You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Goran Šimić.
Alzheimer’s disease (AD) is the most common cause of dementia worldwide (60–70% of cases), affecting over 55 million people. The role of metals in the pathogenesis of AD is still debated. Although previous research has linked changes in essential metal homeostasis and exposure to environmental heavy metals to the pathogenesis of AD, more research is needed to determine the relationship between metals and AD.
- Alzheimer’s disease
- essential metals
- heavy metals
- biomarker
- Mendelian randomization
1. Molecular Mechanisms through Which Metals Contribute to Alzheimer’s Disease Pathology
Increased metal concentration in the brain may contribute to various AD-associated pathological processes including Aβ-aggregation [30[1][2],31], hyperphosphorylation of tau protein [32[3][4],33], neuroinflammation [34][5], oxidative stress [35][6], blood–brain barrier (BBB) impairment [36][7], apoptosis and necrosis of neurons [37[8][9],38], and autophagy [39][10] (Figure 1). Experimental evidence indicates that both essential metals and heavy metals increase the aggregation of Aβ [30,40,41][1][11][12] and the hyperphosphorylation and aggregation of tau protein [33,42,43,44][4][13][14][15]. Furthermore, the exposure of young rats to a mixture of heavy metals induced neuroinflammation dependent on oxidative stress [45][16]. In addition, some essential metals such as Fe [46][17], Cu [47][18], Zn, and calcium (Ca) [39][10] can induce oxidative stress. Fe participates in Fenton reactions and can therefore contribute to the formation of reactive oxygen species [46][17]. Both the observed disruption of the BBB [48,49][19][20] and the apoptosis and necrosis of neurons [37,38][8][9] upon exposure to heavy metals may be preceded by oxidative stress, according to experimental evidence. Neurons are extremely sensitive to oxidative stress. Wang et al. [39][10] proposed that metal ion imbalance could induce oxidative stress, with the following downstream effects: (1) imbalance of protein kinases and phosphatases, increasing tau protein phosphorylation, and (2) imbalance of secretases, resulting in an increase in Aβ production (reviewed in [39][10]). On the other hand, essential metals also serve as cofactors in enzymes that combat oxidative stress. Cu, Zn, and manganese (Mn) are enzyme components of superoxide dismutase enzymes, while selenium is an enzyme component of glutathione peroxidase [50][21].
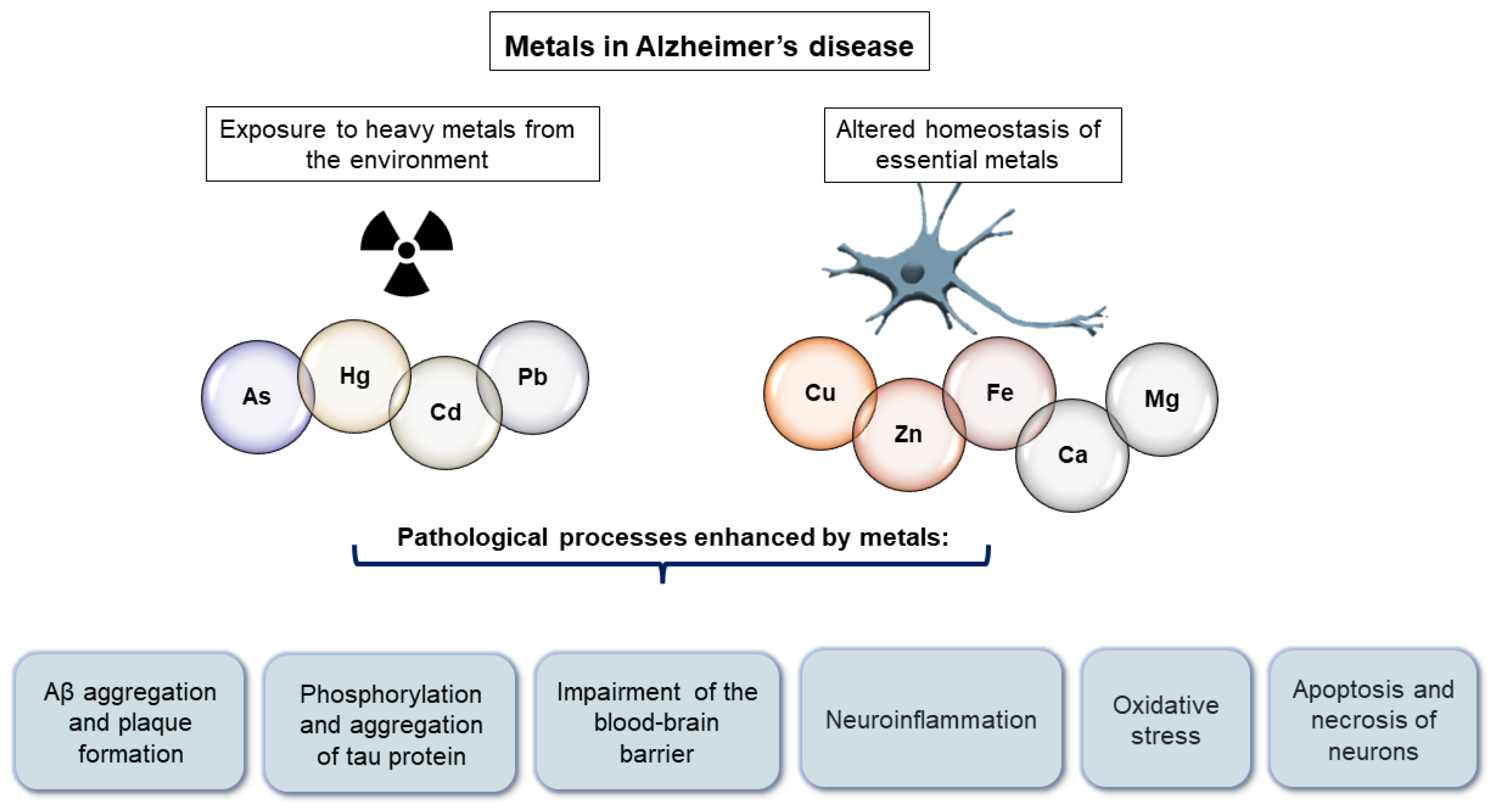
Figure 1.
Pathological processes enhanced by metals in Alzheimer’s disease.
Although there is a substantial body of evidence linking metals to AD-related pathological processes, it is unclear whether disrupted metal homeostasis is involved in the pathogenesis of AD, results from AD pathological processes, or both. Given that AD is a complex disease driven by both genetic and environmental factors, it is unlikely that AD pathogenesis will be explained by a single factor, but rather by the interaction of many.
2. Heavy Metals in Alzheimer’s Disease
Heavy metals including arsenic (As) [51][22], cadmium (Cd) [49][20], lead (Pb) [52][23], and mercury (Hg) [52][23] can cross the BBB and accumulate in the brain, or they can bypass the BBB and enter the brain directly through the olfactory pathway [53][24]. Some researchers have hypothesized that early exposure to heavy metals is associated with the later development of AD. Based on their observations of experimental animals, they concluded that early-life exposure to As [54][25], Pb [55][26], and Cd [56][27] may contribute to the development of neurodegeneration later in life, which is consistent with the developmental hypothesis of AD [57,58,59][28][29][30].
2.1. Arsenic
As is a metalloid that can be ingested through contaminated water, soil, and air, but primarily through drinking contaminated water. More than 220 million people are estimated to consume water that exceeds the permissible level of 10 µg/L [60][31]. Epidemiological studies suggest that As contributes to cognitive impairment [61][32] and an increased risk of AD [62][33], and that elevated As levels in soil are associated with an increase in AD-related mortality [63][34]. As exposure has also been associated with memory impairments in animal studies [64,65,66][35][36][37]. As exposure also increases Aβ levels [67][38], promotes tau hyperphosphorylation [32[3][39][40],68,69], tau aggregation [32][3], oxidative stress caused mainly by mitochondrial dysfunction [70][41], vascular damage [71][42], neuroinflammation [34][5], and apoptosis and the necrosis of neurons [37,38][8][9] (Figure 1). In the majority of human studies, there were no significant differences in As levels between AD patients and the controls, although some studies observed a significant increase in As levels in AD patients [72,73][43][44] and a positive association with CSF AD biomarkers [74][45].
