Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Jakub Jarmula and Version 3 by Catherine Yang.
Skull base surgery has undergone significant progress following key technological developments. From early candle-lit devices to the modern endoscope, refinements in visualization techniques have made endoscopic skull base surgery (ESBS) a standard practice for treating a variety of conditions. The endoscope has also been integrated with other technologies to enhance visualization, including fluorescence agents, intraoperative neuronavigation with augmented reality, and the exoscope.
- skull base surgery
- neuro-oncology
- neuronavigation
- neuroendoscopy
- ultrasonography
1. Illuminance and Brightness
Endoscopic image quality depends on specific image characteristics, including illuminance, brightness, resolution, and dimensionality. Illuminance is the amount of light distributed across a surface area [1][16]. Proper illuminance of anatomic structures is critical for ESBS. A variety of light sources have been developed with different emission spectra. Currently, most endoscopes use xenon light sources that produce excellent illuminance with their high content of blue wavelength light to provide accurate color representation of tissues. However, these light sources carry the risk of thermal tissue damage, especially with high-watt lightbulbs. Light-emitting diode (LED) light sources have also been developed. These instruments carry a lower risk of thermal tissue injury while providing adequate light quality for ESBS [2][17] (Figure 12).
Projecting sufficient light towards the structure of interest (i.e., illuminance) is equally as important as capturing enough returning light to form a recognizable image (i.e., brightness). Brightness is the amount of illuminance perceived by an observer [1][16]. In ESBS, brightness relates to the amount of light radiating from the visualized structures towards the endoscope light sensor, which is then transmitted to the monitor and seen by the surgeon. Brightness is directly proportional to illuminance and inversely proportional to distance squared. Appropriate brightness can be ensured by increasing the endoscope light source illuminance or decreasing the distance between the structure of interest and the endoscope. However, maximal brightness cannot always be ensured. For example, decreasing illuminance or increasing working distance to reduce thermal tissue injury can limit image brightness.
2. Resolution
In addition to brightness, resolution is important for generating precise images during surgery. Currently, endoscopes are available in high definition (HD) (1280 × 720 pixels), full HD (1920 × 1080 pixels), and ultra-HD/4K resolution (3840 × 2160 pixels) with the aim of enhancing the visualization of anatomic structures [4][19]. Additional resolution could be valuable when evaluating detailed anatomy, such as the tumor-tissue interface or intradural features. In response, features such as digital zoom enhance visibility at a fixed distance while maintaining adequate resolution. Digital zoom can improve instrument maneuverability in small spaces, such as the nasal cavity, by allowing enhanced visibility at a longer distance so as other surgical instruments do not collide with the endoscope rod. However, unlike optical zoom, digital zoom reduces the field of view.
It is important to appreciate the effects of viewing distance and screen size on perceived resolution by the surgeon. The recommended viewing distance between the surgeon and monitor ranges between 80–120 cm [5][20]. However, a typical viewing distance can range from 99–152 cm. With a standard 76.2-cm monitor, any improved resolution from an ultra-HD system can be perceived only when standing less than 122 cm away. With a standard viewing distance of 152 cm, any improved resolution from an ultra-HD system can only be perceived with a 101.6-cm or greater size monitor. Therefore, a larger viewing monitor is needed at standard working distances in order to perceive the advantages of a higher resolution system. However, larger monitors require additional physical space in the operating room, and high-resolution systems generate larger image and video files that result in a longer time to transfer these files to the medical chart or data storage device.
Beyond projecting images onto a two-dimensional monitor, certain endoscope systems have been developed to provide three-dimensional (3D) endoscopy [6][22]. Stereoscopic images generated by 3D endoscopy provide depth perception that facilitates surgery and is familiar to those accustomed to working stereoscopically with operating microscopes; however, certain users experience nausea, headache, or visual fatigue, limiting its adoption. Three-dimensional endoscopy presents a learning curve for users because of its differences from traditional endoscopy. In addition, the limitations of 2D images in traditional endoscopy can be overcome by the depth of field gained by seeing dynamic camera motion and by proprioceptive information gained from instruments moving in and out of the surgical corridor. Three-dimensional endoscopy remains a modern technology that requires more investigation to inform optimized settings for safe, effective ESBS.
