Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Dean Liu and Version 2 by Dean Liu.
CurrenItly is needed founded Fenton catalysts with higher reaction rates with catalitic activity similar to the conventional catalysts. The spinel ferrites have attracted increasing interest in recent years due to their low cost, excellent catalytic activity, and magnetic properties that allow a facile separation and reutilization, in addition the presence of two differents metals in an same structure can result in a better catalitic activity. Undwer the same idea the perovskites also result interesting for its use as Fenton catalysts due to the sinergic effect betwen the metals of its structure.
- Fenton
- electro-Fenton
- ORR
- bifunctional catalysts
1. Introduction
There are different methods of synthesis within which the following stand out:
-
Chemical coprecipitation
Chemical coprecipitation is one of the most promising methods thanks to its ease of realization, as well as the possibility of mass production. It is mainly based on the co- precipitation of materials by a pH change. In general, this method consists of a mixture of precursors with the species of M2+ and M3+ (metal), to later precipitate the particles by the addition of a base (KOH, NaOH, or NH4OH). It can be summarized in Equation (1) [1][2][3][4].
2. Sol–Gel Method
This method is one of the most used for the synthesis of nanoparticles due to its relatively low cost, operational simplicity, and high homogeneity of the material obtained. It is based on a series of reactions, summarized in the hydrolysis of a metal alkoxide and the subsequent polycondensation of the hydroxyl groups formed, which produces a three-dimensional matrix[5][6][7], which must subsequently be subjected to thermal processes to improve the crystallinity of the nanoparticles.
3. Hydrothermal/Solvothermal
This process can be defined as a series of chemical reactions that occur in a closed system with one or more precursors in the presence of the solvent (water for the hydrothermal case), at a temperature above the boiling point of this[8]. This process has several advantages such as its operational simplicity, versatility, and low cost, among others[9][10]. An important advantage to highlight is the well-controlled diffusivity within the system[11], which allows good control of the structure and morphology of the synthesized particles.
From these synthesis methods, it has been possible to obtain different new Fenton-type catalysts at the nanometer scale, with different coatings and supports[12][13][14] in order to improve the efficiency of the hetero-Fenton process. It has been established that in the hetero-Fenton process, having a catalyst with a higher amount of transition-metal ions and a higher specific area leads to better Fenton activity[15][16]. Fe3O4 is currently being extensively investigated as a Fenton catalyst[17][18][19] due to its relatively high activity and easy magnetic separation. An essential advantage is the presence of Fe2+ and Fe3+ in a single material, due to its cubic structure where half of the Fe3+ ions occupied all of the tetrahedral sites and the Fe2+ ions are founded in the octahedral, allowing the presence of the two important species in Fenton processes. It has been reported in the literature that transition-metal ions occupying tetrahedral sites are catalytically inert, while those located at octahedral sites tend to determine catalytic activity, which is due to effect that the metal cations in these positions are found exclusively on the surface of the solid and thus take part in reactions to generate OH• radicals [20]. It has been suggested that the activation of H2O2 in the presence of magnetite takes place on the surface of the solid, i.e., the Fe3+-OH[21].
According to crystalline field theory, common metal cations have the following order of preference to occupy octahedral sites, Cr3+ > Ni3+ > Cu3+ > Al3+ > Mg2+ > Fe2+ > Co2+ > Fe3+ > Mn2+ > Zn2+ [22]. The most studied are Fe, Mn, Cu, Ni, Zn, and Co[23][24].
Spinel ferrites have a general formula of A2+B3+2O−24 and a cubic lattice structure, where positions A and B are occupied by divalent and trivalent metal cations, according to the distribution of cations in the lattice, and the spinels can be normal, random, or inverse. In a normal spinel, tetrahedral sites are related to position A and octahedral sites to B. In a reverse spinel, tetrahedral sites are occupied by half of the B cations and the octahedral sites by all of the A cations. This distribution of cations is responsible for determining the magnetic properties of ferrites[25]. There are different types of spinel-structured materials; however, researchers will focus specifically on three of them: copper and cobalt ferrites and iron cobaltite.
Copper ferrite (CuFe2O4) is a well-known material with a reverse-spinel structure, which has a stable structure that reduces metals leaching, together with unique magnetic, electrical, physical, and chemical properties [26][27][28][29]. These properties make them a promising Fenton-type catalyst. Cu+ ions have been reported to have the ability to generate OH• radicals by a mechanism similar to that of Fe2+ according to Equations (2)–(4)[30].