2.2. Cadmium
Humans are exposed to Cd through food, air, and water [75][46]. Smokers have Cd levels that are two to four times higher than nonsmokers [76][47]. Cd may also play a role in the development of AD pathological changes. Cd has been linked in human studies to increased mortality due to AD [77,78][48][49] and cognitive decline [79,80,81][50][51][52]. Ruczaj and Brzoska proposed that Cd primarily exerts its effects by inducing oxidative stress [82][53]. Nevertheless, it also interacts with Aβ [83][54] and increases Aβ aggregation [30,40][1][11], promotes tau hyperphosphorylation [33][4] and aggregation [42][13], impairs the BBB [48[19][20],49], impairs cholinergic transmission and causes the death of cholinergic neurons in the basal forebrain [84][55], and disrupts intracellular cation homeostasis by being an anti-metabolite of Zn and replacing it in Zn enzymes [85][56] (Figure 1). In human studies, there is either an increase [86][57] or no difference [87][58] in Cd levels between AD patients and healthy controls.
2.3. Mercury
Exposure to Hg occurs through food, air, and water, with seafood consumption being the primary source of mercury poisoning [152][59]. Three- to 5-fold increases in Hg levels in the air and water have been documented as a result of industrialization [153][60]. A systematic review [154][61] and meta-analysis [155][62] demonstrated an association between Hg exposure and cognitive decline and progression of AD, but a subsequent report [156][63] did not confirm these findings. In addition, a neuropathological study of 286 brains by Morris et al. revealed no correlation between higher brain Hg levels and neuropathological alterations [152][59]. However, there are multiple molecular mechanisms through which Hg may contribute to the pathogenesis of AD. It promotes Aβ production [157][64] and aggregation [30][1], tau hyperphosphorylation [158,159][65][66] and aggregation [160][67], induces oxidative stress [35][6], and alters calcium homeostasis [161][68] (Figure 1). Human body fluid Hg measurements yielded contradictory results. Both an increase and a decrease were observed in Hg levels between the AD and control subjects, or there was no change. In addition, the CSF Hg level was positively correlated with several CSF AD biomarkers [74][45], whereas the blood Hg level was positively correlated with the CSF Aβ1–42 level [145][69].
2.4. Lead
In addition to food, air, and water, humans are also exposed to lead [29][70] through ingestion. Epidemiological studies have demonstrated that lead exposure contributes to cognitive impairment [162,163][71][72]. Moreover, experimental studies have reported an association between Pb and AD pathological changes. Pb interacts with Aβ [31][2] and increases Aβ production [45,164][16][73] and aggregation [31][2], increases tau hyperphosphorylation [165][74], compromises the BBB [36][7], induces epigenetic modifications by altering the expression of AD-related genes [166[75][76],167], disrupts intracellular cation homeostasis by interfering with Ca homeostasis and replacing Zn ions in Zn enzymes [168][77], and induces oxidative stress [169][78]. In human studies, there was a decrease or no difference in the Pb levels between the AD patients and control subjects, whereas a recent MR study found that higher blood Pb levels were a risk factor for AD [170][79].
2.5. Aluminum
Aluminum (Al), the most abundant metal in the Earth’s crust [171][80], is not an essential element for life; however, in its free, solvated, and trivalent forms, Al3+ is biologically reactive [172][81], accumulating in the central nervous system [173,174][82][83]. In AD-affected brain regions including the entorhinal cortex, hippocampal region, and amygdala, the concentration of Al is higher [175,176][84][85]. Al was co-deposited with fibrillar Aβ in amyloid plaques in a study of brain tissue samples from donors with familial AD (fAD) and the PSEN1-E280A (Glu280Ala) mutation [172,177][81][86]. Cortical Aβ levels are elevated in donors with this mutation, and this mutation is associated with an aggressive etiology of AD [178][87]. Aluminum’s unique association with Aβ and the high levels of Al found in these brain tissues suggest that Al plays a role in the neuropathology of fAD [177][86].
When Al binds to various proteins, oligomerization can occur, resulting in conformational changes that prevent proteases from degrading the proteins. In addition, Al3+ binds strongly to phosphorylated amino acids, causing highly phosphorylated cytoskeleton proteins to aggregate and accumulate [179][88]. As a result, Al induces the apoptotic death of neurons and glial cells. Al-Aβ co-deposition in fAD has been hypothesized, but its association with intraneuronal NFTs has not been confirmed [177[86][89],180], as demonstrated by Mold et al. [181][90]. While Al binding to Aβ in amyloid plaques is anticipated in the early stages of disease progression [177,178[86][87][91],182], an association with tau may occur in later disease stages [177,178,182][86][87][91]. Numerous studies have investigated the association between oral exposure to Al in drinking water and AD [183][92]. According to Martyn et al. [184][93], AD is more prevalent in regions with high levels of Al in their drinking water. In conclusion, even though Al has been proposed as a potential risk factor for AD, there is insufficient evidence to support a causal relationship. Many studies have investigated the association between oral exposure to Al in drinking water and AD; however, more research is required to better understand how genetic, environmental, and lifestyle factors influence the onset and progression of AD.
3. Essential Metals in Alzheimer’s Disease
The homeostasis of essential metals is altered in AD patients [26,27,28][94][95][96]. This term refers to metals that are naturally present in the body and play a role in the function of numerous proteins and enzymes or act as second messengers. Sodium (Na), Ca, and magnesium (Mg) are the most abundant essential metals in the human body, while Fe, Cu, Zn, molybdenum (Mo), cobalt (Co), Mn, and chromium (Cr) are present in trace amounts. Many previous studies have also demonstrated the association between essential metals (primarily Fe, Cu, and Zn) and AD pathological changes.
3.1. Iron
Many biological processes in the body including the brain are regulated by Fe ions. Fe is essential for protein synthesis [197][97], cell growth and differentiation [198[98][99],199], the regulation of Fe-dependent enzymes [200][100], oxygen transport [201][101], and the electron transfer chain in oxidation–reduction reactions [201][101]. Fe is also crucial for the processes of myelination [202][102], development [203][103], and the function of numerous neurotransmitter systems [204][104]. Both amyloid plaques and NFTs have been found to have elevated Fe concentrations [205][105]. Fe is also involved in oxidative stress and the formation of reactive oxygen species in the brains of AD patients via the Fenton reaction [46][17]. Fe also promotes in vitro Aβ aggregation [206][106], tau protein phosphorylation [207,208,209][107][108][109], and tau aggregation [210][110] (Figure 1). It is interesting to note that APP is necessary for the persistence of ferroprotein (iron exporter) on the cell surface, and thus promotes Fe release [211][111].
In meta-analyses, a significant decrease in Fe levels was observed in the plasma [87][58] and serum [129][112] of AD patients, but no significant change was observed in the CSF [129][112]. In contrast, a number of studies observed a correlation between the Fe levels in CSF and various CSF AD biomarkers [74,188,193][45][113][114]. Nonetheless, in many observational studies, there was no difference in the Fe levels between the AD patients and controls.
3.2. Zinc
The brain has a higher Zn concentration than other organs [212][115]. Zn is essential for neurotransmission because, as an antagonist of glutamate NMDA (N-methyl-D-aspartate) receptors, it protects neurons from glutamate-induced excitotoxic damage [213][116]. Zn accumulates in amyloid plaques [214][117], binds to Aβ, and promotes Aβ aggregation and plaque formation [214][117]. Zn also promotes tau protein aggregation [215][118], phosphorylation [216[119][120],217], and translation [217][120] (Figure 1). In meta-analyses, however, a significant decrease in Zn levels was observed in the serum and plasma [123][121] as well as in the hair of AD patients [87][58], whereas there was no significant change in the CSF [123][121] and brain [115][122] levels. To date, MR studies have not identified Zn as a risk factor for AD [218,219,220][123][124][125]. An in vivo study demonstrated positive effects of Zn supplementation in mouse models of AD [221][126], and a small double-blind clinical trial observed the stabilization of cognitive abilities in AD patients after six months [222][127]. Thus, adding Zn to the diet has been suggested to improve the cognitive abilities of AD patients [223][128], whereas Loef et al. found no significant benefit of Zn supplementation in AD [224][129]. In addition, in vivo studies have shown that Zn supplementation promotes the formation of NFTs [225][130] and Aβ deposition [226][131].
