on Thiolysis of Dinitrophenyl Ether. Chem. Commun. 2012, 48, 10529–10531.
- Gong, S.; Zheng, Z.; Guan, X.; Feng, S.; Feng, G. Near-Infrared Mitochondria-Targetable Fluorescent Probe for High-Contrast Bioimaging of H2S. Anal. Chem. 2021, 93, 5700–5708.
- Bae, S.K.; Heo, C.H.; Choi, D.J.; Sen, D.; Joe, E.-H.; Cho, B.R.; Kim, H.M. A Ratiometric Two-Photon Fluorescent Probe Reveals Reduction in Mitochondrial H2S Production in Parkinson’s Disease Gene Knockout Astrocytes. J. Am. Chem. Soc. 2013, 135, 9915–9923.
- Wu, M.-Y.; Li, K.; Hou, J.-T.; Huang, Z.; Yu, X.-Q. A Selective Colorimetric and Ratiometric Fluorescent Probe for Hydrogen Sulfide. Org. Biomol. Chem. 2012, 10, 8342–8347.
- Jose, A.; Sakla, R.; Sharma, N. Sensing and Bioimaging of the Gaseous Signaling Molecule Hydrogen Sulfide by Near-Infrared Fluorescent Probes. ACS Sens. 2020, 5, 3365–3391.
- Yang, Q.-Q.; Tian, Q.-Q.; Ji, N.; Duan, X.-H.; Zhu, X.-H.; Zhang, Y.-L.; He, W. A Novel Fluorescent Probe for the Detection of Sulfur Dioxide Derivatives and Its Application in Biological Imaging. New J. Chem. 2022, 46, 1483–1488.
- Huang, X.; Liu, H.; Zhang, J.; Xiao, B.; Wu, F.; Zhang, Y.; Tan, Y.; Jiang, Y. A Novel Near-Infrared Fluorescent Hydrogen Sulfide Probe for Live Cell and Tissue Imaging. New J. Chem. 2019, 43, 6848–6855.
- Kang, H.; Shamim, M.; Yin, X.; Adluru, E.; Fukuda, T.; Yokomizo, S.; Chang, H.; Park, S.H.; Cui, Y.; Moy, A.J. Tumor-Associated Immune Cell Mediated Tumor Targeting Mechanism with NIR-II Fluorescence Imaging. Adv. Mater. 2021, 34, 2106500.
- Chang, M.J.; Kim, K.; Kang, C.; Lee, M.H. Enhanced Aggregability of AIE-Based Probe through H2S-Selective Triggered Dimerization and Its Applications to Biological Systems. Acs Omega 2019, 4, 7176–7181.
- Dhivya, R.; Kavitha, V.; Gomathi, A.; Keerthana, P.; Santhalakshmi, N.; Viswanathamurthi, P.; Haribabu, J. Dinitrobenzene Ether Reactive Turn-on Fluorescence Probes for the Selective Detection of H2S. Anal. Methods 2021, 14, 58–66.
- Cao, Y.; Wang, L.; Liu, Z.; Sun, C.; Li, Y. Theoretical Study on the Sensing Mechanism of Chalcone-Based Fluorescence Probe for Detecting Hydrogen Sulfide and Biothiols. New J. Chem. 2021, 45, 16906–16912.
- Lin, V.S.; Lippert, A.R.; Chang, C.J. Azide-based fluorescent probes: Imaging hydrogen sulfide in living systems. Methods Enzymol. 2015, 554, 63–80.
- Lippert, A.R.; New, E.J.; Chang, C.J. Reaction-Based Fluorescent Probes for Selective Imaging of Hydrogen Sulfide in Living Cells. J. Am. Chem. Soc. 2011, 133, 10078–10080.
- Essam, Z.M.; Ozmen, G.E.; Setiawan, D.; Hamid, R.R.; El-Aal, R.M.A.; Aneja, R.; Hamelberg, D.; Henary, M. Donor Acceptor Fluorophores: Synthesis, Optical Properties, TD-DFT and Cytotoxicity Studies. Org. Biomol. Chem. 2021, 19, 1835–1846.
- Choi, H.S.; Gibbs, S.L.; Lee, J.H.; Kim, S.H.; Ashitate, Y.; Liu, F.; Hyun, H.; Park, G.; Xie, Y.; Bae, S.; et al. Targeted Zwitterionic Near-Infrared Fluorophores for Improved Optical Imaging. Nat. Biotechnol. 2013, 31, 148–153.
- Cao, J.; Chi, J.; Xia, J.; Zhang, Y.; Han, S.; Sun, Y. Iodinated Cyanine Dyes for Fast Near-Infrared-Guided Deep Tissue Synergistic Phototherapy. ACS Appl. Mater. Interfaces 2019, 11, 25720–25729.