3. Neuronavigation
Neuronavigation utilizes preoperative images and sensors to relate intraoperative coordinates to locations on imaging. Current systems can use optical image guidance, consisting of infrared light-detecting cameras and a reference array attached to the head holder, or electromagnetic tracking, which measures the electromagnetic field generated by a magnetic reference array typically placed on the patient’s forehead [7][23]. Neuronavigation systems undergo calibration and reference these markers to generate images with overlayed location coordinates. Surface contour matching requires identifying points on the patient’s surface with a designated probe to map the patient’s surface features. The computer-generated reconstruction of the patient is then referenced to map the current location of calibrated instruments in relation to preoperative imaging [8][24]. These systems utilize computer modeling to aid the surgeon pre- and intra- operatively.
Outside of the operating room, adaptations of these systems into virtual reality platforms have been incorporation into neurosurgical training [9][26]. For ESBS, these platforms include the Dextroscope (Volume Interactions, Bracco Group, Milan, Italy) and NeuroTouch Endo (National Research Council of Canada, Ottawa, ON, Canada). These virtual reality platforms provide trainees with haptic feedback during part-task training and scenario simulations to promote operative skill development [10][27]. The advantages of virtual reality-based training platforms extend to their potential use in tracking trainee progress to provide feedback on trainee development and training program curricula.
Skull base anatomy presents many challenges, including anatomical variants that can affect surgical orientation. Augmented reality presents surgeons with an additional means of enhancing intraoperative orientation and preventing neurovascular injury [11][29]. Although current systems are focused on incorporating preoperative imaging, the use of intraoperative imaging can provide augmented reality with updated anatomical information in real-time [12][30].
Several augmented reality training models have been specifically developed for ESBS [13][31]. These training models allow trainees to gain exposure to a wide range of skull base pathologies before experiencing surgery with real-life patients. Synthetic tissue models, such as the UpSurgeOn system (UpSurgeOn SRL, Assago, Italy), incorporate augmented reality to provide anatomical exploration that is not otherwise possible with the unaided eye [14][32]. However, this technology is still in its infancy and requires thorough investigation. Advancements in augmented reality, and its growing integration into both neurosurgical training and practice, highlight the increasing importance of these neuronavigation-based technologies.
In ESBS, newer neuronavigation-compatible instruments have increased the utility of these systems. The electronics required for neuronavigation are incorporated in the instrument, which provides greater ergonomics and maneuverability for the surgeon compared to traditional instruments requiring external adaptors. Neuronavigation-compatible instruments also simplify the registration process. For example, malleable suction instruments can be bent during surgery without requiring re-registration. Other available instruments include drills and microdebriders.
However, neuronavigation has notable limitations. Optical image guidance is dependent upon camera line-of-sight and requires the patient to be firmly secured during surgery. Magnetic-based systems are prone to reduced accuracy from interference by external electromagnetic radiation or ferromagnetic instruments. Registration occurring on the face of the patient, as is typically carried out in ESBS, results in greater error at increased depths during surgery [7][23]. These imprecisions can be further exacerbated by brain shift. Re-registration at the site of interest is not without risk, with the potential for prolonged operative time and increased risk of complications, such as cerebrospinal fluid leak and impaired wound healing [15][33].