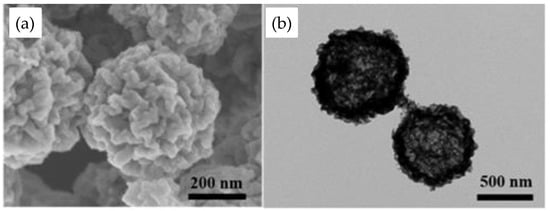
Feng et al.[31] synthesized CuFe2O4 nanoparticles to be used as a Fenton catalyst in the degradation of sulfanilamide. They suggest that CuO is more reactive and effective than Fe3+ for the activation of H2O2 and, which is more important, can work in a higher pH range than conventional iron oxides. Moreover, Zhang et al.[32] observed that the leaching of Cu2+ is 30 times lower in CuFe2O4 than with CuO. The promising performance of the copper spinel ferrite as a Fenton catalyst was pointed out by several authors. Suraj et al. [33] synthesized CuFe2O4 by the chemical coprecipitation method and used it as a heterogeneous Fenton catalyst for the treatment of pulp and paper wastewater, obtaining a 78% elimination of the chemical oxygen demand. Ding et al. [34] demonstrated that the morphology of the spinel is also very important. They synthesized hollow CuFe2O4 spheres with oxygen vacancies (Figure 1), which demonstrated greater degradation of ciprofloxacin than normal CuFe2O4 particles. This better performance of hollow spheres was attributed, among other factors, to the synergistic oxygen vacancies and confinement effects on the catalyst surface. The oxygen vacancies produce highly active electron-rich Cu+ species, which enhanced the H2O2 activation and, thus, the hydroxyl radical generation. In turn, the hollow structure is responsible for concentrating the organic pollutants near the •OH-generator active sites, improving the organic pollutant molecules/•OH radicals contact, and accelerating the degradation. According to Yu et al. [35], the particle size and surface area are more important factors than a crystalline structure for improving the catalytic efficiency of CuFe2O4. López-Ramón et al. [36] evaluated the effect of calcination temperature on the catalytic activity of CuFe2O4 synthesized by the sol–gel method, finding that the calcination temperature has two opposite effects: the activity decreases with increasing temperature due to the increase in crystalline size and cubic-to-tetragonal transformation of ferrite and appearance of hematite; however, the metal leaching decreases with increasing calcination temperatures.

Figure 1. SEM (a) and TEM (b) of CuFe2O4 hollow spheres [34].
Cobalt ferrite (CoFe2O4), like other ferrites, has a high catalytic activity, stable crystal structure, low solubility, magnetic properties, as well as the ease of controlling the leaching of cobalt due to the strong interactions between metals, and the strong redox activity of Co with catalytic properties similar to those of noble metals (Pt, Ir, and Au)[37][38][39][40], which make it a promising material as a Fenton-like catalyst.
Feng et al. [41] synthesized monodispersed CoFe2O4 nanoparticles by a solvothermal method to evaluate them as a Fenton catalyst in the degradation of methylene blue, reaching a concentration decrease of 96.8%. The authors highlighted that the existence of Co2+ ions could favor the decomposition of H2O2 to OH• and subsequently give way to different reactions, as evidenced in Equations (5)–(8).
Sing and Singhal [42] demonstrated that the transition-metal doping of cobalt ferrites is a promising method for tuning the physical characteristics of catalysts and thus, enhancing their catalytic and magnetic properties. For that, the authors synthesized a series of Ru-doped cobalt ferrite nanoparticles by the sol–gel method for the photo-Fenton degradation of red Remazol textile dye, achieving a degradation of approximately 90% within 120 min. The mechanism proposed is based on the photocatalytic and Fenton character of the Ru-modified ferrite. An electron-hole pair is created by the irradiation of cobalt ferrite nanoparticles with visible light. The photogenerated electrons are responsible for the OH• generation from H2O2 and also the reduction of the Fe3+ cation on cobalt ferrite to Fe2+, which further generates OH• radicals in the reaction with hydrogen peroxide. Vinosha et al. [43] also analyzed the photo-Fenton performance of CoFe2O4 nanoparticles obtained by means of chemical coprecipitation, achieving almost total degradation of methylene blue (~99.3% in 75 min) under visible light irradiation. As an outstanding result, they proposed that the pH used in the synthesis was not an impact parameter that affected the morphology of the catalyst; however, it significantly affects the particle size (a more alkaline (pH > 9) medium, larger crystallite size). It has been proposed that the reactions that lead to the formation of CoFe2O4 by the chemical coprecipitation method in an aqueous medium, are those presented in Equations (9) and (10) [44].
