Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Cristina Villamar.
Emerging contaminants (ECs) are causing negative effects on the environment and even on people, so their removal has become a priority worldwide. ECs are organic, pseudo-persistent, and unregulated “new” contaminants detected in water/wastewater in trace concentrations (ng/L–µg/L).
- conventional/non-conventional adsorbents
- nanocomposites
- lipophilic contaminants
1. Introduction
Emerging contaminants (ECs) are organic, pseudo-persistent, and unregulated “new” contaminants detected in water/wastewater in trace concentrations (ng/L–µg/L) [1]. Pharmaceutical and personal care products, hormones, pesticides, and microplastics, among other chemical substances, are some examples of ECs. They reach the environment through effluents from municipal wastewater treatment plants (WWTPs), septic tanks, hospital effluents, livestock activities, and subsurface storage of household and industrial wastes [1,2][1][2]. In fact, several antibiotics, such as azithromycin, amoxicillin, and ciprofloxacin, have been found in influents wastewater from Asian, European, and North American countries at concentrations (ng/L) between 3 and 303,500, 0.4 and 13,625, and 6.1 and 246,100, respectively [3,4][3][4].
After reaching the environment, or even inside the WWTPs, some ECs should be degraded/biodegraded to metabolites or gases. An environmental degradation route of ECs is photochemical transformation, which can occur directly by solar UV radiation absorption [5] and indirectly by photosensitized species reaction [6]. For example, diclofenac has demonstrated being photochemically degraded to 1-(8-chlorocarbazolyl) acetic acid and carbazole. ECs’ biodegradability depends on their bioavailability, being a more viable process for ECs with octanol-water partitioning coefficients (log Kow) between < 1 and > 4 and high polarity (pKa > 0.5) [5,6][5][6]. Ibuprofen, natural estrogens, bisphenol A (BPA), and triclosan are some examples of biodegradable ECs, which generate transformation products or metabolites (e.g., triclosan to 2,8-dichlorodibenzo-p-dioxin under UV light), achieving degradation percentages greater than 50% [6]. However, since ECs with Henry’s constant values from 10−2 to 10−3 mol/m3Pa do not degrade but rather volatilize, ECs such as dibutyl phthalate, di(2-ethylhexyl) phthalate, and nonylphenol have been found in air samples (0.031–0.055 ng/L) [5,6,7][5][6][7]. Another important factor controlling the fate/behavior of ECs (removal/bioavailability/degradation/volatilization/transport) is their ease of being sorbed–desorbed. Sorption–desorption processes are related to the partitioning soils/sediments coefficient measured by Kd [8]. Some antibiotics, hormones, biocides, and artificial sweeteners with low Kd (300–500 L/kg MLSS) have showed insignificant sorption into sludge [1].
Differences between properties/characteristics/behavior/mobility of different ECs have turned them into substances with potential environmental and human risk, despite their low concentrations. Figure 1 shows the main routes of entry of ECs into the environment, their behavior in it, and the possible effects they could cause in different living beings. Conventional WWTPs do not perform effectively for the removal of ECs. Moreover, conventional wastewater treatment processes/systems/technologies are generally not available or efficient for developing countries, and thus it is necessary to search for options that fit each place [9].