4. Robotics
Robotics have had limited use in skull base surgery relative to other surgical fields due to the anatomical constraints of the skull base and its narrow operative corridors. Developed primarily for general surgery, the larger footprint of robotic systems in the operating room, and the larger size of their instruments, has limited their endonasal use. Nevertheless, systems such as the da Vinci robot (Intuitive Surgical Inc., Sunnyvale, CA, USA), have been investigated in skull base surgery [16][17][34,35]. The incorporation of robotics has the potential to address the limitations of endoscopy—2D monocular vision, often requiring two co-surgeons—by providing 3D HD vision, requiring one surgeon, reducing the effects of physiological tremor, and increasing dexterity [18][36]. Robotic systems in conjunction with ESBS have been demonstrated to be feasible [19][37]. They continue to be investigated for improving access to skull base pathologies through combined approaches, such as the transoral (robotic)-transsphenoidal (endoscopic endonasal) approach.
Robotic endoscope holders are also available, which can be adjusted either directly or with a joystick. New systems continue to be investigated preclinically and demonstrate significant progress over earlier models [20][38]. Notably, these systems offer articulation of the endoscope and improved ergonomics [21][39]. They can also reduce surgeon fatigue, improve concentration on tissue manipulation, and increase image stability without requiring a second surgeon [22][40]. The advantages of robotic systems have the potential to further improve the practice of ESBS and its outcomes for patients.
However, robotics in ESBS remain a relatively new development due to their large size relative to the skull base, lack of haptic feedback, and lack of integration with other visualization systems. These systems may also disrupt the surgical collaboration inherent to four-handed, two-surgeon approaches. Currently, the published literature is limited to preclinical studies. Increased research on the utility of these promising systems is required, especially in the clinical context [23][41].
5. Intraoperative Imaging
5.1. Real-Time Fluorescence Agents
Fluorescence agents have been used in ESBS to aid in the visualization of blood vessels and tumors [24][42]. These agents work through a mechanism involving electron excitation through the absorption of higher-energy light and the subsequent emission of lower-energy light as the electron returns to its ground state. Fluorescence agents can demarcate tumors from healthy tissue to aid in greater tumor resection [25][43].
Indocyanine green (ICG) is a fluorophore that binds to plasma proteins in the intravascular space and has a very favorable safety profile [26][44]. Being contained within the intravascular space, ICG has been used to visualize and avoid damage to blood vessels during surgery. ICG has been also studied in a variety of tumors, most notably pituitary adenoma. Fluorescence intensity measured within one minute of ICG infusion can differentiate healthy tissue compared to the pituitary adenoma. Other techniques have also been developed, such as second-window ICG, where high-dose ICG infusion 24 h before surgery can concentrate fluorescence within the pituitary adenoma [27][45].
5-aminolevulinic acid (5-ALA) is a fluorophore acting through the porphyrin synthesis pathway that is approved for intraoperative visualization of high-grade glioma [28][46]. 5-ALA has been studied in ESBS for multiple conditions, though results suggest limited utility in specific tumor types [29][47]. This agent can enhance the detection of tumor tissue otherwise difficult to visualize due to its location near the optic canal. Compared to a microscope, the proximity of an endoscope to the tissue can improve fluorescent signal detection for deeply located pathologies. Future knowledge about the properties and lesion-specific pharmacokinetics of 5-ALA can improve its use in ESBS [30][48].
On Target Laboratories (OTL)-38 is a fluorophore that targets folate receptor alpha [31][49]. This agent has been promising in detecting folate receptor-overexpressing tumors. In one prospective study, OTL-38 intraoperatively demonstrated high sensitivity and specificity in detecting non-functioning pituitary adenomas [32][50]. Evaluation of tumor resection margins revealed improved resection with OTL-38 compared to unaided visual inspection. However, one major limitation of OTL-38 is the lack of known folate receptor expression levels preoperatively, which may reduce sensitivity and increase the occurrence of false negative results [33][51]. These agents, and additional fluorophores such as sodium fluorescein, continue to be researched to improve visualization and patient outcomes in ESBS.
5.2. Ultrasonography
Ultrasonography is based on the reverse piezoelectric effect, where an electrical current causes vibration of a crystal lattice, producing high-frequency sound waves that are reflected to a transducer to recreate an image [34][52].