In turn, Iron cobaltite (FeCo2O4) has been also studied in environmental remediation and energy storage, thanks to its electrical properties and electrochemical performance [45]. In the energy storage field, Mohamed et al. demonstrated that iron cobaltite nanorods show a better capacity and lower overpotential as the cathode of lithium−O2 batteries than other metal cobaltites (Mn, Ni, and Zn) [46] because the FeCo2O4 surface has the highest number of electropositive Co3+ active sites that improve the oxygen adsorption and Fe2+ in the tetrahedral site that favors the release of electrons to reduce oxygen. Yadav et al. [47] demonstrated that iron cobaltites are also efficient for supercapacitive and photocatalytic applications due to the valence states of the Fe3+/Fe2+ and Co3+/Co2+ species, which are considered active catalytic sites. These nanoflake-like iron cobaltites present a capacitance as high as 1230 F g−1 (5 mV s−1) with a good rate capability and superior cycling stability and also show a good photocatalytic performance achieving up to 94.19% degradation of crystal violet dye under sunlight illumination. However, despite this, very little work has been carried out with reference to their evaluation as a Fenton-like catalyst [48][49]. Zhang et al. [48] synthesized nitrogen-containing carbon/FeCo2O4 composites and analyzed their performances as Fenton catalysts for the degradation of methylene blue obtaining almost 100% removal in 10 min without pH adjustment, which was attributed to the uniform distribution of bimetals and nitrogen doping, which ensured the exposure of sites with high catalytic activity. Zhao et al. [49] analyzed the behavior of FeCo2O4/g–C3N4 as a photo-Fenton catalyst in the degradation of rhodamine B (RhB), obtaining 98% degradation in 45 min, which was attributed to a synergetic interaction between photocatalytic and Fenton-like reactions and the effective separation of the photogenerated charges. Therefore, it is proposed that the use of iron cobaltite as a pristine catalyst (without doping or support) in the Fenton reaction may be promising for future applications.
In general, spinel ferrites turn out to be attractive materials for catalytic activities in Fenton processes, mainly because they manage to improve the generation reaction of Fe2+ and achieve a synergistic effect between metal ions with valences similar to those of iron involved in the process (M2+, M3+, M: Metal).
Perovskites are other types of materials with promising catalytic activity that can be synthesized by the abovementioned methods (Chemical coprecipitation, sol–gel, and hydro/solvothermal) [50][51][52][53]. Perovskites can be defined as a type of mixed oxide with different formulations, binary (ABO), ternary (AA’BO or ABB’O), and quaternary (AA’BB’O), where A and B are cations sites occupied by alkali metals, alkaline-earth metals or rare-earth metals and transition metals, respectively [54].
Some perovskites have been studied in different Fenton-type reactions, for example, Carrasco-Díaz et al. [55] removed paracetamol by Fenton reaction using LaCu1−xMxO3 (M = Mn, Ti) as the catalyst and determined that the most active catalyst was the one that contained the highest amount of Cu2+ at the surface. Moreover, they found that the titanium and manganese species seem not to be responsible for the improvement of activity with respect to the sample LaCuO3. Li et al. [56] synthetized a Ca1−xFeO3−δ perovskite and determined that the A-site cation can distort the FeO6 octahedra in the perovskite and regulate the oxygen vacancies (OV) concentration; in this way, an A-site cation deficient of Ca0.9FeO3-δ results in an improved H2O2 activation for the degradation of tetracycline by a Fenton-like process. Similarly, Xie et al. [57] found that the copper incorporation in LaCoO3 perovskite improved the electro-Fenton activity due to the enhancement of redox activity and oxygen vacancies, but in this case, by the substitution of B-site elements, which synergistically promoted the activation of hydrogen peroxide to a hydroxyl radical (•OH). On the other hand, Rusevova et al. [58] degraded phenol via heterogeneous Fenton-like reactions using iron-containing LaFeO3, and BiFeO3 perovskites, and made a comparison with data reported in the literature using, as a catalyst, nano-sized Fe(II, III) oxide particles, determining that the perovskites synthetized had a higher catalytic activity. Zhao et al. [59] determined that BiFeO3 supported in carbon aerogel (BFO/CA) with a three-dimensional (3D) structure improves the catalytic activity and stability of BiFeO3, resulting in an interesting strategy for the development of advanced catalysts for its possible application in Fenton processes.