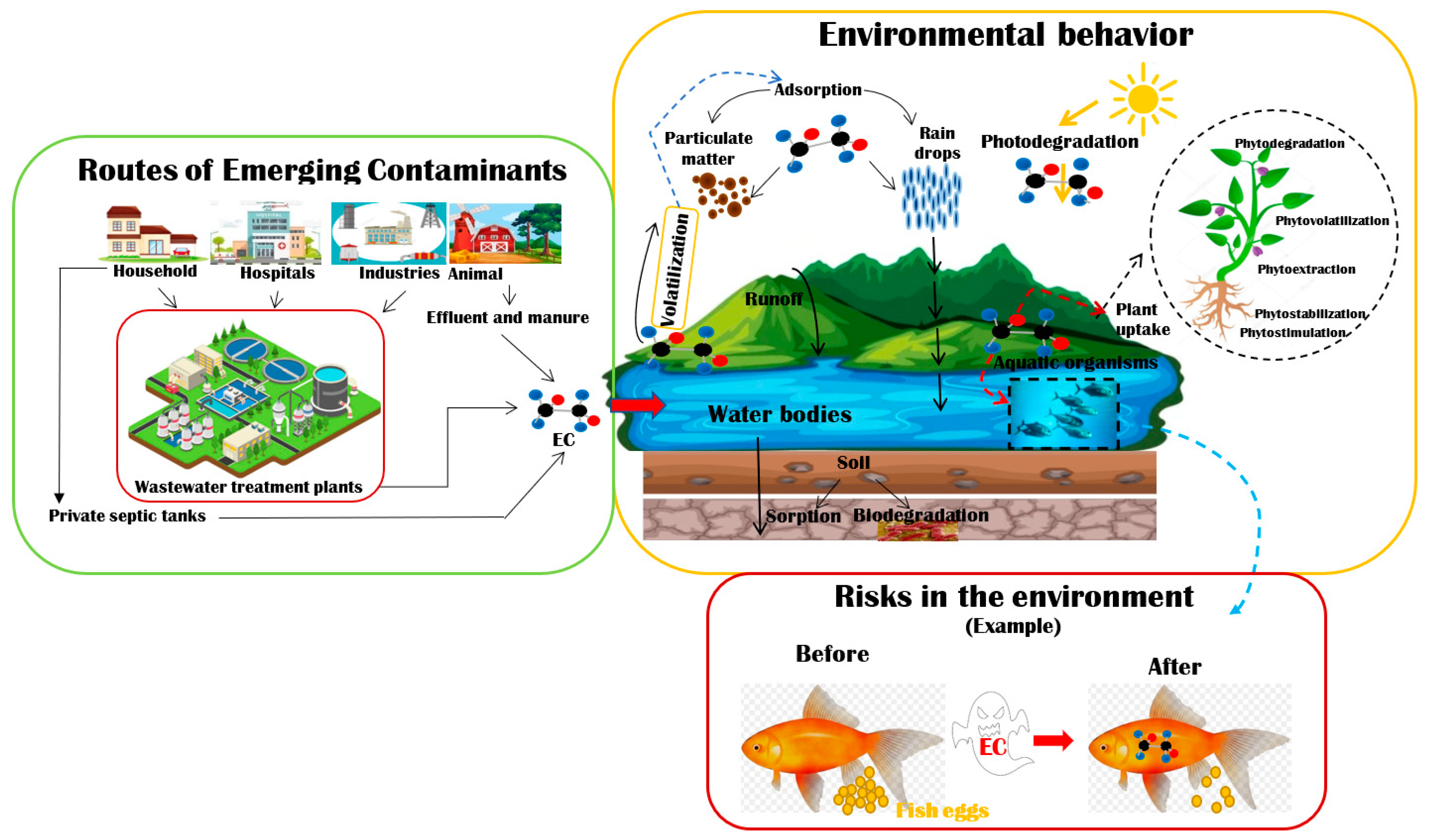
Figure 1.
Routes of entry of ECs into the environment, its behavior, and effects.
Adsorption has proven to be the most effective, sustainable, renewable, and selective method with low cost and energy consumption [10]. It allows the removal of contaminants such as heavy metals (metal/adsorbent: nickel, lead/nano polypyrrole-polyethyleneimine [11,12][11][12]; zinc/modified fruit peels, graphene oxide + magnetite [13,14][13][14]) and dyes (dye/adsorbent: methylene blue, acid orange 10/activated carbon from oil palm waste; methylene blue and crystal violet/Bauhinia forficata residual fruit) [10,15][10][15]. Activated carbon is the most used adsorbent, but the high costs of this adsorbent, added to the loss of efficiency in the regeneration process, have made it necessary to search for other alternatives [16].