3.3. Copper
Normal brain function requires optimal Cu levels, as indicated by the disruption of its metabolism. Patients with Menkes syndrome, for example, suffer from intellectual deficits and neurodegeneration. This disorder is caused by a sex-linked mutation of the ATP7A gene on the X chromosome (which encodes a protein involved in the transmembrane transfer of Cu ions) and is characterized by the decreased absorption of Cu in the intestine, and consequently, a decreased concentration of Cu in the cytosol of all body cells except in the intestines and kidneys [227][132]. In Wilson’s disease, excessive Cu accumulation in the body is associated with psychosis, parkinsonism, and dementia [228,229][133][134]. Cu homeostasis is also impaired in AD [28][96]. Cu promotes the formation and accumulation of Aβ-oligomers by binding to Aβ [41][12]. Cu chelation can prevent the cytotoxic effect of the Cu-Aβ complex [230][135]. Cu accumulates in plaques [231[136][137],232], and the interaction between Cu and APP has been demonstrated [232][137]. Cu can induce both the phosphorylation and aggregation of tau [42,43][13][14] (Figure 1) and its interaction with apolipoprotein E (ApoE) contributes to the pathogenesis of AD. ApoE2 has the highest binding affinity for divalent Cu, Zn, and Fe ions, while ApoE4 has the lowest [233,234][138][139]. In meta-analyses, Cu levels in the serum of AD patients increased significantly [87[58][140][141][142],92,122,133], whereas Cu levels in the brains of AD patients decreased [92][140]. Recent MR studies [218,220][123][125] have surprisingly found that higher Cu levels are protective against AD risk.
3.4. Calcium
Ca is an indispensable second messenger that regulates hundreds of signaling pathways crucial for the normal functioning of memory and cognition-related cells and networks [235][143]. Many neurodegenerative diseases including AD [236][144] are characterized by a disruption of cellular Ca signaling. The excessive entry of Ca ions through ionotropic glutamate receptors is a known mechanism of excitotoxic neuronal death [237,238][145][146]. Ca homeostasis disruption promotes Aβ and tau pathology [239][147]. However, human studies have produced contradictory results, with both decreased [240,241][148][149] and increased Ca [186,242][150][151] being risk factors. In recent MR studies, higher Ca levels were shown to reduce the risk of AD [241[149][152],243], or no association between Ca levels and AD risk has been observed [218,220][123][125].
3.5. Manganese
Mn is a crucial element for protein synthesis, lipid and glucose metabolism, and oxidative stress protection [244][153]. However, Mn is also an environmental toxin, and elevated Mn levels have been linked to diminished cognitive performance [187,245,246][154][155][156]. A rise in Mn levels has also been observed in patients with AD [109][157]. Nonetheless, a meta-analysis by Du et al. [89][158] revealed a significant decrease in Mn levels between AD and the controls.
3.6. Magnesium
Human studies have demonstrated that Mg deficiency impairs memory [247][159] and that Mg supplementation can improve memory in dementia patients [248,249,250][160][161][162]. In addition, a decrease in Mg concentration has been observed in the tissues of AD patients [251,252][163][164]. However, no change in Mg concentration was observed in the brains of AD patients in some studies (reviewed in [253][165]). Mg influences the processing and transport of APP, with low Mg levels favoring the β-secretase pathway and high Mg levels favoring the α-secretase pathway [254][166], whereas the treatment of experimental animals with Mg sulfate reduces tau phosphorylation and influences the maintenance of cognitive functions and synaptic plasticity [255][167]. According to the meta-analysis by Du et al. [89][158], the serum and plasma Mg concentrations were lower in the AD patients than in the controls, whereas the CSF Mg concentrations did not differ between groups. Thomassen et al. [95][168] did not find an association between the plasma Mg levels and the risk of AD in a study involving more than 100,000 participants. Kieboom et al. demonstrated that both low and high Mg concentrations were associated with an increased risk of dementia. They concluded that the relationship between Mg and the risk of dementia was U-shaped rather than linear [108][169].
3.7. Other Essential Metals
AD also perturbs the homeostasis of Na, K, and Co. Previous studies have associated elevated Na levels with AD [27,96,256,257][95][170][171][172]. Both increased [102][173] and decreased [195][174] K levels have been associated with AD, whereas in some studies, no change in the K levels was observed in AD. Co is an essential component of vitamin B12 and is an environmental toxin. Zheng et al. showed that mice exposed to Co develop age-related neurodegeneration [258][175].
References
- Yano, K.; Hirosawa, N.; Sakamoto, Y.; Katayama, H.; Moriguchi, T. Aggregations of amyloid beta-proteins in the presence of metal ions. Toxicol. Lett. 2003, 144, 134.
- Wallin, C.; Sholts, S.B.; Österlund, N.; Luo, J.; Jarvet, J.; Roos, P.M.; Ilag, L.; Gräslund, A.; Wärmländer, S.K.T.S. Alzheimer’s disease and cigarette smoke components: Effects of nicotine, PAHs, and Cd(II), Cr(III), Pb(II), Pb(IV) ions on amyloid-β peptide aggregation. Sci. Rep. 2017, 7, 14423.
- Wisessaowapak, C.; Visitnonthachai, D.; Watcharasit, P.; Satayavivad, J. Prolonged arsenic exposure increases tau phosphorylation in differentiated SH-SY5Y cells: The contribution of GSK3 and ERK1/2. Environ. Toxicol. Pharmacol. 2021, 84, 103626.
- Shati, A.A.; Alfaifi, M.Y. Trans-resveratrol Inhibits Tau Phosphorylation in the Brains of Control and Cadmium Chloride-Treated Rats by Activating PP2A and PI3K/Akt Induced-Inhibition of GSK3β. Neurochem. Res. 2019, 44, 357–373.
- Mao, J.; Yang, J.; Zhang, Y.; Li, T.; Wang, C.; Xu, L.; Hu, Q.; Wang, X.; Jiang, S.; Nie, X.; et al. Arsenic trioxide mediates HAPI microglia inflammatory response and subsequent neuron apoptosis through p38/JNK MAPK/STAT3 pathway. Toxicol. Appl. Pharmacol. 2016, 303, 79–89.
- Augusti, P.R.; Conterato, G.M.M.; Somacal, S.; Sobieski, R.; Spohr, P.R.; Torres, J.V.; Charão, M.F.; Moro, A.M.; Rocha, M.P.; Garcia, S.C.; et al. Effect of astaxanthin on kidney function impairment and oxidative stress induced by mercuric chloride in rats. Food Chem. Toxicol. 2008, 46, 212–219.
- Gu, H.; Territo, P.R.; Persohn, S.A.; Bedwell, A.A.; Eldridge, K.; Speedy, R.; Chen, Z.; Zheng, W.; Du, Y. Evaluation of chronic lead effects in the blood brain barrier system by DCE-CT. J. Trace Elem. Med. Biol. 2020, 62, 126648.
- Chattopadhyay, S.; Bhaumik, S.; Purkayastha, M.; Basu, S.; Nag Chaudhuri, A.; Das Gupta, S. Apoptosis and necrosis in developing brain cells due to arsenic toxicity and protection with antioxidants. Toxicol. Lett. 2002, 136, 65–76.
- Bashir, S.; Sharma, Y.; Irshad, M.; Gupta, S.D.; Dogra, T.D. Arsenic-induced cell death in liver and brain of experimental rats. Basic Clin. Pharmacol. Toxicol. 2006, 98, 38–43.
- Wang, L.; Yin, Y.L.; Liu, X.Z.; Shen, P.; Zheng, Y.G.; Lan, X.R.; Lu, C.B.; Wang, J.Z. Current understanding of metal ions in the pathogenesis of Alzheimer’s disease. Transl. Neurodegener. 2020, 9, 10.
- Syme, C.D.; Viles, J.H. Solution 1H NMR investigation of Zn2+ and Cd2+ binding to amyloid-beta peptide (Abeta) of Alzheimer’s disease. Biochim. Biophys. Acta 2006, 1764, 246–256.
- Jiao, Y.; Han, D.; Yang, P. Molecular modeling of the inhibitory mechanism of copper(II) on aggregation of amyloid β-peptide. Sci. China Ser. B 2005, 48, 580.
- Kitazawa, M.; Cheng, D.; Laferla, F.M. Chronic copper exposure exacerbates both amyloid and tau pathology and selectively dysregulates cdk5 in a mouse model of AD. J. Neurochem. 2009, 108, 1550–1560.
- Zhou, L.-X.; Du, J.-T.; Zeng, Z.-Y.; Wu, W.-H.; Zhao, Y.-F.; Kanazawa, K.; Ishizuka, Y.; Nemoto, T.; Nakanishi, H.; Li, Y.-M. Copper (II) modulates in vitro aggregation of a tau peptide. Peptides 2007, 28, 2229–2234.
- Jiang, L.F.; Yao, T.M.; Zhu, Z.L.; Wang, C.; Ji, L.N. Impacts of Cd(II) on the conformation and self-aggregation of Alzheimer’s tau fragment corresponding to the third repeat of microtubule-binding domain. Biochim. Biophys. Acta 2007, 1774, 1414–1421.