In summary, perovskites are materials similar to spinel ferrites, and the contribution of two different metal species (formation of OVs) can be of interest to the Fenton process.
References
- Jiang, Wanquan; Yang, H.C.; Yang, S.Y.; Horng, H.E.; Hung, J.C.; Chen, Y.C.; Hong, Chin-Yih Preparation and properties of superparamagnetic nanoparticles with narrow size distribution and biocompatible. Magnetism and Magnetic Materials 2004, 283, 210-214, https://doi.org/10.1016/j.jmmm.2004.05.022.
- Lin, Chia-Chang; Lai, Yu-Po; Wu, Kuan-Yi A high-productivity process for mass-producing Fe3O4 nanoparticles by co-precipitation in a rotating packed bed. Powder Technology 2022, 395, 369-376, https://doi.org/10.1016/j.powtec.2021.09.036.
- Lin, Chia-Chang; Lee, Cheng-Yen Adsorption of ciprofloxacin in water using Fe3O4 nanoparticles formed at low temperature and high reactant concentrations in a rotating packed bed with co-precipitation. Materials Chemistry and Physics 2020, 240, 122049, https://doi.org/10.1016/j.matchemphys.2019.122049.
- Rao, M.N.; Sultana, Razia; Kota, Sri Harsha Solid and Hazardous Waste Management: Chapter 5. Hazardous Waste. Science and Engineering 2017, 1, 159-207, https://doi.org/10.1016/B978-0-12-809734-2.00005-5.
- Praveen, P; Viruthagiri, G; Mugundan, S; Shanmugam, N Structural, optical and morphological analyses of pristine titanium di-oxide nanoparticles – Synthesized via sol–gel route. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2014, 117, 622-629, https://doi.org/10.1016/j.saa.2013.09.037.
- Dubey, R.S. Temperature-dependent phase transformation of TiO2 nanoparticles synthesized by sol-gel method. Materials Letters 2018, 215, 312-317, https://doi.org/10.1016/j.matlet.2017.12.120.
- Zanurin, A; Johari, N.A.; Mas Ayu, H; Redzuan, N.; Izman, S Research progress of sol-gel ceramic coating: A review. materialstoday: PROCEEDINGS 2022, 48, 1849-1854, https://doi.org/10.1016/j.matpr.2021.09.203.
- Demazeau, G Solvothermal and hydrothermal processes: the main physico-chemical factors involved and new trends. Chemical Intermediates 2011, 37, 107-123, https://doi.org/10.1007/s11164-011-0240-z.
- Krajian, H; Abdallah, B; Kakhia, M; Alkafri, N Hydrothermal growth method for the deposition of ZnO films: Structural, chemical and optical studies. Microelectronics Reliability 2021, 125, 114352, https://doi.org/10.1016/j.microrel.2021.114352.
- Demyanets, L.N.; Lyutin, V.I. Status of hydrothermal growth of bulk ZnO: Latest issues and advantages. Crystal Growth 2008, 310, 993-999, https://doi.org/10.1016/j.jcrysgro.2007.11.145.
- Yadav, Sarita; Sharma, Ambika Importance and challenges of hydrothermal technique for synthesis of transition metal oxides and composites as supercapacitor electrode materials. Energy Storage 2021, 44, 103295, https://doi.org/10.1016/j.est.2021.103295.
- Guo, Xiaojun; Wang, Kebai; Xu, Yanan Tartaric acid enhanced CuFe2O4-catalyzed heterogeneous photo-Fenton-like degradation of methylene blue. Materials Science and Engineering: B 2019, 245, 75-84, https://doi.org/10.1016/j.mseb.2019.05.015.
- He, H.-Y.; Lu, J Highly photocatalytic activities of magnetically separable reduced graphene oxide-CoFe2O4 hybrid nanostructures in dye photodegradation. Separation and Purification Technology 2017, 172, 374-381, https://doi.org/10.1016/j.seppur.2016.08.040.
- Boepple, Matthias; Zhu, Zhigang; Hu, Xiaobing; Weimar, Udo; Barsan, Nicolae Impact of heterostructures on hydrogen sulfide sensing: Example of core-shell CuO/CuFe2O4 nanostructures. Sensors and Actuators B: Chemical 2020, 321, 128523, https://doi.org/10.1016/j.snb.2020.128523.