2. Characteristics, Concentration, and Toxicity of Some ECs in Water Bodies
2.1. Pharmaceuticals and Personal Care Products (PPCPs)
There are more than 3000 substances (50–150 g/inhab) used as analgesics/anti-inflammatories, antibiotics, contraceptives, antidepressants, and pressure regulators, among others [17]. Meanwhile, around 2000 chemical compounds are used as personal care products, which include fragrances, preservatives, disinfectants, and sun protection agents, among others [18]. Indeed, the PPCPs production has reached about 20 million tons/year [19]. PPCPs have been found in surface water (up to 10,000 mg/L), groundwater (up to 100 mg/L), influents (75–73,730 ng/L), and effluents (24–4800 ng/L) of WWTP, sludge, sediments, and even in living beings (e.g., triclosan in fish at 2100 ng/g) [20,21][20][21]. In general, PPCPs can be made of several complex molecules with different structures and shapes, which vary widely according to their molecular weight (88.5–> 900.0 g/mol) [4,14][4][14]. Moreover, they are polar molecules with ionizable groups, i.e., in the solid phase, they have different adsorption mechanisms (e.g., ion exchange). Most of them are lipophilic [4]. However, PPCPs have log Kow values in a wide range (−2.4–13.1), from acid to basic substances under environmental conditions (pKa = 0.6–13.9) [4,22][4][22]. PPCPs reach Kd values between 1.9 and 39,000, are partially/completely soluble in water (0.02–3.12 × 105 mg/L, T = 20 °C) [4[4][19],19], and have dissipation times between <3 and up to 300 days [18]. Recurrent discharges of PPCPs could cause endocrine problems, genotoxicity, aquatic toxicity, and resistance in pathogenic microorganisms [23]. For instance, diclofenac at concentrations between 5 and 50 mg/L can increase the plasma vitellogenin in fish. It has even caused effects on steppe eagles and vultures [1,18][1][18]. Meanwhile, ciprofloxacin, tetracycline, ampicillin, trimethoprim, erythromycin, and sulfamethoxazole can increase the antibiotic resistance of E. coli and Xanthomonas maltophilia [18,19][18][19]. Personal care products, such as benzophenone-3 threaten coral reefs and 4-methylbenzylidene camphor, have been demonstrated to generate embryonic malformations in frogs. Additionally, triclosan produces adverse effects on the first stages of the frog’s life [21,24,25][21][24][25]. However, PPCPs also affect humans, as their presence has been detected in breast milk, blood, and urine of children [21]. Furthermore, benzyl paraben and benzophenone-4 were found in the placenta, which could indicate a transfer from mother to fetus [26].2.2. Pesticides
Until 2020, a use of around 3.5 million tons/year of pesticides was estimated, but only less than 0.1% was used for plants [27]. Some pesticides, such as glyphosate or its main metabolite (amino methyl-phosphonic acid), have been found in surface waters (0.02–6.0 g/L), soil (15.9–1025.5 kg/kg), deep waters (0.1 µg/L), and sediments (0.1–100 mg/L). Moreover, pesticides with high vapor pressure (1.51 × 10−7–1.29 × 10−1 Pa)/high volatility (e.g., pentachlorophenol) are released to the air during their application (between 5 and 90% of them), moving long distances and even reaching pristine areas [28,29,30][28][29][30]. They have been found in rainfall, e.g., methyl parathion, and also near agricultural sites where they were not applied (~23 µg/L) [28]. According to their molecular structure, pesticides have different chemical properties, reaching weak to strong acid character (pKa = 0.7–9.1), medium to high solubility in water (2.0–1.2 × 106 mg/L, T = 20–25 °C), very low to very high bioaccumulation (log Kow = −4.6–8.0), and high persistence (7 