Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Carla Wunderle and Version 2 by Lindsay Dong.
Inflammation has been identified as a key driver for disease-related malnutrition, leading to anorexia, reduced food intake, muscle catabolism, and insulin resistance, which are stimulating a catabolic state. Interesting data suggest that inflammation also modulates the response to nutritional treatment. Patients with high inflammation show no response to nutritional interventions, while patients with lower levels of inflammation do. This may explain the contradictory results of nutritional trials to date and the lack of effect in more severly ill patients.
- malnutrition
- inflammation
- nutrition
- biomarker
- treatment response
1. Introduction
Disease-related malnutrition (DRM) is a common syndrome in patients with acute and chronic illnesses. Prevalence rates are approximately 30% among medical inpatients and rise higher among the elderly or critically ill [1][2][1,2]. Left untreated, DRM is associated with poor outcomes, such as higher mortality and prolonged intensive care unit (ICU) and hospital stays [3][4][3,4]. Inflammation, undernutrition-driven catabolism, and inadequate dietary intake are key drivers of DRM [1]. While medical inpatients with malnutrition have been shown to benefit from nutritional treatment, this may not be equally true for other patient populations, such as those in the ICU [1][5][1,5].
The focus on inflammation as a key driver of DRM has grown due to the growing understanding of DRM and its pathophysiology. Recent studies have shown associations between inflammatory processes measured by inflammation biomarkers, such as C-reactive protein (CRP), and responses to nutritional therapy [6][7].
2. Malnutrition and Inflammation
2.1. Malnutrition—Risk Factors and Diagnosis
DRM is multifactorial: risk factors include polypharmacy, disease-related inflammatory mechanisms, compromised intake or assimilation of nutrients, immobility associated muscle wasting, older age, and social isolation [1][7][1,10]. In addition to an already high prevalence of malnutrition upon admission, nutritional states may be further aggravated during hospitalization due to illness-related loss of appetite, fasting for diagnostic tests, drug-induced side effects, diseases that affect gastrointestinal function, or other factors associated with hospitalization [1]. To diagnose malnutrition, the Global Leadership Initiative on Malnutrition (GLIM) proposes a two-step approach consisting of nutritional risk screening followed by a more thorough evaluation. There is no one universal screening method for malnutrition but rather a number of different tools which have been validated for different settings, including the NRS-2002 [8][11], SGA [9][12], MUST [10][13] or MNA-SF [11][14]. If nutritional risk is identified, a nutritional assessment to confirm a diagnosis should be performed, including etiological (reduced food intake or assimilation and disease burden/inflammation) and phenotypic (non-volitional weight loss, low BMI and reduced muscle mass) criterions. According to GLIM, a diagnosis of malnutrition is fulfilled if one etiological and one phenotypic criterion apply for the patient [12][15].2.2. Malnutrition—Classification
The European Society of Clinical Nutrition and Metabolism (ESPEN)PEN proposes three etiological groups: DRM with inflammation, DRM without inflammation, and malnutrition/undernutrition without disease (Figure 1a). DRM with inflammation can be divided further into acute and chronic forms. Chronically malnourished patient groups typically affected by inflammation (and thus cachexia) include patients with cancer, chronic obstructive pulmonary disease (COPD), inflammatory bowel diseases, congestive heart failure, chronic kidney disease, and other end-stage organ diseases. Inflammation in these patients is often milder, with CRP levels of up to 40 mg/l. DRM with inflammation due to acute disease or injury typically affects critically ill patients or post major surgery, and is accompanied by higher levels of CRP [13][8]. The American Society for Parenteral and Enteral Nutrition (ASPEN) uses a similar approach, categorizing according to (I) social and environmental circumstances, (II) chronic illness and (III) acute illness or injury (Figure 1b) [14][16]. The distinction between inflammatory and noninflammatory forms of malnutrition reflects their different phenotypes.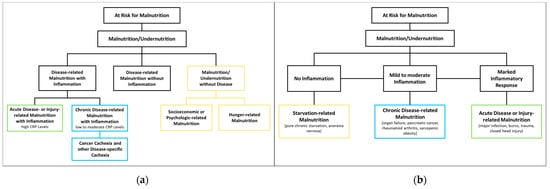
Figure 1. Classification of Malnutrition by (a) European Society of Clinical Nutrition and Metabolism (ESPEN) [13][8] and (b) American Society for Parenteral and Enteral Nutrition (ASPEN) [14][16].
2.3. Malnutrition—Therapy and Clinical Outcomes
Malnutrition has been shown to be a risk factor for adverse outcomes such as increased mortality, a higher risk of readmission within 30 days, prolonged hospital and ICU stays, loss of function, and infection rates [2][3][4][2,3,4]. While the possible benefits of nutritional therapy in malnourished patients have long been unclear [15][16][6,17], recent evidence in favor of applying nutritional therapy in medical inpatient settings has been growing, in part due to large-scale RCTs including the EFFORT [17][18] and the NOURISH trials [18][19].