- Liu, Xiaojuan; Li, Shizhen; Akinwolemiwa, Bamidele; Hu, Di; Wi, Tao Low-crystalline transition metal oxide/hydroxide on MWCNT by Fenton-reaction-inspired green synthesis for lithium ion battery and OER electrocatalysis. Electrochimica Acta 2021, 387, 138559, https://doi.org/10.1016/j.electacta.2021.138559.
- Cheng, Zhuoying; Li, Shaopeng; Nguyen, Tat Thang; Gao, Xing; Luo, SuYue; Guo, Minghui Biochar loaded on MnFe2O4 as Fenton catalyst for Rhodamine B removal: Characterizations, catalytic performance, process optimization and mechanism. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2021, 631, 127651, https://doi.org/10.1016/j.colsurfa.2021.127651.
- Zhou, Tianhong; Huang, Xingxing; Zhai, Tianjiao; Ma, Kai; Zhang, Hongwei; Zhang, Guozhen Fabrication of novel three-dimensional Fe3O4-based particles electrodes with enhanced electrocatalytic activity for Berberine removal. Chemosphere 2022, 287, 132397, Chemosphere.
- Yang, Ruixia; Peng, Qiaohong; Yu, Bing; Shen, Youqing; Cong, Hailin Yolk-shell Fe3O4@MOF-5 nanocomposites as a heterogeneous Fenton-like catalyst for organic dye removal. Separation and Purification Technology 2021, 267, 118620, https://doi.org/10.1016/j.seppur.2021.118620.
- Zhu, Xianyi; Zhang, Lihua; Zou, Guanglong; Chen, Qin; Guo, Yuanlong; Liang, Songmiao; Hu, Lijie; North, Michael; Xie, Haibo Carboxylcellulose hydrogel confined-Fe3O4 nanoparticles catalyst for Fenton-like degradation of Rhodamine B. International Journal of Biological Macromolecules 2021, 180, 792-803, https://doi.org/10.1016/j.ijbiomac.2021.04.067.
- Jacobs, J.P.; Maltha, A; Reintjes, J.G.H.; Drimal, J.; Ponec, V.; Brongersma, H.H. The Surface of Catalytically Active Spinels. Catalysis 1994, 147, 294-300, https://doi.org/10.1006/jcat.1994.1140.
- So, H.L.; Lin, K.Y.; Chu, W Triclosan removal by heterogeneous Fenton-like process: Studying the kinetics and surface chemistry of Fe3O4 as catalyst. Environmental Chemical Engineering 2019, 7, 103432, https://doi.org/10.1016/j.jece.2019.103432.
- Miller, A Distribution of Cations in Spinels. Applied Physics 1959, 30, S24-S25, https://doi.org/10.1063/1.2185913.
- Han, Xing; Liu, Shiye; Huo, Xiangtao; Cheng, Fangqin; Zhang, Mei; Guo, Min Facile and large-scale fabrication of (Mg,Ni)(Fe,Al)2O4 heterogeneous photo-Fenton-like catalyst from saprolite laterite ore for effective removal of organic contaminants. Hazardous Materials 2020, 392, 122295, https://doi.org/10.1016/j.jhazmat.2020.122295.
- Wang, Yuru; Tian, Dongfan; Chu, Wei; Li, Minrui; Lu, Xinwei Nanoscaled magnetic CuFe2O4 as an activator of peroxymonosulfate for the degradation of antibiotics norfloxacin. Separation and Purification Technology 2019, 212, 536-544, https://doi.org/10.1016/j.seppur.2018.11.051.
- Shilpa Amulya, M.A.; Nagaswarupa, H.P.; Anil Kumar, M.R.; Ravikumar, C.R.; Kusuma, K.B.; Prashantha, S.C. Evaluation of bifunctional applications of CuFe2O4 nanoparticles synthesized by a sonochemical method. Physics and Chemistry of Solids 2021, 148, 109756, https://doi.org/10.1016/j.jpcs.2020.109756.
- Gao, Jinsuo; Wu, Shouchun; Han, Yanling; Tan, Feng; Shi, Yong; Liu, Mengyao; Li, Xinyong 3D mesoporous CuFe2O4 as a catalyst for photo-Fenton removal of sulfonamide antibiotics at near neutral pH. Colloid and Interface Science 2018, 524, 409-416, https://doi.org/10.1016/j.jcis.2018.03.112.
