Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Jose Alberto Gallegos-Infante.
Skin inflammation occurs as an immune response to various stimuli such as ultraviolet light, irritants, or any type of skin barrier injury. Finding safe and effective drugs to combat skin inflammation remains a research challenge.
- topical anti-inflammatory
- skin inflammation
- in vitro
- ex vivo
- cell culture
1. Introduction
Inflammation is a physiological defense response to various stimuli, such as infection and tissue damage. Inflammation can be acute, for example, in response to tissue damage, or chronic, with pathological consequences [2][1].
Ultraviolet (UV) rays, trauma, irritants, infections, or any type of barrier disruption trigger a coordinated immune response to maintain skin homeostasis. The immune cells present in the skin are the main ones responsible for restoring homeostasis, but they can also be effector cells in histopathological processes such as dermatitis and psoriasis [11,15][2][3].
The epidermis contains immune cells, mainly CD8+ effector T cells and LCs [16][4]. The dermis contains a more diverse population of immune cells, including CD4+ helper T cells, γδ-T cells, dermal dendritic cells, innate lymphocytes, pDC cell, NK cells, macrophages, mast cells, and fibroblasts [10,11,17][2][5][6]. Likewise, the dermis is drained of lymphatic and blood vessels, which are pathways for cell migration [16][4].
Although the skin’s immune system is strong, human skin harbors a rich and diverse collection of bacteria, fungi, and viruses. This group of microorganisms constitutes the skin microbiota. In recent years, the skin microbiota has been shown to play an important role in inflammatory skin conditions [18][7].
2. Keratinocytes as Triggers of Inflammation in the Skin
Damaged keratinocytes send out the first signals called alarmins, which consist of high mobility group 1 box protein (HMGB1), heat shock protein (HSP), antimicrobial peptides (defensins, cathelicidin, calgranulin A/B), interleukin (IL)-1a, IL-33, and IL-8 [15][3]. These endogenous molecules are considered to be damage-associated molecular patterns (DAMPs) recognized by pattern recognition receptors (PRRs) present in both immune and nonimmune cells to elicit an immune response, with Toll-like receptors (TLRs) being the major subset of the PRRs receptor family. PRRs can also recognize molecules derived from the presence of pathogens through pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide [19][8]. Activation of the pathway leads to translocation of the nuclear factor-kappa B (NF-κB) into the nucleus for transcription of downstream target genes, such as the proinflammatory cytokines IL-1, IL-6, tumor necrosis factor alpha (TNF-α), the chemokine IL-8, or the enzyme cyclooxygenase-2 (COX-2) [20][9].
In addition to TLRs, keratinocytes also express other receptors in response to injury, such as the IL-1 receptor (IL-1R) and tumor necrosis factor receptor 1 (TNFR1) [15][3].
The family IL-1 is divided into three subfamilies: IL-1, IL-18, and IL-36. IL-36 induces the production of other proinflammatory cytokines and antimicrobial peptides (AMP) as well as its own expression in an autocrine manner. IL-18 Like IL-1β is synthesized as an inactive precursor that is activated by the enzyme caspase-1. These cytokines are ligands of the IL-1R subfamily of receptors that contain the Toll/IL-1R domain [21][10].
After stimulation with IL-1 or TLR ligands, myeloid differentiation factor 88 (MyD88) recruits IL-1R-associated kinases (IRAK) and TNF receptor-associated factor 6 (TRAF6) for the assembly of the MyD88 signaling complex. Once activated in the MyD88 complex, it enables phosphorylation of the inhibitory protein IkB. This releases NF-κB from IkB binding in the cytoplasm and translocates it to the nucleus [15][3].
TNF acts on keratinocytes by binding to TNFR1, which attracts the TNFR1-associated death domain protein (TRADD). TRADD interacts with TNF receptor-associated factor 2 (TRAF2) through its binding domain to the N-terminal of TRAF2 and with receptor-interacting protein (RIP) through the death domain. TRAF2 and RIP mediate the recruitment of IKK (IκB kinase), an essential component of the NFκB activation pathway [22][11].
Another response mechanism of keratinocytes and immune cells to the presence of DAMPs, and PAMPs, is the activation of a large multiprotein oligomer complex in the cytoplasm called an inflammasome. Inflammasomes belong to the NOD-like receptor family, which is composed of 3 subfamilies: NOD (nucleotide-binding oligomerization domain), NLRC (NOD-like receptor caspase activation and recruitment CARD domain containing), and NLRP (NOD-like receptor pyrin domain containing), which is associated with the formation of inflammasomes. There are 14 different NLRPs known, of which NLRP3 has been the best studied to date [23][12]. This multimeric complex is formed by NLRP3, an adapter protein apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and procaspase 1. Its assembly activates caspase-1, which activates IL-1β precursors, IL-18 to their active forms (Figure 1) [17][6].