Ultrasonography has not yet gained wide adoption in ESBS. Ultrasound probes have been assessed in pituitary tumor resection, with limited ability to evaluate skull base anatomical structures [35][53]. The recent development of smaller-sized probes with improved resolution may increase the utility of this technique in ESBS. One retrospective study found increased extent of resection and fewer complications for patients who underwent ultrasound-guided pituitary adenoma resection compared to traditional surgery [36][54]. However, the published experience is limited, and prospective studies remain to be reported.
Color Doppler ultrasonography, which labels fluid velocity with color, can identify vascular structures in the skull base, such as the internal carotid artery. New improvements in probe portability and resolution have led to the color Doppler microvascular probe. Compared to traditional Doppler probes, color Doppler microvascular probes have shown greater promise in identifying key vascular structures during ESBS [37][55]. However, image resolution and accurate structure identification are limited. The development of ultrasound contrast agents may further improve ultrasonography in ESBS. Contrast-enhanced ultrasonography has been reported for a variety of skull base pathologies [38][56]. This technique resulted in successful visualization of lesion tissue and high- and low- flow blood vessels compared to traditional ultrasonography. However, these contrast agents have not been tested using an endoscopic approach.
5.3. Computed Tomography and Magnetic Resonance Imaging
Imaging modalities, such as computer tomography (CT) and magnetic resonance imaging (MRI), have enhanced the study and diagnosis of skull base conditions. CT uses X-rays and a mathematical process termed reconstruction to transform three-dimensional structures into two-dimensional cross-sectional images. MRI is based on nuclear magnetic resonance, primarily of hydrogen atoms, in an applied magnetic field that is detected and reconstructed into cross-sectional images without the use of ionizing radiation [39][58].
These imaging modalities have been used during surgery to assess skull base anatomy. Intraoperative CT and MRI have been used to evaluate residual tumor tissue, leading to improved extent of resection and progression-free survival [40][59]. Currently, intraoperative CT may be performed using mobile units, while intraoperative MRI requires designated imaging suites. One advantage of intraoperative imaging is the possibility of performing neuronavigation system re-registration during surgery, which is particularly useful in open cranial surgery where significant brain shift can occur. Re-registration provides updated imaging information to the surgeon and can identify intraoperative changes to anatomical structure [7][23]. However, intraoperative imaging has its limitations in ESBS. CT causes exposure to ionizing radiation, which limits its use in certain patient populations, including children and pregnant women [41][60]. MRI is time-consuming compared to other imaging modalities and can prolong surgery duration by up to 40 min [42][61].
6. Neuroanatomy
The evolution of ESBS has required revisiting known anatomical structures through the perspective of endoscopic endonasal approaches [43][62]. New endoscopic approaches have invited a fundamental reevaluation of skull base anatomy and established procedures. Improved anatomical knowledge has informed surgical techniques and made endoscopic approaches a first-line choice for certain conditions, such as craniopharyngiomas [44][63]. Detailed descriptions of the microsurgical anatomy of the skull base, and the common sites of its pathologies, have enabled surgeons to appropriately plan and perform ESBS.
One example of the reevaluation of the microsurgical neuroanatomy is the cavernous sinus (Figure 28). The division of the cavernous sinus and middle fossa into triangles has been traditionally used for transcranial open approaches, however, this technique is not adequate for endoscopic endonasal approaches. Recently, a 360-degree division of potential spaces of tumor extension was described to provide anatomical guidance for surgical approach selection [43][62].