days–>5 years) [28,30][28][30]. Pesticides mainly affect non-target organisms, e.g., atrazine (concentrations in water >200 ng/L) [29] causes sex change in male frogs and affected/altered the reproductive system and fertility of mice, fish, and humans [28,29,31][28][29][31]. Another widely used herbicide is glyphosate, which affects the entire food chain, delaying periphytic colonization and reducing the abundance of aquatic organisms such as Pseudokirchneriella subcapitata and Lemma minor (EC50-7d = 11.2–46.9 and EC50-4d = 64.7–270.0 mg a.i./L, respectively) [30,31][30][31]. Paraquat produced neurotoxicity and systemic and pulmonary inflammation (inhalation for 16 days) in rats [32]. Furthermore, its use in edible crops is related to antibiotic resistance in humans, even it was classified as a carcinogenic by the World Health Organization [33]. The byproducts/metabolites of pesticides also cause negative effects [28]. For example, aminomethylphosphonic acid produced acute toxicity in Vibrio fischeri at concentrations between 50 and 167 mg/L [30].2.3. Steroid Hormones
The human population generates about 30,000 and 700 kg/year of natural and synthetic estrogens, respectively. Natural estrogens are excreted by the adrenal cortex, testes, ovaries, and placenta of humans and animals (e.g., estrone/E1, 17β-estradiol/E2, estriol/E3) [34]. Meanwhile, synthetic hormones are synthesized from cholesterol (e.g., 17α-ethinylestradiol/EE2) [34,35][34][35]. In specific, synthetic estrogens come from oral contraceptives. However, the estrogen generated by cattle is much higher, amounting to 83,000 kg/year in the US and the European Union alone [36]. WWTPs or feedlot effluents are the main pathways for hormones entering soil, surface water, sediment, and groundwater. They have been found within WWTP influents (>802 ng/L), WWTP effluents (>275 ng/L), surface waters (0.04–667 ng/L), groundwater (5–> 1000 ng/L), drinking water (up to 3500 ng/L), and livestock waste (14–533 ng/g) [3,4][3][4]. Steroid hormones are characterized by having a molecular weight between 242 and >296 g/mol, being poorly soluble in water (8.8–441.0 mg/L, at 20 °C), with low to moderate hydrophobicity (log Kow = 1.6–4.7), weak acid character (pKa = 10.3–18.9), and non-volatile (vapor pressure= 9 × 10−13–3 × 10−8 Pa) [34,36][34][36]. Despite their low persistence (thalf-life = 0.2–9.0 days in water/sediments) compared with other ECs, they cause negative effects on ecosystems and humans (cancer and infertility) by their endocrine-disrupting character [4,36][4][36]. Chronic exposure of fathead minnow to EE2 (5–6 ng/L) led to feminization of male fish by the development of the ovary cavity, and impaired oogenesis in females [4,34][4][34]. E2 at 1 ng/L induced changes in vitellogenin production in males, and produced the feminization of some species of male fish (1–10 ng/L) [34,35][34][35]. Levonorgestrel at 6.5 ng/L inhibited spermatogenesis, reduced fish egg production and reproduction, increased female weight and length, and promoted female masculinization [34]. Likewise, EE2 at 10 ng/L affected cardiac function in bullfrog tadpoles [34], reducing the fish biomass and interrupting the aquatic food chain [36].2.4. Micellaneous ECs