- Saravanakumar, B; Ramachandran, S.P; Ravi, G; Ganesh, V; Guduru, Ramesh K; Yuvakkumar, R Electrochemical performances of monodispersed spherical CuFe2O4 nanoparticles for pseudocapacitive applications. Vacuum 2019, 168, 108798, https://doi.org/10.1016/j.vacuum.2019.108798.
- Wu, Xingping; Xia, Fan; Nan, Zhaodong Facile synthesis of double-mesoporous-shelled hollow spheres of Cu–CuFe2O4/SiO2 composite as excellent Fenton catalyst. Materials Chemistry and Physics 2020, 242, 122490, https://doi.org/10.1016/j.matchemphys.2019.122490.
- Li, Jun; Ren, Yi; Ji, Fangzhou; Lai, Bo Heterogeneous catalytic oxidation for the degradation of p-nitrophenol in aqueous solution by persulfate activated with CuFe2O4 magnetic nano-particles. Chemical Engineering Journal 2017, 324, 63-73, https://doi.org/10.1016/j.cej.2017.04.104.
- Fontecha-Cámara, María A; Moreno-Castilla, Carlos; López-Ramón, María Victoria; Álvarez, Miguel A Mixed iron oxides as Fenton catalysts for gallic acid removal from aqueous solutions. Applied Catalysis B: Environmental 2016, 196, 207-215, https://doi.org/10.1016/j.apcatb.2016.05.032.
- Feng, Yong, Liao, Changzhong; Shih, Kaimin Copper-promoted circumneutral activation of H2O2 by magnetic CuFe2O4 spinel nanoparticles: Mechanism, stoichiometric efficiency, and pathway of degrading sulfanilamide. Chemosphere 2016, 154, 573-582, https://doi.org/10.1016/j.chemosphere.2016.04.019.
- Zhang, Tao; Zhu, Haibo; Croué, Jean-Philippe Production of Sulfate Radical from Peroxymonosulfate Induced by a Magnetically Separable CuFe2O4 Spinel in Water: Efficiency, Stability, and Mechanism. Environmental Science and Technology 2013, 47, 2784-2791, https://doi.org/10.1021/es304721g.
- Suraj, P; Vijyendra, Kumar; Thakur, C; Ghosh, Prabir Taguchi optimization of COD removal by heterogeneous Fenton process using copper ferro spinel catalyst in a fixed bed reactor—RTD, kinetic and thermodynamic study. Environmental Chemical Engineering 2019, 7, 102859, https://doi.org/10.1016/j.jece.2018.102859.
- Ding, Rong-Rong; Li, Wen-Qiang; He, Chuan-Shu; Wang, Yi-Ran; Liu, Xiao-Cheng; Zhou, Guan-Nan; Mu, Yang Oxygen vacancy on hollow sphere CuFe2O4 as an efficient Fenton-like catalysis for organic pollutant degradation over a wide pH range. Applied Catalysis B: Environmental 2021, 291, 120069, https://doi.org/10.1016/j.apcatb.2021.120069.
- Yu, Deyou; Ni, Huagang; Wang, Lili; Wu, Minghua; Yang, Xiaosu Nanoscale-confined precursor of CuFe2O4 mediated by hyperbranched polyamide as an unusual heterogeneous Fenton catalyst for efficient dye degradation. Cleaner Production 2018, 186, 146-154, https://doi.org/10.1016/j.jclepro.2018.03.134.
- López-Ramón, María V; Álvarez, Miguel A; Moreno-Castilla, Carlos; Fontecha-Cámara, María A; Yebra-Rodríguez, África; Bailón-García, Esther Effect of calcination temperature of a copper ferrite synthesized by a sol-gel method on its structural characteristics and performance as Fenton catalyst to remove gallic acid from water. Colloid and Interface Science 2018, 511, 193-202, https://doi.org/10.1016/j.jcis.2017.09.117.
- Xu, Mengjuan; Li, Jun; Yan, Yan; Zhao, Xiuge; Yan, Jianfei; Zhang, Yunhong; Lai, Bo; Chen, Xi; Song, Liping Catalytic degradation of sulfamethoxazole through peroxymonosulfate activated with expanded graphite loaded CoFe2O4 particles. Chemical Engineering Journal 2019, 369, 403-413, https://doi.org/10.1016/j.cej.2019.03.075.