- Ashok, A.; Rai, N.K.; Tripathi, S.; Bandyopadhyay, S. Exposure to As-, Cd-, and Pb-mixture induces Aβ, amyloidogenic APP processing and cognitive impairments via oxidative stress-dependent neuroinflammation in young rats. Toxicol. Sci. 2015, 143, 64–80.
- Kanti Das, T.; Wati, M.R.; Fatima-Shad, K. Oxidative Stress Gated by Fenton and Haber Weiss Reactions and Its Association with Alzheimer’s Disease. Arch. Neurosci. 2015, 2, e20078.
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163.
- Branca, J.J.V.; Maresca, M.; Morucci, G.; Mello, T.; Becatti, M.; Pazzagli, L.; Colzi, I.; Gonnelli, C.; Carrino, D.; Paternostro, F.; et al. Effects of Cadmium on ZO-1 Tight Junction Integrity of the Blood Brain Barrier. Int. J. Mol. Sci. 2019, 20, 6010.
- Zhang, T.; Xu, Z.; Wen, L.; Lei, D.; Li, S.; Wang, J.; Huang, J.; Wang, N.; Durkan, C.; Liao, X.; et al. Cadmium-induced dysfunction of the blood-brain barrier depends on ROS-mediated inhibition of PTPase activity in zebrafish. J. Hazard. Mater. 2021, 412, 125198.
- Wolonciej, M.; Milewska, E.; Roszkowska-Jakimiec, W. Trace elements as an activator of antioxidant enzymes. Postep. Hig. Med. Dosw. 2016, 70, 1483–1498.
- Charlet, L.; Chapron, Y.; Faller, P.; Kirsch, R.; Stone, A.T.; Baveye, P.C. Neurodegenerative diseases and exposure to the environmental metals Mn, Pb, and Hg. Coord. Chem. Rev. 2012, 256, 2147–2163.
- Garza-Lombó, C.; Pappa, A.; Panayiotidis, M.I.; Gonsebatt, M.E.; Franco, R. Arsenic-induced neurotoxicity: A mechanistic appraisal. J. Biol. Inorg. Chem. 2019, 24, 1305–1316.
- Sunderman Jr, F. Nasal toxicity, carcinogenicity, and olfactory uptake of metals. Ann. Clin. Lab. Sci. 2001, 31, 3–24.
- Niño, S.A.; Vázquez-Hernández, N.; Arevalo-Villalobos, J.; Chi-Ahumada, E.; Martín-Amaya-Barajas, F.L.; Díaz-Cintra, S.; Martel-Gallegos, G.; González-Burgos, I.; Jiménez-Capdeville, M.E. Cortical Synaptic Reorganization Under Chronic Arsenic Exposure. Neurotox. Res. 2021, 39, 1970–1980.
- Bihaqi, S.W.; Bahmani, A.; Subaiea, G.M.; Zawia, N.H. Infantile exposure to lead and late-age cognitive decline: Relevance to AD. Alzheimers Dement. 2014, 10, 187–195.
- Gustin, K.; Tofail, F.; Vahter, M.; Kippler, M. Cadmium exposure and cognitive abilities and behavior at 10 years of age: A prospective cohort study. Environ. Int. 2018, 113, 259–268.
- Borenstein, A.R.; Copenhaver, C.I.; Mortimer, J.A. Early-life risk factors for Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2006, 20, 63–72.
- Miller, D.B.; O’Callaghan, J.P. Do early-life insults contribute to the late-life development of Parkinson and Alzheimer diseases? Metabolism 2008, 57 (Suppl. S2), 244–249.
- Gauvrit, T.; Benderradji, H.; Buée, L.; Blum, D.; Vieau, D. Early-Life Environment Influence on Late-Onset Alzheimer’s Disease. Front. Cell Dev. Biol. 2022, 10, 834661.
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850.
- O’Bryant, S.E.; Edwards, M.; Menon, C.V.; Gong, G.; Barber, R. Long-term low-level arsenic exposure is associated with poorer neuropsychological functioning: A Project FRONTIER study. Int. J. Environ. Res. Public Health 2011, 8, 861–874.
- Yang, Y.W.; Liou, S.H.; Hsueh, Y.M.; Lyu, W.S.; Liu, C.S.; Liu, H.J.; Chung, M.C.; Hung, P.H.; Chung, C.J. Risk of Alzheimer’s disease with metal concentrations in whole blood and urine: A case-control study using propensity score matching. Toxicol. Appl. Pharmacol. 2018, 356, 8–14.
- Li, X.L.; Zhan, R.Q.; Zheng, W.; Jiang, H.; Zhang, D.F.; Shen, X.L. Positive association between soil arsenic concentration and mortality from Alzheimer’s disease in mainland China. J. Trace Elem. Med. Biol. 2020, 59, 126452.
- Ma, L.; Zhang, C.; Liu, W. Effects of arsenic on the offspring development in mice. Zhonghua Yu Fang Yi Xue Za Zhi 1994, 28, 20–23.
- Tyler, C.R.; Allan, A.M. The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human and Rodent Studies: A Review. Curr. Environ. Health Rep. 2014, 1, 132–147.
- Samad, N.; Jabeen, S.; Imran, I.; Zulfiqar, I.; Bilal, K. Protective effect of gallic acid against arsenic-induced anxiety-/depression- like behaviors and memory impairment in male rats. Metab. Brain Dis. 2019, 34, 1091–1102.
- Zarazúa, S.; Bürger, S.; Delgado, J.M.; Jiménez-Capdeville, M.E.; Schliebs, R. Arsenic affects expression and processing of amyloid precursor protein (APP) in primary neuronal cells overexpressing the Swedish mutation of human APP. Int. J. Dev. Neurosci. 2011, 29, 389–396.
- Giasson, B.I.; Sampathu, D.M.; Wilson, C.A.; Vogelsberg-Ragaglia, V.; Mushynski, W.E.; Lee, V.M.Y. The environmental toxin arsenite induces tau hyperphosphorylation. Biochemistry 2002, 41, 15376–15387.
- Pakzad, D.; Akbari, V.; Sepand, M.R.; Aliomrani, M. Risk of neurodegenerative disease due to tau phosphorylation changes and arsenic exposure via drinking water. Toxicol. Res. 2021, 10, 325–333.
- Hassani, S.; Yaghoubi, H.; Khosrokhavar, R.; Jafarian, I.; Mashayekhi, V.; Hosseini, M.J.; Shahraki, J. Mechanistic view for toxic effects of arsenic on isolated rat kidney and brain mitochondria. Biologia 2015, 70, 683–689.
- Tsinovoi, C.L.; Xun, P.; McClure, L.A.; Carioni, V.M.O.; Brockman, J.D.; Cai, J.; Guallar, E.; Cushman, M.; Unverzagt, F.W.; Howard, V.J.; et al. Arsenic Exposure in Relation to Ischemic Stroke: The Reasons for Geographic and Racial Differences in Stroke Study. Stroke 2018, 49, 19–26.
- Koseoglu, E.; Kutuk, B.; Nalbantoglu, O.U.; Koseoglu, R.; Kendirci, M. Arsenic and selenium measurements in nail and hair show important relationships to Alzheimer’s disease in the elderly. J. Trace Elem. Med. Biol. 2021, 64, 126684.
- Strumylaite, L.; Kregzdyte, R.; Kucikiene, O.; Baranauskiene, D.; Simakauskiene, V.; Naginiene, R.; Damuleviciene, G.; Lesauskaite, V.; Zemaitiene, R. Alzheimer’s Disease Association with Metals and Metalloids Concentration in Blood and Urine. Int. J. Environ. Res. Public Health 2022, 19, 7309.
- Babić Leko, M.; Mihelčić, M.; Jurasović, J.; Nikolac Perković, M.; Španić, E.; Sekovanić, A.; Orct, T.; Zubčić, K.; Langer Horvat, L.; Pleić, N.; et al. Heavy Metals and Essential Metals Are Associated with Cerebrospinal Fluid Biomarkers of Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 467.
- Satarug, S.; Moore, M.R. Adverse Health Effects of Chronic Exposure to Low-Level Cadmium in Foodstuffs and Cigarette Smoke. Environ. Health Perspect. 2004, 112, 1099–1103.
- ATSDR. Toxicological Profile for Cadmium; Agency for Toxic Substances & Disease Registry, Department of Health and Human Services, Public Health Service: Atlanta, GA, USA, 2012.
- Min, J.Y.; Min, K.B. Blood cadmium levels and Alzheimer’s disease mortality risk in older US adults. Environ. Health A Glob. Access Sci. Source 2016, 15, 69.