The use/consumption of different products such as fire retardants, food additives, plasticizers, solvent stabilizers, surfactants/detergents, industrial additives (fluorinated organic compounds), and corrosion inhibitors is considerable worldwide [3,37][3][37]. In fact, the worldwide production of surfactants was projected to 24.2 million tons for 2022 [38], and plasticizers such as BPA had a production of 2.0 million metric tons/year [5]. Moreover, around 159,000 metric tons/year of synthetic non-nutritional sweeteners are consumed [39,40][39][40]. The physicochemical characteristics of these substances are very varied due to the differences between them, which also makes their behavior in the environment different. For instance, artificial sweeteners present between medium and very high solubility (4–1000 g/L, 20 °C), from low to high acid character (pKa = 1.9–11.8), and very low bioaccumulation (log Kow = −1.61–0.91) [6,39][6][39]. However, other substances such as BPA (plasticizer), nonylphenol (detergent). and Tris(2-chloroethyl) phosphate (TCEP, fire retardant) have from low to high bioaccumulation with log Kow values of 3.2, 4.4, and 1.8, respectively [5,41][5][41]. Moreover, BPA and phthalates are semi-volatiles, so they easily move into the environment. They have a short half-life (5–18 days) in air because they could be photodegraded [5]. Likewise, fluorinated organic compounds have longer chains exhibiting persistence, from moderately solubility (e.g., perfluoro octane sulfonate = 1.08 g/L) to high solubility (e.g., perfluorooctanoic acid > 20 mg/L) in water, long-distance mobility, high to very high bioaccumulation (e.g., log Kow of perfluoro octane sulfonate = 4.5–6.9), and toxic effects [42,43][42][43]. In turn, plasticizers are commonly found in surface water (<1–12,000 ng/L), runoff (50–2410 ng/L), and other water sources [5,7][5][7]. They include bisphenol type -A/-S/-F and phthalates [5,7,41][5][7][41]. Fluorinated organic compounds have been detected in surface water (0.09–578,970 ng/L), groundwater (0.17–8.54 ng/L), WWTP influents (65–112 µg/L) and WWTPs effluents (43–78 µg/L), runoff (~2 ng/L), and drinking water (~54 ng/L) [5,41][5][41]. Furthermore, traces of fluorides (0.023–>1600 ng/g) have been found in some species of fish, amphibians, crustaceans, seals, whales, and polar bears [41]. Artificial sweeteners were found in drinking water (36–2400 ng/L), surface water (0.03–9600 ng/L), groundwater (non-detected–33,600 μg/L), seawater (200–393 ng/L), and lakes (non-detected–780 ng/L) [6,39][6][39]. Ethoxylated alcohol (surfactant) has been reported to affect fish and invertebrates. In fathead minnows, it affects egg production/larval survival, with a non-observed effect concentration (NOEC) ~0.73 mg/L. In species such as bluegills, it affects survival and growth at concentrations ~5.7 mg/L [44]. Non-ionic surfactants and nonylphenol ethoxylates have exhibited acute toxic effects (EC50 = 1.1–25.4 mg/L) on tadpoles of four Australian and two exotic frogs [39,44][39][44]. In turn, artificial sweeteners such as aspartame (2000–50,000 mg/L) have been reported to cause cancer in rats and headaches, nausea, and vomiting in humans. Meanwhile, 5% sucralose produced thymus shrinkage and migraine in rats and humans, respectively [39].2.5. Microplastics
Microplastics (size = 1–5000 nm) are classified as primary (microbeads from personal care products and cosmetics) and secondary (degradation/fragmentation/leaching of plastics) [45]. Approximately 4130 tons/year of microbeads are used in different personal care products in EU countries plus Norway and Switzerland [45]. In total, plastic waste has reached values around 6300 million tons (oceans = 1.6–4.1%) between 1950 and 2015 [45,46][45][46]. More than 400,000 tons microplastics/year (95% from WWTPs) could enter the environment [45]. They were found in lakes and rivers (0.05–320 particles/L) and sediments from shore, water, and benthic at concentrations of 75–1300 particles/m2, 2.5–25,800.0 particles/m3, and 6.2–980 particles/kg, respectively [45]. A problem associated with microplastics is