- Lovett, W.R.; Al Hamd, A.; Casa, S.; Henary, M. Synthesis of PH-Sensitive Benzothiazole Cyanine Dye Derivatives Containing a Pyridine Moiety at the Meso Position. Dyes Pigments 2021, 190, 109268.
- Li, Y.; Gu, B.; Su, W.; Duan, X.; Xu, H.; Huang, Z.; Li, H.; Yao, S. A Simple and Efficient Fluorescent Probe for the Rapid Detection of H2S in Living Cells and on Agar Gels. Anal. Methods 2017, 9, 3290–3295.
- Liu, Y.; Meng, F.; He, L.; Liu, K.; Lin, W. A Dual-Site Two-Photon Fluorescent Probe for Visualizing Lysosomes and Tracking Lysosomal Hydrogen Sulfide with Two Different Sets of Fluorescence Signals in the Living Cells and Mouse Liver Tissues. Chem. Commun. 2016, 52, 7016–7019.
- Chen, F.; Han, D.; Liu, H.; Wang, S.; Li, K.-B.; Zhang, S.; Shi, W. A Tri-Site Fluorescent Probe for Simultaneous Sensing of Hydrogen Sulfide and Glutathione and Its Bioimaging Applications. Analyst 2018, 143, 440–448.
- Ren, M.; Deng, B.; Kong, X.; Zhou, K.; Liu, K.; Xu, G.; Lin, W. A TICT-Based Fluorescent Probe for Rapid and Specific Detection of Hydrogen Sulfide and Its Bio-Imaging Applications. Chem. Commun. 2016, 52, 6415–6418.
- Li, S.-J.; Li, Y.-F.; Liu, H.-W.; Zhou, D.-Y.; Jiang, W.-L.; Ou-Yang, J.; Li, C.-Y. A Dual-Response Fluorescent Probe for the Detection of Viscosity and H2S and Its Application in Studying Their Crosstalk Influence in Mitochondria. Anal. Chem. 2018, 90, 9418–9425.
- Zhang, Y.; Zhang, B.; Li, Z.; Wang, L.; Ren, X.; Ye, Y. Endoplasmic Reticulum Targeted Fluorescent Probe for the Detection of Hydrogen Sulfide Based on a Twist-Blockage Strategy. Org. Biomol. Chem. 2019, 17, 8778–8783.
- Feng, X.; Zhang, T.; Liu, J.-T.; Miao, J.-Y.; Zhao, B.-X. A New Ratiometric Fluorescent Probe for Rapid, Sensitive and Selective Detection of Endogenous Hydrogen Sulfide in Mitochondria. Chem. Commun. 2016, 52, 3131–3134.
- Yang, Y.; He, L.; Xu, K.; Lin, W. A Ratiometric Fluorescent Chemosensor for the Convenient Monitoring of Hydrogen Sulfide Concentration by the Dual Fluorescence Fluctuation Mode of Two Distinct Emission Bands in Living Cells and Zebrafish. New J. Chem. 2019, 43, 10926–10931.
- Zhang, Y.; Chen, Y.; Yang, B.; Xue, X.; He, W.; Guo, Z. FRET Based Fluorescent Ratiometric Probes for Rapid Detection of Endogenous Hydrogen Sulphide in Living Cells. Analyst 2020, 145, 4233–4238.
- Zhu, H.; Liu, C.; Yuan, R.; Wang, R.; Zhang, H.; Li, Z.; Jia, P.; Zhu, B.; Sheng, W. A Simple Highly Specific Fluorescent Probe for Simultaneous Discrimination of Cysteine/Homocysteine and Glutathione/Hydrogen Sulfide in Living Cells and Zebrafish Using Two Separated Fluorescence Channels under Single Wavelength Excitation. Analyst 2019, 144, 4258–4265.
- Lan, J.-S.; Zeng, R.-F.; Liu, Y.; Xiang, Y.-W.; Jiang, X.; Liu, L.; Xie, S.-S.; Ding, Y.; Zhang, T. A Near-Infrared Nile Red Fluorescent Probe for the Discrimination of Biothiols by Dual-Channel Response and Its Bioimaging Applications in Living Cells and Animals. Analyst 2019, 144, 3676–3684.
- Jing, X.; Yu, F.; Lin, W. A PET-Based Lysosome-Targeted Turn-on Fluorescent Probe for the Detection of H2S and Its Bioimaging Application in Living Cells and Zebrafish. New J. Chem. 2019, 43, 16796–16800.
More