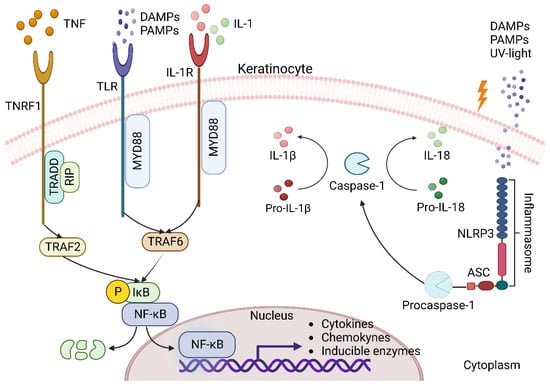
Figure 1. Keratinocytes as triggers of inflammation. During acute inflammation, keratinocytes, particularly with the canonical NF-κβ pathway, respond through several mechanisms that include proinflammatory molecules such as IL-1 cytokines, TNF-α, and recognition of molecular patterns PAMPs and DAMPs by various receptors (TNFR1, TLR, IL-1R, and NLRP3). When TNFR1 interacts with TNF, it recruits the adaptor protein TRADD, which in turn recruits the protein RIP and TRAF2. The receptors TLR and IL-1R, when interacting with their ligands, recruit MyD88 and TRAF6 to form a signaling complex. All this leads to the activation of the IKK complex, which enables the phosphorylation of the inhibitory protein IkB for the release of NF-κB, which translocates to the nucleus and upregulates the expression of cytokines, chemokines and inducible enzymes. On the other hand, the NLRP3 receptor recognizes PAMPs and DAMPs in the cytoplasm to assemble the inflammasome with ASC and procaspase-1 and trigger activation of caspase-1, which contributes to inflammation through proteolytic processing of IL-1β and IL-18 these cytokines may act as a positive feedback loop for NF-κB activation and amplify the inflammatory response.
Cytokines and chemokines produced by keratinocytes recruit and attract neutrophils to the site of injury, where they remain for 2 to 5 days before becoming apoptotic in the absence of infection. In addition to killing pathogens, neutrophils secrete TNF-α, IL-1β, and IL-6, which promote keratinocyte proliferation and the immune response [24][13] and stimulate monocytes to differentiate into M1 macrophages [25][14].
3. Dendritic Cells
The DCs of the skin can be classified according to their location in the different skin layers: LCs, which are constitutively located between keratinocytes in the epidermis, and dermal DCs, which are located in the dermis just below the dermal–epidermal junction and are scattered throughout the skin compartment [26][15]. In addition to their different location in the skin, the different types of DC may have specific functional properties, such as secretion of proinflammatory mediators (inflammatory DC), production of type I interferon (pDC), or cross-presentation (DC103) [11][2].
LCs are characterized by high expression of the major histocompatibility complex class II (MHCII), the presence of Langerin + Birbeck granules, and are located in the epidermis and are responsible for the uptake, processing and presentation of antigens to lymphocytes in the local lymph nodes [27][16].
4. Macrophages
Macrophages can be distinguished according to their pro-inflammatory (M1) and anti-inflammatory (M2) functions. The unique ability of macrophages to generate these types of polar opposite responses provides primary protection to the host and maintains tissue homeostasis. M1 macrophages are characterized by phagocytic activity and expression of certain proinflammatory cytokines such as TNFα, IL-1β, and IL-6 and proinflammatory mediators such as inducible nitric oxide synthase (iNOS) [28][17].
5. Mast Cells
Mast cells are located in the dermal layer. They require cytokines such as IL-3, IL-4, IL-9 and IL-10 to induce and promote their proliferation. They contain granules with preformed mediators such as histamine, sulfated proteoglycans, serotonin, and tryptase and/or chymase. They produce and release large amounts of histamine, especially in allergic reactions. They also produce large amounts of prostaglandin D2 and leukotrienes, lipid-derived inflammatory mediators, and cytokines such as TNFα and IL-1β [29][18].
6. T Cells
During inflammation, the immigration of T lymphocytes into the skin is facilitated and promoted by the local production of proinflammatory cytokines and chemokines. In the skin, a proportion of recruited T cells become resident memory T cells (TRMs), which are long-lived and distinct from their circulating counterparts. These cells provide local surveillance and are maintained in the tissue [30,31][19][20].
T cells are classified on the basis of their T receptors (TCRs) into αβ-T cells, which make up 99%, and γδ-T cells, which make up about 1%. αβ-T cells are in turn divided into CD8+ and CD4+ depending on the expression of the respective co-receptor [32][21].