that those with low density (910–2200 kg/m3) could move hydrophobic contaminants long distances [47]. Dichlorodiphenyltrichloroethane (DDT), polycyclic aromatic hydrocarbons, chlorinated benzenes, polychlorinated biphenyls (PCBs), organo-halogenated pesticides, and endocrine disruptors have been found in microplastics such as polyethylene, polypropylene, PVC, and polystyrene [47]. BPA, polybrominated diphenyl ethers and phthalates, and microplastic additives also have been found in plastic debris at concentrations from 0.1 to 700 ng/g, up to 990 ng/g, and up to 3940 ng/g, respectively [48]. Microplastics produce digestive and locomotion problems, and changes in metabolic profiles in organisms, such as planktonic, crustaceans (e.g., Daphnia magna), fish (e.g., zebrafish), turtles, and whales [47,49][47][49]. These ECs could even compromise human health since they have been found in consumer species such as brown shrimp [47,48][47][48]. The surface area/shape/size/texture of microplastics is also related to toxicity. Particularly, the smallest microplastics in the form of fibers and a greater surface area have been reported to generate greater toxicity [48]. High-density polyethylene (0–80 μm) accumulated in the lysosomal system of blue mussels after 1.0 h of exposure. In addition, earthworms of the species Lumbricus terrestris exposed to 450 and 600 g/kg of polyethylene increased their mortality rate between 8 and 25%, respectively. There was also a decrease in their growth and negative effects on the construction of burrows [42]. Polypropylene fibers were more toxic than polyethylene spheres for Hyalella Azteca [45]. Among the most used methods to eliminate microplastics from aqueous medium are physical (sedimentation, membrane filtration), chemical (photocatalytic oxidation, coagulation, ozonation), and biological (conventional activated sludge systems, activated sludge systems + membrane bioreactor, microorganism biodegradation) [44,47,48,49,50][44][47][48][49][50]. The use of materials is also essential in the elimination of this type of EC, mainly through magnetic separation. Nanomaterials such as magnetic carbon nanotubes and magnetite have been used efficiently [51]. Shia et al. (2022) removed polyethylene, polypropylene, polystyrene, and polyethylene terephthalate (size: 200–900 μm), reaching efficiencies between 62.8 and 86.9% [52]. Table 1 shows the features of some ECs and their concentrations for different water sources. It is observed that there is a wide range of ECs found in different water bodies in their common concentrations (0.01 ng/L–>6010 mg/L). The EC characteristics are also very varied (log Kow = −3.4–13.9, water solubility = 0.49–21,600 mg/L), which could be a complication when it comes to removing them from water. Lipophilic substances (high log Kow) and low water solubility such as carbamazepine (anticonvulsant), triclosan (disinfectant), EE2 (hormones), and musk xylene (fragrance) suggest higher removal efficiency through adsorption processes. Meanwhile, hydrophilic substances (low log Kow) and high-water solubility, such as caffeine (stimulant), clofibric acid (lipid regulator), atenolol (beta blocker), and glyphosate (herbicide), are more difficult to remove through adsorption processes. This was verified in the research carried out by León et al. [53], in which triclosan (48.6–76.4%) was more easily removed than caffeine (40.1–67.4%) using agro-industrial residues, under the same operating conditions. Therefore, lipophilic ECs can be removed more easily than hydrophilic by adsorption process.Table 1.
Physical and chemical characteristics of some ECs and their concentrations in water bodies.