2.4. Inflammation in Malnutrition
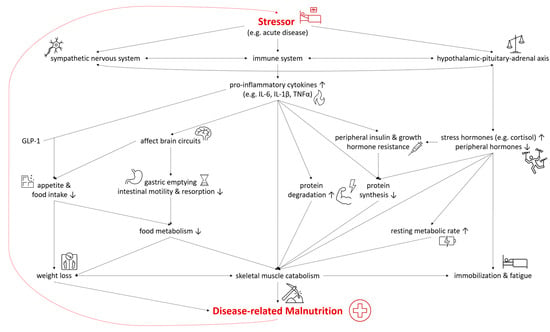
In both DRM with acute and chronic inflammation, the sympathetic nervous system, the immune system, and the hypothalamic–pituitary–adrenal axis are activated as a systemic response to a stressor and disease [19][20][22,23]. As they are connected both anatomically and functionally, they interact in the response to the stressor [21][24]. The modulation of the hypothalamic–pituitary–adrenal axis stimulates the release of stress hormones, including cortisol, catecholamines, and suppresses other hormones regulating sex, thyroid, and other peripheral functions [19][22]. In malnutrition, the deiodination of thyroxine (T4) to triiodothyronine (T3) was shown to be down regulated—a process called “low T3 syndrome” which is an adaptive metabolic mechanism to reduce energy expenditure and prevent catabolism [22][25]. Catecholamines and cortisol increase glycogenolysis and gluconeogenesis in the liver while simultaneously inducing peripheral insulin resistance and inhibiting glucose from entering cells [19][22]. Furthermore, insulin-dependent glucose transporters in peripheral tissues are downregulated, causing stress hyperglycemia [23][26]. Pro-inflammatory cytokines including interleukin 6 (IL-6), interleukin 1β (IL-1β) and tumor necrosis factor α (TNF-α) are released, triggering several mechanisms which contribute to the pathogenesis of malnutrition (Figure 2). Pro-inflammatory cytokines also affect brain circuits which control food intake, cause delayed gastric emptying and increase skeletal muscle catabolism [1][20][24][25][1,23,27,28]. Furthermore, researchers have identified an interaction of pro-inflammatory cytokines (mainly IL-6 and IL-1β) and gut tissue-released glucagon-like peptide-1 (GLP-1), resulting in reduced food intake and unintentional weight loss [26][29]. Muscle degradation is triggered by decreased synthesis of muscle protein and the increased degradation of proteins such as myosin heavy chains [27][9]. These endocrine changes further advance catabolism and lead to fatigue and immobilization [1][20][1,23]. The combination of these mechanisms leads to compromised food metabolism, a hypercatabolic state, and eventually to DRM.
Figure 2. Selected Pathways in the Interplay of Inflammation and the Pathophysiology of Disease-related Malnutrition. IL-6, interleukin 6; IL-1β, interleukin 1β; TNF-α, tumor necrosis factor α; GLP-1, glucagon-like Peptide-1.
3. Is Nutrition a Friend? How Nutrition influences Inflammation
3.1. Anti-Inflammatory Potential of Nutrients and Other Food Components
3.1.1. Omega-3 and Omega-6 Fatty Acids
Polyunsaturated fatty acids (PUFA), especially omega-3 (n-3 FA) and omega-6 fatty acids (n-6 FA), are amongst the most studied macronutrients in this context. As the human body is not able to synthesize these essential elements, they must be ingested from an outside source. Long-chain n-3 FA (LC n-3 FA) such as eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), or docosahexaenoic acid (DHA) are found in aquatic organisms or can be metabolized from plant-derived α-linolenic acid (ALA) [28][36]. While n-6 FA linolenic acid (LA) is commonly found in vegetable oils such as sunflower oil, long-chain n-6 FA (LC n-6 FA), arachidonic acid (AA) is found in animal products such as meat or egg or is biosynthesized from LA. LC n-3 FA and LC n-6 FA are then used as substrates for mediators such as prostaglandins, thromboxanes, and leukotrienes. While these n-6 FA products are considered pro-inflammatory, products within the n-3 FA pathway are considered anti-inflammatory. Due to the role of the same enzymes in both pathways, n-3 FA possesses the potential to competitively reduce the metabolism of n-6 FA to pro-inflammatory mediators. However, results on the pro-inflammatory effect of n-6 FA are conflicting, as some studies did not find a significant association with inflammation biomarkers or even reported anti-inflammatory effects [29][30][37,38]. This is also reflected in the literature-based DII (described in detail below) which calculated an anti-inflammatory potential for n-6 FA [31][39]. The effects of n-3 and n-6 FA have been extensively studied in relation to cardiovascular diseases [32][40], as well as other chronic illnesses associated with inflammation such as rheumatoid arthritis [33][41] or cancer cachexia [34][35][42,43].