CD8+ T lymphocytes are sentinel cells that can recruit other lymphocytes in the skin. They also exert antiviral effects on the skin via the interferon-γ (IFN-γ) mechanism. Skin CD8+ T cells in atopic dermatitis are a potent source of IL-13, IFN-γ, and IL-22, suggesting a pathogenic contribution to inflammation in the disease [30][19].
The three main types of CD4+ T cells, TH1, TH2, and TH17, are found in the skin in various inflammatory diseases. For example, when the skin is infected with intracellular organisms, TH1 cells are present to produce IFN-γ and lymphotoxins and activate macrophages to kill intracellular organisms. TH1 cell responses have been associated with autoimmune and immune diseases such as psoriasis, while TH2 cell responses have been associated with allergic diseases such as asthma and atopic dermatitis. However, TH17 cells have been shown to play a role in both psoriasis and atopic dermatitis [11][2].
γδ-T lymphocytes maintain skin homeostasis by balancing keratinocyte differentiation and proliferation with destruction of infected or malignant cells. Cutaneous γδ-T cells isolated from psoriasis patients produced more IL-17 after IL-23 stimulation. These cytokines lead to the recruitment of numerous lymphocytes, neutrophils, and myeloid cells, creating a positive feedback loop that maintains skin inflammation and stimulates epidermal hyperplasia [33][22].
Invariant natural killer cells (iKNT) are involved in hypersensitivity reactions because they produce IL-4 in response to haptens in the skin, leading to the accumulation of sensitized iNKT cells. They exhibit antitumor activity and are also characterized by the ability to self-recognize and rapidly release cytokines such as IFN-γ [29][18].
7. The Skin Microbiome in Inflammatory Skin Diseases
The skin microbiome comprises the totality of microorganisms (microbiota), their genomes and environmental factors. Four dominant bacterial phyla living on the skin are: Actinobacteria, Firmicutes, Proteobacteria, and Bacteroidetes, with Corynebacterium, Cutibacterium, and Staphylococcus being the most prevalent among the more than 40 bacterial genera identified [34][23].
Staphylococcus epidermidis accounts for more than 90% of the total resident aerobic microbiota and has many mutualistic anti-inflammatory effects that promote barrier function and inhibit colonization by potentially pathogenic strains. These include production of antimicrobial peptides, immunomodulatory properties, and increased expression of tight junction proteins. Many of these effects are mediated by activation of innate immune receptors on keratinocytes and other local immune cells via Toll-like TLRs [18][7].
Naik et al. [35][24] found that resident commensals in mice are required for optimal IL-1 signaling in the skin to promote local effector responses. These findings support the idea that defects in T cell function in the steady state or during inflammation are the result of impaired dialog with skin commensals.
A change in the composition of the microbiome can lead to changes in the reactivity of the immune system and thus to the development of inflammatory diseases such as atopic dermatitis, seborrheic dermatitis, psoriasis, acne and rosacea [36][25]. Thus, microbiome studies in patients with atopic dermatitis showed a decrease in bacterial community biodiversity and a significant increase in Staphylococcus aureus colonization compared to healthy individuals, in whom this species rarely invades [37][26]. On the other hand, the most common bacteria harboring psoriatic lesions are those of the phylum Firmicutes, which are present in a higher proportion in the psoriatic skin than in the skin of healthy individuals [38][27].
References
- Kindt, T.J.; Goldsby, R.A.; Osborne, B.A.; Kuby, J. Kuby Immunology, 6th ed.; W.H. Freeman: New York, NY, USA, 2007; pp. 350–356.
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin Immune Sentinels in Health and Disease. Nat. Rev. Immunol. 2009, 9, 679–691.
- Juráňová, J.; Franková, J.; Ulrichová, J. The Role of Keratinocytes in Inflammation. J. Appl. Biomed. 2017, 15, 169–179.
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms Regulating Skin Immunity and Inflammation. Nat. Rev. Immunol. 2014, 14, 289–301.
- Cruz, M.S.; Diamond, A.; Russell, A.; Jameson, J.M. Human Aβ and Γδ T Cells in Skin Immunity and Disease. Front. Immunol. 2018, 9, 1304.
- Di Meglio, P.; Perera, G.K.; Nestle, F.O. The Multitasking Organ: Recent Insights into Skin Immune Function. Immunity 2011, 35, 857–869.
- Balato, A.; Cacciapuoti, S.; Di Caprio, R.; Marasca, C.; Masarà, A.; Raimondo, A.; Fabbrocini, G. Human Microbiome: Composition and Role in Inflammatory Skin Diseases. Arch. Immunol. Ther. Exp. 2019, 67, 1–18.
- Frevert, C.W.; Felgenhauer, J.; Wygrecka, M.; Nastase, M.V.; Schaefer, L. Danger-Associated Molecular Patterns Derived From the Extracellular Matrix Provide Temporal Control of Innate Immunity. J. Histochem. Cytochem. 2018, 66, 213–227.