- Peng, Q.; Bakulski, K.M.; Nan, B.; Park, S.K. Cadmium and Alzheimer’s disease mortality in U.S. adults: Updated evidence with a urinary biomarker and extended follow-up time. Environ. Res. 2017, 157, 44–51.
- Souza-Talarico, J.N.; Marcourakis, T.; Barbosa, F.; Moraes Barros, S.B.; Rivelli, D.P.; Pompéia, S.; Caramelli, P.; Plusquellec, P.; Lupien, S.J.; Catucci, R.F.; et al. Association between heavy metal exposure and poor working memory and possible mediation effect of antioxidant defenses during aging. Sci. Total Environ. 2017, 575, 750–757.
- Li, H.; Wang, Z.; Fu, Z.; Yan, M.; Wu, N.; Wu, H.; Yin, P. Associations between blood cadmium levels and cognitive function in a cross-sectional study of US adults aged 60 years or older. BMJ Open 2018, 8, e020533.
- Huang, G.; Ren, G. Interaction between ω-6 fatty acids intake and blood cadmium on the risk of low cognitive performance in older adults from National Health and Nutrition Examination Survey (NHANES) 2011-2014. BMC Geriatr. 2022, 22, 292.
- Ruczaj, A.; Brzóska, M.M. Environmental exposure of the general population to cadmium as a risk factor of the damage to the nervous system: A critical review of current data. J. Appl. Toxicol. 2022, 43, 66–88.
- Notarachille, G.; Arnesano, F.; Calò, V.; Meleleo, D. Heavy metals toxicity: Effect of cadmium ions on amyloid beta protein 1–42. Possible implications for Alzheimer’s disease. Biometals 2014, 27, 371–388.
- del Pino, J.; Zeballos, G.; Anadón, M.J.; Moyano, P.; Díaz, M.J.; García, J.M.; Frejo, M.T. Cadmium-induced cell death of basal forebrain cholinergic neurons mediated by muscarinic M1 receptor blockade, increase in GSK-3β enzyme, β-amyloid and tau protein levels. Arch. Toxicol. 2016, 90, 1081–1092.
- Li, X.; Lv, Y.; Yu, S.; Zhao, H.; Yao, L. The effect of cadmium on Aβ levels in APP/PS1 transgenic mice. Exp. Ther. Med. 2012, 4, 125–130.
- Yadav, J.; Verma, A.K.; Ahmad, M.K.; Garg, R.K.; Shiuli; Mahdi, A.A.; Srivastava, S. Metals toxicity and its correlation with the gene expression in Alzheimer’s disease. Mol. Biol. Rep. 2021, 48, 3245–3252.
- Li, K.; Li, A.; Mei, Y.; Zhao, J.; Zhou, Q.; Li, Y.; Yang, M.; Xu, Q. Trace elements and Alzheimer dementia in population-based studies: A bibliometric and meta-analysis. Environ. Pollut. 2023, 318, 120782.
- Morris, M.C.; Brockman, J.; Schneider, J.A.; Wang, Y.; Bennett, D.A.; Tangney, C.C.; van de Rest, O. Association of Seafood Consumption, Brain Mercury Level, and APOE ε4 Status with Brain Neuropathology in Older Adults. JAMA 2016, 315, 489–497.
- Engstrom, D.R.; Fitzgerald, W.F.; Cooke, C.A.; Lamborg, C.H.; Drevnick, P.E.; Swain, E.B.; Balogh, S.J.; Balcom, P.H. Atmospheric Hg emissions from preindustrial gold and silver extraction in the Americas: A reevaluation from lake-sediment archives. Environ. Sci. Technol. 2014, 48, 6533–6543.
- Mutter, J.; Curth, A.; Naumann, J.; Deth, R.; Walach, H. Does inorganic mercury play a role in Alzheimer’s disease? A systematic review and an integrated molecular mechanism. J. Alzheimer’s Dis. 2010, 22, 357–374.
- Xu, L.; Zhang, W.; Liu, X.; Zhang, C.; Wang, P.; Zhao, X. Circulatory Levels of Toxic Metals (Aluminum, Cadmium, Mercury, Lead) in Patients with Alzheimer’s Disease: A Quantitative Meta-Analysis and Systematic Review. J. Alzheimer’s Dis. 2018, 62, 361–372.
- Olayinka, O.; Olayinka, O.O.; Alemu, B.T.; Akpinar-Elci, M.; Grossberg, G.T. Toxic Environmental Risk Factors for Alzheimer’s Disease: A Systematic Review. Aging Med. Healthc. 2019, 10, 4–17.
- Alattia, J.-R.; Kuraishi, T.; Dimitrov, M.; Chang, I.; Lemaitre, B.; Fraering, P.C. Mercury is a direct and potent γ-secretase inhibitor affecting Notch processing and development in Drosophila. FASEB J. 2011, 25, 2287–2295.
- Olivieri, G.; Brack, C.; Müller-Spahn, F.; Stähelin, H.B.; Herrmann, M.; Renard, P.; Brockhaus, M.; Hock, C. Mercury induces cell cytotoxicity and oxidative stress and increases beta-amyloid secretion and tau phosphorylation in SHSY5Y neuroblastoma cells. J. Neurochem. 2000, 74, 231–236.
- Fujimura, M.; Usuki, F.; Sawada, M.; Takashima, A. Methylmercury induces neuropathological changes with tau hyperphosphorylation mainly through the activation of the c-jun-N-terminal kinase pathway in the cerebral cortex, but not in the hippocampus of the mouse brain. Neurotoxicology 2009, 30, 1000–1007.
- Yang, D.-J.; Shi, S.; Zheng, L.-F.; Yao, T.-M.; Ji, L.-N. Mercury(II) promotes the in vitro aggregation of tau fragment corresponding to the second repeat of microtubule-binding domain: Coordination and conformational transition. Biopolymers 2010, 93, 1100–1107.
- Yin, X.; Sun, J.; Mei, Y.; Guo, X.; Chen, S.-I.; Wang, Z.-I.; Yang, L. Effect of Hg2+ on voltage-dependent calcium channels and intracellular free calcium in trigeminal ganglion neurons of rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2008, 26, 542–545.
- Hock, C.; Drasch, G.; Golombowski, S.; Müller-Spahn, F.; Willershausen-Zönnchen, B.; Schwarz, P.; Hock, U.; Growdon, J.H.; Nitsch, R.M. Increased blood mercury levels in patients with Alzheimer’s disease. J. Neural Transm. 1998, 105, 59–68.
- Elonheimo, H.M.; Andersen, H.R.; Katsonouri, A.; Tolonen, H. Environmental Substances Associated with Alzheimer’s Disease-A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 11839.
- Weisskopf, M.G.; Wright, R.O.; Schwartz, J.; Spiro, A.; Sparrow, D.; Aro, A.; Hu, H. Cumulative lead exposure and prospective change in cognition among elderly men: The VA Normative Aging Study. Am. J. Epidemiol. 2004, 160, 1184–1193.
- Wright, R.O.; Tsaih, S.W.; Schwartz, J.; Spiro, A.; McDonald, K.; Weiss, S.T.; Hu, H. Lead exposure biomarkers and mini-mental status exam scores in older men. Epidemiology 2003, 14, 713–718.
- Gu, H.; Wei, X.; Monnot, A.D.; Fontanilla, C.V.; Behl, M.; Farlow, M.R.; Zheng, W.; Du, Y. Lead exposure increases levels of β-amyloid in the brain and CSF and inhibits LRP1 expression in APP transgenic mice. Neurosci. Lett. 2011, 490, 16–20.
- Bihaqi, S.W.; Eid, A.; Zawia, N.H. Lead exposure and tau hyperphosphorylation: An in vitro study. Neurotoxicology 2017, 62, 218–223.
- Bakulski, K.M.; Rozek, L.S.; Dolinoy, D.C.; Paulson, H.L.; Hu, H. Alzheimer’s disease and environmental exposure to lead: The epidemiologic evidence and potential role of epigenetics. Curr. Alzheimer Res. 2012, 9, 563–573.
- Wang, T.; Zhang, J.; Xu, Y. Epigenetic Basis of Lead-Induced Neurological Disorders. Int. J. Environ. Res. Public Health 2020, 17, 4878.
- Andrade, V.M.; Aschner, M.; Marreilha dos Santos, A.P. Neurotoxicity of Metal Mixtures. Adv. Neurobiol. 2017, 18, 227–265.