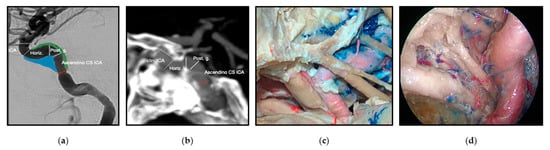
Figure 28. The cavernous sinus spaces and the cavernous segment of the internal carotid artery (ICA) from imaging, microsurgical transcranial and endoscopic endonasal perspectives. (a) Angiogram, lateral view of the ICA. (b) CT angiogram, lateral view of the ICA. The relationship of the ICA with the sphenoid bone is observed. (c) Cadaveric dissection demonstrating a superolateral view of the middle fossa and ICA via a transcranial approach. (d) Cadaveric dissection demonstrating an endoscopic endonasal view of the relationship of the cavernous ICA with the nerves on the lateral wall of the cavernous sinus. Reprinted/adapted with permission from Ref. [3][18]. 2022, Erion Junior de Andrade, M.D., M.Sc.
Detailed descriptions of the skull base are especially important for areas with complex anatomy and multiple available approaches. The anterior, middle, and posterior cranial fossa can be accessed from the front of the skull near the eye (transorbital), from the sides of the skull base (transpterygoid), from the back of the skull base (transcondylar), via the region near the pituitary gland (parasellar), and near where the spinal cord exits the cranium (clival and petroclival) [43][44][45][46][47][48][49][50][62,63,64,65,66,67,68,69]. Knowledge of neuroanatomy continues to grow with the development of expanded endoscopic approaches that encompass greater areas of the skull base [51][70]. The increasing application of endoscopic approaches in pediatric surgery has further refined the current understanding of skull base development [52][71]. The knowledge of surgical micro-neuroanatomy should be used in combination with available technology as the mainstay of any surgical procedure.
7. The Exoscope
In addition to the endoscope, work has been performed to develop the exoscope: a telescopic intraoperative visualization device with HD video resolution [53][72] (Figure 39, Video S1). Unlike the endoscope, the exoscope is positioned outside the body of the patient. It provides greater magnification, generates a wider focal distance, incorporates image enhancement, and provides 3D visualization. These features can improve anatomical visualization, increase surgeon comfort with better ergonomics, and facilitate teaching [54][73].
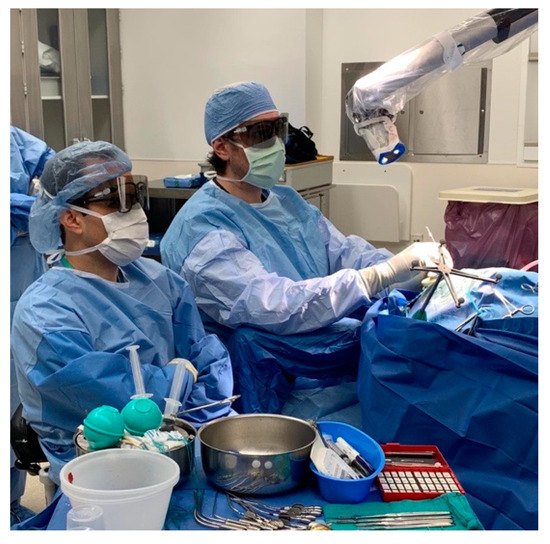
Figure 39. The surgical exoscope in use during a neuro-surgical procedure. Reprinted/adapted with permission from Ref. [55]. 2022, Pablo F. Recinos, M.D.
The surgical exoscope in use during a neuro-surgical procedure. Reprinted/adapted with permission from Ref. [12]. 2022, Pablo F. Recinos, M.D.
Exoscopy is a relatively new development in skull base surgery and further study is required to understand its strengths and limitations. Identified limitations include tissue differentiation, specifically bleeding tissue, the loss of stereoscopy, and the need for improved integration with existing technologies, such as fluorescence agents and endoscopy [56][74]. Endoscopes used with exoscopes have been reported to improve the visualization of operative blind spots. The exo-endoscopic approach can provide not only a wider field of view, but also better instrument positioning and improved viewing perspective for shared observation by operating room personnel [57][75]. Continued operative experience with the exo-endoscopic approach can further advance the complementary use of these technologies in ESBS [58][76].