- Guo, Xiaojun; Li, Hairu; Zhao, Shengguo Fast degradation of Acid Orange II by bicarbonate-activated hydrogen peroxide with a magnetic S-modified CoFe2O4 catalyst. Taiwan Institute of Chemical Engineers 2015, 55, 90-100, https://doi.org/10.1016/j.jtice.2015.03.039.
- Yao, Yunjin; Yang, Zeheng; Zhang, Dawei; Peng, Wenchao; Sun, Hongqi; Wang, Shaobin Magnetic CoFe2O4–Graphene Hybrids: Facile Synthesis, Characterization, and Catalytic Properties. I&EC research 2012, 51, 6044-6051, https://doi.org/10.1021/ie300271p.
- Hong, Peidong; Li, Yulian; He, Junyong; Saeed, Abdul; Zhang, Kaisheng; Wang, Chengming; Kong, Lingtao; Liu, Jinhuai Rapid degradation of aqueous doxycycline by surface CoFe2O4/H2O2 system: behaviors, mechanisms, pathways and DFT calculation. Applied Surface Science 2020, 526, 146557, https://doi.org/10.1016/j.apsusc.2020.146557.
- Feng, X; Mao, G.Y; Bu, F.X; Cheng, X.L; Jiang, D.M; Jiang, J.S Controlled synthesis of monodisperse CoFe2O4 nanoparticles by the phase transfer method and their catalytic activity on methylene blue discoloration with H2O2. Magnetism and Magnetic Materials 2013, 343, 126-132, https://doi.org/10.1016/j.jmmm.2013.05.001.
- Singh, Sneha; Singhal, Sonal Transition metal doped cobalt ferrite nanoparticles: Efficient photocatalyst for photodegradation of textile dye. materialstoday:PROCEEDINGS 2019, 14, 453-460, https://doi.org/10.1016/j.matpr.2019.04.168.
- Annie Vinosha, P; Jerome Das, S Investigation on the role of pH for the structural, optical and magnetic properties of cobalt ferrite nanoparticles and its effect on the photo-fenton activity. materialstoday:PROCEEDINGS 2018, 5, 8662-8671, https://doi.org/10.1016/j.matpr.2017.12.291.
- Kachi, Wajeeh; Al-Shammari, Ahmed Majeed; Zainal, Israa G Cobalt Ferrite Nanoparticles: Preparation, characterization and salinized with 3-aminopropyl triethoxysilane. Energy Procedia 2019, 157, 1353-1365, https://doi.org/10.1016/j.egypro.2018.11.300.
- Sun, Zhiming; Liu, Xiaorui; Dong, Xiongbo; Zhang, Xiangwei; Tan, Ye; Yuan, Fang; Zheng, Shuilin; Chunquan, Li Synergistic activation of peroxymonosulfate via in situ growth FeCo2O4 nanoparticles on natural rectorite: Role of transition metal ions and hydroxyl groups. Chemosphere 2021, 263, 127965, https://doi.org/10.1016/j.chemosphere.2020.127965.
- Mohamed, Saad Gomaa; Tsai, Yuan-Quei; Chen, Chih-Jung; Tsai, Yi-Ting; Hung, Tai-Feng; Chang, Wen-Sheng; Liu, Ru-Shi Ternary Spinel MCo2O4 (M = Mn, Fe, Ni, and Zn) Porous Nanorods as Bifunctional Cathode Materials for Lithium–O2 Batteries. ACS:APPLIED MATERIALS & INTERFACES 2015, 22, 12038-12046, https://doi.org/10.1021/acsami.5b02180.
- Yadav, A.A; Hunge, Y.M; Kulkarni, S.B Synthesis of multifunctional FeCo2O4 electrode using ultrasonic treatment for photocatalysis and energy storage applications. Ultrasonics Sonochemistry 2019, 58, 104663, https://doi.org/10.1016/j.ultsonch.2019.104663.
- Zhang, Ting; Ma, Qingqing; Zhou, Mengqi; Li, Changyu; Sun, Jianchao; Shi, Weijie; Ai, Shiyun Degradation of methylene blue by a heterogeneous Fenton reaction catalyzed by FeCo2O4-N-C nanocomposites derived by ZIFs. Powder Technology 2021, 383, 212-219, https://doi.org/10.1016/j.powtec.2021.01.051.
- Zhao, Lei; Yang, Dan; Ma, Lili; Feng, Xueting; Ding, Hanming An efficient heterogeneous catalyst of FeCo2O4/g-C3N4 composite for catalytic peroxymonosulfate oxidation of organic pollutants under visible light. Colloids and Surfaces A: Physicochemical and Engineering Aspects 2021, 610, 125725, https://doi.org/10.1016/j.colsurfa.2020.125725.