- Patra, R.C.; Rautray, A.K.; Swarup, D. Oxidative Stress in Lead and Cadmium Toxicity and Its Amelioration. Vet. Med. Int. 2011, 2011, 457327.
- Li, C.; Zhang, Y.; Liang, J.; Wu, C.; Zou, X. Assessing the Association Between Lead Pollution and Risk of Alzheimer’s Disease by Integrating Multigenomics. Front. Neurosci. 2022, 16, 880105.
- Masten, S.; Carson, B.L. Aluminum Compounds Review of Toxicological Literature Abridged Final Report; Integrated Laboratory Systems: Morrisville, NC, USA, 2000.
- Exley, C.; Mold, M.J. The binding, transport and fate of aluminium in biological cells. J. Trace Elem. Med. Biol. 2015, 30, 90–95.
- Exley, C.; Mold, M.J. Aluminium in human brain tissue: How much is too much? J. Biol. Inorg. Chem. 2019, 24, 1279–1282.
- Exley, C.; Mold, M.J. Imaging of aluminium and amyloid β in neurodegenerative disease. Heliyon 2020, 6, e03839.
- Walton, J.R. Brain lesions comprised of aluminum-rich cells that lack microtubules may be associated with the cognitive deficit of Alzheimer’s disease. Neurotoxicology 2009, 30, 1059–1069.
- Walton, J.R. Aluminum in hippocampal neurons from humans with Alzheimer’s disease. Neurotoxicology 2006, 27, 385–394.
- Mold, M.; Linhart, C.; Gómez-Ramírez, J.; Villegas-Lanau, A.; Exley, C. Aluminum and Amyloid-β in Familial Alzheimer’s Disease. J. Alzheimers. Dis. 2020, 73, 1627–1635.
- Lopera, F.; Ardilla, A.; Martínez, A.; Madrigal, L.; Arango-Viana, J.C.; Lemere, C.A.; Arango-Lasprilla, J.C.; Hincapié, L.; Arcos-Burgos, M.; Ossa, J.E.; et al. Clinical Features of Early-Onset Alzheimer Disease in a Large Kindred with an E280A Presenilin-1 Mutation. JAMA 1997, 277, 793–799.
- Díaz-Nido, J.; Avila, J. Aluminum induces the in vitro aggregation of bovine brain cytoskeletal proteins. Neurosci. Lett. 1990, 110, 221–226.
- Mirza, A.; King, A.; Troakes, C.; Exley, C. Aluminium in brain tissue in familial Alzheimer’s disease. J. Trace Elem. Med. Biol. 2017, 40, 30–36.
- Mold, M.J.; O’Farrell, A.; Morris, B.; Exley, C. Aluminum and Tau in Neurofibrillary Tangles in Familial Alzheimer’s Disease. J. Alzheimer’s Dis. Rep. 2021, 5, 283.
- Mold, M.J.; O’Farrell, A.; Morris, B.; Exley, C. Aluminum and Neurofibrillary Tangle Co-Localization in Familial Alzheimer’s Disease and Related Neurological Disorders. J. Alzheimer’s Dis. 2020, 78, 139.
- Flaten, T.P. Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res. Bull. 2001, 55, 187–196.
- Martyn, C.N.; Osmond, C.; Edwardson, J.A.; Barker, D.J.P.; Harris, E.C.; Lacey, R.F. Geographical relation between Alzheimer’s disease and aluminum in drinking water. Lancet 1989, 333, 59–62.
- Bush, A.I. The metal theory of Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 33 (Suppl. S1), S277–S281.
- Babić Leko, M.; Jurasović, J.; Nikolac Perković, M.; Španić, E.; Sekovanić, A.; Orct, T.; Lukinović Škudar, V.; Bačić Baronica, K.; Kiđemet-Piskač, S.; Vogrinc, Ž.; et al. The Association of Essential Metals with APOE Genotype in Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 82, 661–672.
- Zubčić, K.; Hof, P.R.; Šimić, G.; Jazvinšćak Jembrek, M. The Role of Copper in Tau-Related Pathology in Alzheimer’s Disease. Front. Mol. Neurosci. 2020, 13, 572308.
- Jia, M.; Haldar, S.; Khan, M.A.; Sharma, S.D.; Merrick, W.C.; Theil, E.C.; Goss, D.J. Fe2+ binds iron responsive element-RNA, selectively changing protein-binding affinities and regulating mRNA repression and activation. Proc. Natl. Acad. Sci. USA 2012, 109, 8417–8422.
- Pfeifhofer-Obermair, C.; Tymoszuk, P.; Nairz, M.; Schroll, A.; Klais, G.; Demetz, E.; Engl, S.; Brigo, N.; Weiss, G. Regulation of Th1 T Cell Differentiation by Iron via Upregulation of T Cell Immunoglobulin and Mucin Containing Protein-3 (TIM-3). Front. Immunol. 2021, 12, 637809.
- Pourcelot, E.; Lénon, M.; Mobilia, N.; Cahn, J.Y.; Arnaud, J.; Fanchon, E.; Moulis, J.M.; Mossuz, P. Iron for proliferation of cell lines and hematopoietic progenitors: Nailing down the intracellular functional iron concentration. Biochim. Biophys. Acta -Mol. Cell Res. 2015, 1853, 1596–1605.
- Andreini, C.; Putignano, V.; Rosato, A.; Banci, L. The human iron-proteome. Metallomics 2018, 10, 1223–1231.
- Hirota, K. An intimate crosstalk between iron homeostasis and oxygen metabolism regulated by the hypoxia-inducible factors (HIFs). Free Radic. Biol. Med. 2019, 133, 118–129.
- Schulz, K.; Kroner, A.; David, S. Iron efflux from astrocytes plays a role in remyelination. J. Neurosci. 2012, 32, 4841–4847.
- Beard, J. Iron deficiency alters brain development and functioning. J. Nutr. 2003, 133, 1468S–1472S.
- Agarwal, K.N. Iron and the brain: Neurotransmitter receptors and magnetic resonance spectroscopy. Br. J. Nutr. 2001, 85 (Suppl. S2), S147–S150.
- Smith, M.A.; Harris, P.L.; Sayre, L.M.; Perry, G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. USA 1997, 94, 9866–9868.
- Mantyh, P.W.; Ghilardi, J.R.; Rogers, S.; DeMaster, E.; Allen, C.J.; Stimson, E.R.; Maggio, J.E. Aluminum, iron, and zinc ions promote aggregation of physiological concentrations of beta-amyloid peptide. J. Neurochem. 1993, 61, 1171–1174.
- Egaña, J.T.; Zambrano, C.; Nuñez, M.T.; Gonzalez-Billault, C.; Maccioni, R.B. Iron-induced oxidative stress modify tau phosphorylation patterns in hippocampal cell cultures. Biometals 2003, 16, 215–223.
- Lovell, M.A.; Xiong, S.; Xie, C.; Davies, P.; Markesbery, W.R. Induction of hyperphosphorylated tau in primary rat cortical neuron cultures mediated by oxidative stress and glycogen synthase kinase-3. J. Alzheimer’s Dis. 2004, 6, 659–671; discussion 673–681.
- Wan, W.; Cao, L.; Kalionis, B.; Murthi, P.; Xia, S.; Guan, Y. Iron Deposition Leads to Hyperphosphorylation of Tau and Disruption of Insulin Signaling. Front. Neurol. 2019, 10, 607.
- Yamamoto, A.; Shin, R.-W.; Hasegawa, K.; Naiki, H.; Sato, H.; Yoshimasu, F.; Kitamoto, T. Iron (III) induces aggregation of hyperphosphorylated tau and its reduction to iron (II) reverses the aggregation: Implications in the formation of neurofibrillary tangles of Alzheimer’s disease. J. Neurochem. 2002, 82, 1137–1147.
- Wong, B.X.; Tsatsanis, A.; Lim, L.Q.; Adlard, P.A.; Bush, A.I.; Duce, J.A. β-Amyloid precursor protein does not possess ferroxidase activity but does stabilize the cell surface ferrous iron exporter ferroportin. PLoS ONE 2014, 9, e114174.
- Tao, Y.; Wang, Y.; Rogers, J.T.; Wang, F. Perturbed iron distribution in Alzheimer’s disease serum, cerebrospinal fluid, and selected brain regions: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2014, 42, 679–690.
- Shams, M.; Martola, J.; Charidimou, A.; Granberg, T.; Ferreira, D.; Westman, E.; Wintermark, M.; Iv, M.; Larvie, M.; Kristoffersen Wiberg, M.; et al. Cerebrospinal Fluid Metals and the Association with Cerebral Small Vessel Disease. J. Alzheimer’s Dis. 2020, 78, 1229–1236.