| EC Type | EC Subtype | Contaminant | Water Solubility [mg/L] |
Log Kow | Concentration Found [ng/L] |
Type of Water | Reference |
|---|---|---|---|---|---|---|---|
| PPCPs | Stimulant | Caffeine | 21,600 | −0.07 | 753,500–1.0 × 106 | Surface water | [22,54,55][22][54][55] |
| 20–23,970 | Ground water | ||||||
| 500–5000 | Drinking water | ||||||
| Anti-inflammatory | Ibuprofen | 21 | 0.35 | 13.5–89,500 | Effluent WWTP | [19,22,54,56][19][22][54][56] | |
| Anticonvulsant | Carbamazepine | 112–236 | 13.90 | 589–3.5 × 108 | Influent WWTP | [19,57,58][19][57][58] | |
| 1200–6.6 × 107 | Effluent WWTP | ||||||
| Lipid regulator | Clofibric acid | 214,650 | 2.88 | nd–420 | Influent WWTP | [19,59][19][59] | |
| Antibiotic | Ciprofloxacin | 650 | 0.28 | 2200–14,000 | Influent WWTP | [19,60,61][19][60][61] | |
| 1100–44,000 | Hospital wastewater | ||||||
| Diagnostic Contrast Media | Iopromide | 23.8 | −2.1 | 780–11,4000 | River water | [19,62][19][62] | |
| 1170–4030 | Urban effluents | ||||||
| Antidepressant | Diazepan | 50 | 3.08 | nd–100 | Surface water | [19,63][19][63] | |
| nd–100 | Influent WWTP | ||||||
| Beta blocker | Atenolol | 300 | 0.16 | 90–255 | Influent WWTP | [19,64][19][64] | |
| Fragrance | Musk xylene | 0.49–1.0 | 4.40 | 200,000–400,000 | Drinking water | [19,65][19][65] | |
| Disinfectant | Triclosan | 10.00 | 4.76 | 200–1854 | Influent WWTP | [24,[25,2454]][25][54] | |
| Pesticides | Herbicide | Glyphosate | 15.70 | −3.40 | up to 6.01 × 109 | Surface water | [30,66][30][66] |
| Insecticide | Acetamiprid | 2950 | 0.80 | 0.08–249 | Surface water | [27,29][27][29] | |
| Clothianidin | 304 | 0.70 | 1.0–740 | Surface water | [27,29,67,68][27][29][67][68] | ||
| Thiamethoxam | 4100 | −0.13 | 1.0–914 | Surface water | |||
| Steroid hormones | Natural | E2 | 13 | 2.45 | 3.8–188.0 | Influent WWTP | [19,58,60,69][19][58][60][69] |
| E1 | 13 | 3.43 | 12–196 | Effluent WWTP | [23] | ||
| 17α-estradiol | 13.3 | 4.01 | 6.4–12.6 | ||||
| Synthetic | EE2 | 4.8 | 3.67 | 0.59–5.6 | |||
| Industrial and chemical substances | Anticorrosive | Methylbenzotriazole | 366 | 2.72 | nd–2900 | Effluent WWTP | [29] |
| Plasticizer | BPA | 120 | 2.2–3.4 | 0.01–8800 | Bottled water | [5,70][5][70] | |
| 140–12,000 | Surface waster | ||||||
| Microplastics | - | - | - | - | 8.3–3.1 × 105 * | Effluent WWTP | [45,49][45][49] |
| 0.27–30 * | Lake water | ||||||
| 0.05–320 * | River water |
Notes: Units for data with * = million pieces/m3.
References
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990.
- Delgado, N.; Bermeo, L.; Hoyos, D.A.; Peñuela, G.A.; Capparelli, A.; Marino, D.; Navarro, A.; Casas-Zapata, J.C. Occurrence and removal of pharmaceutical and personal care products using subsurface horizontal flow constructed wetlands. Water Res. 2020, 187, 116448.
- Stuart, M.; Lapworth, D. Emerging Organic Contaminants in Groundwater. In Smart Sensors, Measurement and Instrumentation; Mukhopadhyay, S., Mason, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 259–284. ISBN 9783642370069.
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207.
- Wilkinson, J.; Hooda, P.S.; Barker, J.; Barton, S.; Swinden, J. Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field. Environ. Pollut. 2017, 231, 954–970.
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641.
- Wilkinson, J.L.; Hooda, P.S.; Swinden, J.; Barker, J.; Barton, S. Spatial (bio) accumulation of pharmaceuticals, illicit drugs, plasticisers, per fl uorinated compounds and metabolites in river sediment, aquatic plants and benthic organisms *. Environ. Pollut. 2018, 234, 864–875.