- Shan, Zelin; Ma, Fang; You, Shijie; Shan, Lianbin; Kong, Deyong; Guo, Haijuan; Cui, Chongwei Enhanced visible light photo-Fenton catalysis by lanthanum-doping BiFeO3 for norfloxacin degradation. Environmental Research 2023, 216, 114588, https://doi.org/10.1016/j.envres.2022.114588.
- Chen, Xiangjun; Qiao, Jinshuo; Wang, Zhenhua; Sun, Wang; Sun, Kening Layered perovskites with exsolved Co-Fe nanoalloy as highly active and stable anodes for direct carbon solid oxide fuel cells. Alloys and Compounds 2023, 940, 168872, https://doi.org/10.1016/j.jallcom.2023.168872.
- Torregrosa-Rivero, Verónica; Sánchez-Adsuar, María-Salvadora; Illán-Gómez, María-José Exploring the effect of using carbon black in the sol-gel synthesis of BaMnO3 and BaMn0.7Cu0.3O3 perovskite catalysts for CO oxidation. Catalysis Today 2023, 419, 114145, https://doi.org/10.1016/j.cattod.2023.02.005.
- Nguyen, Anh Tien; Phung, Viet Duc; Mittova, Valentina Olegovna; Ngo, Hai Dang; Vo, Thuan Ngoc; Le Thi, My Linh; Nguyen, Van Hoang; Mittova, Irina Yakovlevna; Phung Le, My Loan; Ahn, Yong Nam; et al.Kim, Il TaeNguyen, Tuan Loi Fabricating nanostructured HoFeO3 perovskite for lithium-ion battery anodes via co-precipitation. Scripta Materialia 2022, 207, 114259, https://doi.org/10.1016/j.scriptamat.2021.114259.
- Alrozi, Rasyidah; Zubir, Nor Aida; Abu Bakar, Noor Fitrah; Ladewig, Bradley P; Motuzas, Julius; Hanif Abu Bakar, Noor Hana; Wang, David K; Diniz da Costa, João Functional role of B-site substitution on the reactivity of CaMFeO3 (M = Cu, Mo, Co) perovskite catalysts in heterogeneous Fenton-like degradation of organic pollutant. Taiwan Institute of Chemical Engineers 2023, 143, 104675, https://doi.org/10.1016/j.jtice.2023.104675.
- Carrasco-Díaz, M.R; Castillejos-López, E; Cerpa-Naranjo, A; Rojas-Cervantes, M.L Efficient removal of paracetamol using LaCu1−xMxO3 (M = Mn, Ti) perovskites as heterogeneous Fenton-like catalysts. Chemical Engineering Journal 2016, 304, 408-418, https://doi.org/10.1016/j.cej.2016.06.054.
- Li, Jinxin; Ma, Wencheng; Zhong, Dan; Li, Kefei; Ma, Jun; Zhang, Shaobo; Du, Xuan Oxygen vacancy concentration modulation of perovskite-based heterogeneous catalysts for Fenton-like oxidation of tetracycline. Cleaner Production 2022, 362, 132469, https://doi.org/10.1016/j.jclepro.2022.132469.
- Xie, Liangbo; Liu, Xiaohui; Chang, Jing; Zhang, Cailing; Li, Yi; Zhang, He; Zhan, Sihui; Hu, Wenping Enhanced redox activity and oxygen vacancies of perovskite triggered by copper incorporation for the improvement of electro-Fenton activity. Chemical Engineering Journal 2022, 428, 131352, https://doi.org/10.1016/j.cej.2021.131352.
- Rusevova, Klara; Köferstein, Roberto; Rosell, Mònica; Richnow, Hans H; Kopinke, Frank-dieter; Georgi, Anett LaFeO3 and BiFeO3 perovskites as nanocatalysts for contaminant degradation in heterogeneous Fenton-like reactions. Chemical Engineering Journal 2014, 239, 322-331, https://doi.org/10.1016/j.cej.2013.11.025.
- Zhao, Hongying; Cao, Jinlei; Lv, Huanli; Wang, Yanbin; Zhao, Guohua 3D nano-scale perovskite-based composite as Fenton-like system for efficient oxidative degradation of ketoprofen. Catalysis Communications 2013, 41, 87-90, https://doi.org/10.1016/j.catcom.2013.07.017.
More