- Hayden, M.S.; Ghosh, S. NF-κB in Immunobiology. Cell Res. 2011, 21, 223–244.
- Garlanda, C.; Riva, F.; Bonavita, E.; Gentile, S.; Mantovani, A. Decoys and Regulatory “Receptors” of the IL-1/Toll-Like Receptor Superfamily. Front. Immunol. 2013, 4, 180.
- Pobezinskaya, Y.L.; Liu, Z. The Role of TRADD in Death Receptor Signaling. Cell Cycle 2012, 11, 871–876.
- Montaño Estrada, L.D.; Fortoul Van der Goes, T.I.; Rendón Huerta, E.P. ¿Qué son los inflamosomas? El NLRP3 como ejemplo. Rev. Fac. Med. UNAM 2016, 60, 42–49.
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790.
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of Wound Healing. Curr. Derm. Rep. 2018, 7, 350–358.
- Tomura, M.; Hata, A.; Matsuoka, S.; Shand, F.H.W.; Nakanishi, Y.; Ikebuchi, R.; Ueha, S.; Tsutsui, H.; Inaba, K.; Matsushima, K.; et al. Tracking and Quantification of Dendritic Cell Migration and Antigen Trafficking between the Skin and Lymph Nodes. Sci. Rep. 2014, 4, 6030.
- Deckers, J.; Hammad, H.; Hoste, E. Langerhans Cells: Sensing the Environment in Health and Disease. Front. Immunol. 2018, 9, 93.
- Mills, C.D. Anatomy of a Discovery: M1 and M2 Macrophages. Front. Immunol. 2015, 6, 212.
- Nguyen, A.V.; Soulika, A.M. The Dynamics of the Skin’s Immune System. Int. J. Mol. Sci. 2019, 20, 1811.
- Kortekaas Krohn, I.; Aerts, J.L.; Breckpot, K.; Goyvaerts, C.; Knol, E.; Van Wijk, F.; Gutermuth, J. T-cell Subsets in the Skin and Their Role in Inflammatory Skin Disorders. Allergy 2022, 77, 827–842.
- Seidel, J.A.; Vukmanovic-Stejic, M.; Muller-Durovic, B.; Patel, N.; Fuentes-Duculan, J.; Henson, S.M.; Krueger, J.G.; Rustin, M.H.A.; Nestle, F.O.; Lacy, K.E.; et al. Skin Resident Memory CD8+ T Cells Are Phenotypically and Functionally Distinct from Circulating Populations and Lack Immediate Cytotoxic Function. Clin. Exp. Immunol. 2018, 194, 79–92.
- Matos, T.R.; O’Malley, J.T.; Lowry, E.L.; Hamm, D.; Kirsch, I.R.; Robins, H.S.; Kupper, T.S.; Krueger, J.G.; Clark, R.A. Clinically Resolved Psoriatic Lesions Contain Psoriasis-Specific IL-17–Producing Aβ T Cell Clones. J. Clin. Investig. 2017, 127, 4031–4041.
- Cai, Y.; Shen, X.; Ding, C.; Qi, C.; Li, K.; Li, X.; Jala, V.R.; Zhang, H.; Wang, T.; Zheng, J.; et al. Pivotal Role of Dermal IL-17-Producing Γδ T Cells in Skin Inflammation. Immunity 2011, 35, 596–610.
- Grice, E.A.; Kong, H.H.; Conlan, S.; Deming, C.B.; Davis, J.; Young, A.C.; Bouffard, G.G.; Blakesley, R.W.; Murray, P.R.; Green, E.D.; et al. Topographical and Temporal Diversity of the Human Skin Microbiome. Science 2009, 324, 1190–1192.
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S.; et al. Compartmentalized Control of Skin Immunity by Resident Commensals. Science 2012, 337, 1115–1119.
- Ferček, I.; Lugović-Mihić, L.; Tambić-Andrašević, A.; Ćesić, D.; Grginić, A.G.; Bešlić, I.; Mravak-Stipetić, M.; Mihatov-Štefanović, I.; Buntić, A.-M.; Čivljak, R. Features of the Skin Microbiota in Common Inflammatory Skin Diseases. Life 2021, 11, 962.
- Bourrain, M.; Ribet, V.; Calvez, A.; Lebaron, P.; Schmitt, A.-M. Balance between Beneficial Microflora and Staphylococcus Aureus Colonisation: In Vivo Evaluation in Patients with Atopic Dermatitis during Hydrotherapy. Eur. J. Dermatol. 2013, 23, 786–794.
- Fahlén, A.; Engstrand, L.; Baker, B.S.; Powles, A.; Fry, L. Comparison of Bacterial Microbiota in Skin Biopsies from Normal and Psoriatic Skin. Arch. Dermatol. Res. 2012, 304, 15–22.
More