- Strozyk, D.; Launer, L.J.; Adlard, P.A.; Cherny, R.A.; Tsatsanis, A.; Volitakis, I.; Blennow, K.; Petrovitch, H.; White, L.R.; Bush, A.I. Zinc and copper modulate Alzheimer Abeta levels in human cerebrospinal fluid. Neurobiol. Aging 2009, 30, 1069–1077.
- Frederickson, C.J. Neurobiology of Zinc and Zinc-Containing Neurons. Int. Rev. Neurobiol. 1989, 31, 145–238.
- Takeda, A.; Takada, S.; Ando, M.; Itagaki, K.; Tamano, H.; Suzuki, M.; Iwaki, H.; Oku, N. Impairment of recognition memory and hippocampal long-term potentiation after acute exposure to clioquinol. Neuroscience 2010, 171, 443–450.
- Bush, A.I.; Pettingell, W.H.; de Paradis, M.; Tanzi, R.E.; Wasco, W. The amyloid beta-protein precursor and its mammalian homologues. Evidence for a zinc-modulated heparin-binding superfamily. J. Biol. Chem. 1994, 269, 26618–26621.
- Mo, Z.-Y.; Zhu, Y.-Z.; Zhu, H.-L.; Fan, J.-B.; Chen, J.; Liang, Y. Low micromolar zinc accelerates the fibrillization of human tau via bridging of Cys-291 and Cys-322. J. Biol. Chem. 2009, 284, 34648–34657.
- An, W.-L.; Bjorkdahl, C.; Liu, R.; Cowburn, R.F.; Winblad, B.; Pei, J.-J. Mechanism of zinc-induced phosphorylation of p70 S6 kinase and glycogen synthase kinase 3beta in SH-SY5Y neuroblastoma cells. J. Neurochem. 2005, 92, 1104–1115.
- Pei, J.-J.; An, W.-L.; Zhou, X.-W.; Nishimura, T.; Norberg, J.; Benedikz, E.; Götz, J.; Winblad, B. P70 S6 kinase mediates tau phosphorylation and synthesis. FEBS Lett. 2006, 580, 107–114.
- Ventriglia, M.; Brewer, G.J.; Simonelli, I.; Mariani, S.; Siotto, M.; Bucossi, S.; Squitti, R. Zinc in Alzheimer’s Disease: A Meta-Analysis of Serum, Plasma, and Cerebrospinal Fluid Studies. J. Alzheimer’s Dis. 2015, 46, 75–87.
- de Wilde, M.C.; Vellas, B.; Girault, E.; Yavuz, A.C.; Sijben, J.W. Lower brain and blood nutrient status in Alzheimer’s disease: Results from meta-analyses. Alzheimer’s Dement. 2017, 3, 416–431.
- Meng, L.; Wang, Z.; Ming, Y.C.; Shen, L.; Ji, H.F. Are micronutrient levels and supplements causally associated with the risk of Alzheimer’s disease? A two-sample Mendelian randomization analysis. Food Funct. 2022, 13, 6665–6673.
- Wang, Z.; Meng, L.; Shen, L.; Ji, H.F. Impact of modifiable risk factors on Alzheimer’s disease: A two-sample Mendelian randomization study. Neurobiol. Aging 2020, 91, 167.e11–167.e19.
- Cheng, W.W.; Zhu, Q.; Zhang, H.Y. Mineral Nutrition and the Risk of Chronic Diseases: A Mendelian Randomization Study. Nutrients 2019, 11, 378.
- Corona, C.; Masciopinto, F.; Silvestri, E.; Del Viscovo, A.; Lattanzio, R.; La Sorda, R.; Ciavardelli, D.; Goglia, F.; Piantelli, M.; Canzoniero, L.M.T.; et al. Dietary zinc supplementation of 3xTg-AD mice increases BDNF levels and prevents cognitive deficits as well as mitochondrial dysfunction. Cell Death Dis. 2010, 1, e91.
- Brewer, G.J. Copper excess, zinc deficiency, and cognition loss in Alzheimer’s disease. Biofactors 2012, 38, 107–113.
- Brewer, G.J. Alzheimer’s disease causation by copper toxicity and treatment with zinc. Front. Aging Neurosci. 2014, 6, 92.
- Loef, M.; von Stillfried, N.; Walach, H. Zinc diet and Alzheimer’s disease: A systematic review. Nutr. Neurosci. 2012, 15, 2–12.
- Craven, K.M.; Kochen, W.R.; Hernandez, C.M.; Flinn, J.M. Zinc Exacerbates Tau Pathology in a Tau Mouse Model. J. Alzheimers. Dis. 2018, 64, 617–630.
- Wang, C.Y.; Wang, T.; Zheng, W.; Zhao, B.L.; Danscher, G.; Chen, Y.H.; Wang, Z.Y. Zinc overload enhances APP cleavage and Aβ deposition in the Alzheimer mouse brain. PLoS ONE 2010, 5, e15349.
- Kodama, H.; Murata, Y.; Kobayashi, M. Clinical manifestations and treatment of Menkes disease and its variants. Pediatr. Int. 1999, 41, 423–429.
- Merle, U.; Schaefer, M.; Ferenci, P.; Stremmel, W. Clinical presentation, diagnosis and long-term outcome of Wilson’s disease: A cohort study. Gut 2007, 56, 115–120.
- Žigman, T.; Petković Ramadža, D.; Šimić, G.; Barić, I. Inborn Errors of Metabolism Associated with Autism Spectrum Disorders: Approaches to Intervention. Front. Neurosci. 2021, 15, 624.
- You, H.; Tsutsui, S.; Hameed, S.; Kannanayakal, T.J.; Chen, L.; Xia, P.; Engbers, J.D.T.; Lipton, S.A.; Stys, P.K.; Zamponi, G.W. Aβ neurotoxicity depends on interactions between copper ions, prion protein, and N-methyl-D-aspartate receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 1737–1742.
- Lovell, M.A.; Robertson, J.D.; Teesdale, W.J.; Campbell, J.L.; Markesbery, W.R. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998, 158, 47–52.
- Ayton, S.; Lei, P.; Bush, A.I. Metallostasis in Alzheimer’s disease. Free Radic. Biol. Med. 2013, 62, 76–89.
- Miyata, M.; Smith, J.D. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and β-amyloid peptides. Nat. Genet. 1996, 14, 55–61.
- Moir, R.D.; Atwood, C.S.; Romano, D.M.; Laurans, M.H.; Huang, X.; Bush, A.I.; Smith, J.D.; Tanzi, R.E. Differential effects of apolipoprotein E isoforms on metal-induced aggregation of A beta using physiological concentrations. Biochemistry 1999, 38, 4595–4603.
- Squitti, R.; Ventriglia, M.; Simonelli, I.; Bonvicini, C.; Costa, A.; Perini, G.; Binetti, G.; Benussi, L.; Ghidoni, R.; Koch, G.; et al. Copper Imbalance in Alzheimer’s Disease: Meta-Analysis of Serum, Plasma, and Brain Specimens, and Replication Study Evaluating ATP7B Gene Variants. Biomolecules 2021, 11, 960.
- Wang, Z.-X.; Tan, L.; Wang, H.-F.; Ma, J.; Liu, J.; Tan, M.-S.; Sun, J.-H.; Zhu, X.-C.; Jiang, T.; Yu, J.-T. Serum Iron, Zinc, and Copper Levels in Patients with Alzheimer’s Disease: A Replication Study and Meta-Analyses. J. Alzheimer’s Dis. 2015, 47, 565–581.
- Bucossi, S.; Ventriglia, M.; Panetta, V.; Salustri, C.; Pasqualetti, P.; Mariani, S.; Siotto, M.; Rossini, P.M.; Squitti, R. Copper in Alzheimer’s disease: A meta-analysis of serum, plasma, and cerebrospinal fluid studies. J. Alzheimer’s Dis. 2011, 24, 175–185.
- Kawamoto, E.M.; Vivar, C.; Camandola, S. Physiology and Pathology of Calcium Signaling in the Brain. Front. Pharmacol. 2012, 3, 61.
- Woods, N.K.; Padmanabhan, J. Neuronal Calcium Signaling and Alzheimer’s Disease. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1193–1217.
- Olney, J.W. Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 1969, 164, 719–721.
- Sattler, R.; Tymianski, M. Molecular Mechanisms of Glutamate Receptor-Mediated Excitotoxic Neuronal Cell Death. Mol. Neurobiol. 2001, 24, 107–130.
- Tong, B.C.K.; Wu, A.J.; Li, M.; Cheung, K.H. Calcium signaling in Alzheimer’s disease & therapies. Biochim. Biophys. Acta -Mol. Cell Res. 2018, 1865, 1745–1760.
- Sato, K.; Mano, T.; Ihara, R.; Suzuki, K.; Tomita, N.; Arai, H.; Ishii, K.; Senda, M.; Ito, K.; Ikeuchi, T.; et al. Lower Serum Calcium as a Potentially Associated Factor for Conversion of Mild Cognitive Impairment to Early Alzheimer’s Disease in the Japanese Alzheimer’s Disease Neuroimaging Initiative. J. Alzheimer’s Dis. 2019, 68, 777–788.
- Shi, Y.; Liu, R.; Guo, Y.; Li, Q.; Zhou, H.; Yu, S.; Liang, H.; Li, Z. An Updated Mendelian Randomization Analysis of the Association Between Serum Calcium Levels and the Risk of Alzheimer’s Disease. Front. Genet. 2021, 12, 1602.
- Ma, L.Z.; Wang, Z.X.; Wang, Z.T.; Hou, X.H.; Shen, X.N.; Ou, Y.N.; Dong, Q.; Tan, L.; Yu, J.T. Serum Calcium Predicts Cognitive Decline and Clinical Progression of Alzheimer’s Disease. Neurotox. Res. 2021, 39, 609–617.
- Kern, J.; Kern, S.; Blennow, K.; Zetterberg, H.; Waern, M.; Guo, X.; Börjesson-Hanson, A.; Skoog, I.; Östling, S. Calcium supplementation and risk of dementia in women with cerebrovascular disease. Neurology 2016, 87, 1674–1680.
- He, Y.; Zhang, H.; Wang, T.; Han, Z.; Ni, Q.B.; Wang, K.; Wang, L.; Zhang, Y.; Hu, Y.; Jin, S.; et al. Impact of Serum Calcium Levels on Alzheimer’s Disease: A Mendelian Randomization Study. J. Alzheimer’s Dis. 2020, 76, 713–724.
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxid. Med. Cell. Longev. 2018, 2018, 7580707.
- Mohammed, R.S.; Ibrahim, W.; Sabry, D.; El-Jaafary, S.I. Occupational metals exposure and cognitive performance among foundry workers using tau protein as a biomarker. Neurotoxicology 2020, 76, 10–16.
- Rolle-McFarland, D.; Liu, Y.; Mostafaei, F.; Zauber, S.E.; Zhou, Y.; Li, Y.; Fan, Q.; Zheng, W.; Nie, L.H.; Wells, E.M. The association of bone, fingernail and blood manganese with cognitive and olfactory function in Chinese workers. Sci. Total Environ. 2019, 666, 1003–1010.
- Barahona, A.J.; Bursac, Z.; Veledar, E.; Lucchini, R.; Tieu, K.; Richardson, J.R. Relationship of Blood and Urinary Manganese Levels with Cognitive Function in Elderly Individuals in the United States by Race/Ethnicity, NHANES 2011-2014. Toxics 2022, 10, 191.
- Balmuș, I.M.; Strungaru, S.A.; Ciobica, A.; Nicoara, M.N.; Dobrin, R.; Plavan, G.; Ștefănescu, C. Preliminary Data on the Interaction between Some Biometals and Oxidative Stress Status in Mild Cognitive Impairment and Alzheimer’s Disease Patients. Oxid. Med. Cell. Longev. 2017, 2017, 7156928.
- Du, K.; Zheng, X.; Ma, Z.T.; Lv, J.Y.; Jiang, W.J.; Liu, M.Y. Association of Circulating Magnesium Levels in Patients with Alzheimer’s Disease From 1991 to 2021: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2022, 13, 799824.
- Bardgett, M.E.; Schultheis, P.J.; McGill, D.L.; Richmond, R.E.; Wagge, J.R. Magnesium deficiency impairs fear conditioning in mice. Brain Res. 2005, 1038, 100–106.
- Cherbuin, N.; Kumar, R.; Sachdev, P.S.; Anstey, K.J. Dietary Mineral Intake and Risk of Mild Cognitive Impairment: The PATH through Life Project. Front. Aging Neurosci. 2014, 6, 4.
- Glick, J. Use of magnesium in the management of dementias. Med. Sci. Res. 1990, 18, 831–833.
- Ozturk, S.; Cillier, A.E. Magnesium supplementation in the treatment of dementia patients. Med. Hypotheses 2006, 67, 1223–1225.
- Andrási, E.; Igaz, S.; Molnár, Z.; Makó, S. Disturbances of magnesium concentrations in various brain areas in Alzheimer’s disease. Magnes. Res. 2000, 13, 189–196.
- Vural, H.; Demirin, H.; Kara, Y.; Eren, I.; Delibas, N. Alterations of plasma magnesium, copper, zinc, iron and selenium concentrations and some related erythrocyte antioxidant enzyme activities in patients with Alzheimer’s disease. J. Trace Elem. Med. Biol. 2010, 24, 169–173.
- Cilliers, K. Trace element alterations in Alzheimer’s disease: A review. Clin. Anat. 2021, 34, 766–773.
- Yu, J.; Sun, M.; Chen, Z.; Lu, J.; Liu, Y.; Zhou, L.; Xu, X.; Fan, D.; Chui, D. Magnesium modulates amyloid-beta protein precursor trafficking and processing. J. Alzheimer’s Dis. 2010, 20, 1091–1106.
- Xu, Z.-P.; Li, L.; Bao, J.; Wang, Z.-H.; Zeng, J.; Liu, E.-J.; Li, X.-G.; Huang, R.-X.; Gao, D.; Li, M.-Z.; et al. Magnesium Protects Cognitive Functions and Synaptic Plasticity in Streptozotocin-Induced Sporadic Alzheimer’s Model. PLoS ONE 2014, 9, e108645.
- Thomassen, J.Q.; Tolstrup, J.S.; Nordestgaard, B.G.; Tybjærg-Hansen, A.; Frikke-Schmidt, R. Plasma Concentrations of Magnesium and Risk of Dementia: A General Population Study of 102 648 Individuals. Clin. Chem. 2021, 67, 899–911.
- Kieboom, B.C.T.; Licher, S.; Wolters, F.J.; Ikram, M.K.; Hoorn, E.J.; Zietse, R.; Stricker, B.H.; Ikram, M.A. Serum magnesium is associated with the risk of dementia. Neurology 2017, 89, 1716–1722.
- Souza, L.A.C.; Trebak, F.; Kumar, V.; Satou, R.; Kehoe, P.G.; Yang, W.; Wharton, W.; Earley, Y.F. Elevated cerebrospinal fluid sodium in hypertensive human subjects with a family history of Alzheimer’s disease. Physiol. Genom. 2020, 52, 133–142.
- Faraco, G.; Hochrainer, K.; Segarra, S.G.; Schaeffer, S.; Santisteban, M.M.; Menon, A.; Jiang, H.; Holtzman, D.M.; Anrather, J.; Iadecola, C. Dietary salt promotes cognitive impairment through tau phosphorylation. Nature 2019, 574, 686–690.
- Mohamed, S.A.; Herrmann, K.; Adlung, A.; Paschke, N.; Hausner, L.; Frölich, L.; Schad, L.; Groden, C.; Ulrich, H.K. Evaluation of Sodium (23 Na) MR-imaging as a Biomarker and Predictor for Neurodegenerative Changes in Patients with Alzheimer’s Disease. In Vivo 2021, 35, 429–435.
- Vintimilla, R.M.; Large, S.E.; Gamboa, A.; Rohlfing, G.D.; O’Jile, J.R.; Hall, J.R.; O’Bryant, S.E.; Johnson, L.A. The Link between Potassium and Mild Cognitive Impairment in Mexican-Americans. Dement. Geriatr. Cogn. Dis. Extra 2018, 8, 151–157.
- Mielke, M.M.; Zandi, P.P.; Blennow, K.; Gustafson, D.; Sjögren, M.; Rosengren, L.; Skoog, I. Low serum potassium in mid life associated with decreased cerebrospinal fluid Abeta42 in late life. Alzheimer Dis. Assoc. Disord. 2006, 20, 30–36.
- Zheng, F.; Li, Y.; Zhang, F.; Sun, Y.; Zheng, C.; Luo, Z.; Wang, Y.L.; Aschner, M.; Zheng, H.; Lin, L.; et al. Cobalt induces neurodegenerative damages through Pin1 inactivation in mice and human neuroglioma cells. J. Hazard. Mater. 2021, 419, 126378.
More