- Zheng, C.; Feng, S.; Liu, P.; Fries, E.; Wang, Q.; Shen, Z.; Liu, H.; Zhang, T. Sorption of Organophosphate Flame Retardants on Pahokee Peat Soil. Clean-Soil Air Water 2016, 44, 1163–1173.
- Catherine, H.N.; Tan, K.-H.; Shih, Y.; Doong, R.; Manu, B.; Ding, J. Surface interaction of tetrabromobisphenol A, bisphenol A and phenol with graphene-based materials in water: Adsorption mechanism and thermodynamic effects. J. Hazard. Mater. Adv. 2023, 9, 100227.
- Baloo, L.; Isa, M.H.; Sapari, N.B.; Jagaba, A.H.; Wei, L.J.; Yavari, S.; Razali, R.; Vasu, R. Adsorptive removal of methylene blue and acid orange 10 dyes from aqueous solutions using oil palm wastes-derived activated carbons. Alex. Eng. J. 2021, 60, 5611–5629.
- Birniwa, A.H.; Abubakar, A.S.; Huq, A.K.O.; Mahmud, H.N.M.E. Polypyrrole-polyethyleneimine (Ppy-PEI) nanocomposite: An effective adsorbent for nickel ion adsorption from aqueous solution. J. Macromol. Sci. Part A Pure Appl. Chem. 2021, 58, 206–217.
- Birniwa, A.H.; Kehili, S.; Ali, M.; Musa, H.; Ali, U.; Rahman, S.; Kutty, M.; Jagaba, A.H.; Sa, S.; Tag-eldin, E.M.; et al. Polymer-Based Nano-Adsorbent for the Removal of Lead Ions: Kinetics Studies and Optimization by Response Surface Methodology. Separations 2022, 9, 356.
- Castro, D.; Rosas-Laverde, N.M.; Aldás, M.B.; Almeida-Naranjo, C.; Guerrero, V.H.; Pruna, A.I. Chemical modification of agro-industrial waste-based bioadsorbents for enhanced removal of Zn(II) ions from aqueous solutions. Materials 2021, 14, 2134.
- Almeida-Naranjo, C.E.; Morillo, B.; Aldás, M.B.; Garcés, N.; Debut, A.; Guerrero, V.H. Zinc removal from synthetic waters using magnetite/graphene oxide composites. Remediation 2023, 33, 135–150.
- Sellaoui, L.; Bouzidi, M.; Franco, D.S.P.; Alshammari, A.S.; Gandouzi, M.; Georgin, J.; Mohamed, N.B.H.; Erto, A.; Badawi, M. Exploitation of Bauhinia angmuir residual fruit powder for the adsorption of cationic dyes. Chem. Eng. J. 2023, 456, 141033.
- Paredes-Laverde, M.; Silva-agredo, J.; Torres-palma, R.A. Removal of norfloxacin in deionized, municipal water and urine using rice (Oryza sativa) and coffee (Coffee angmu) husk wastes as natural adsorbents. J. Environ. Manag. 2018, 213, 98–108.
- Quesada, H.B.; Alves Baptista, A.T.; Cusioli, L.F.; Seibert, D.; de Oliveira Bezerra, C.; Bergamasco, R. Surface water pollution by pharmaceuticals and an alternative of Removal by low-cost adsorbents: A review. Chemosphere 2019, 222, 766–780.
- Ebele, A.J.; Abou-Elwafa Abdallah, M.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16.
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640.
- Brausch, J.M.; Rand, G.M. A review of personal care products in the aquatic environment: Environmental concentrations and toxicity. Chemosphere 2011, 82, 1518–1532.
- Dhillon, G.S.; Kaur, S.; Pulicharla, R.; Brar, S.K.; Cledón, M.; Verma, M.; Surampalli, R.Y. Triclosan: Current status, occurrence, environmental risks and bioaccumulation potential. Int. J. Environ. Res. Public Health 2015, 12, 5657–5684.
- Kaur, H.; Bansiwal, A.; Hippargi, G.; Pophali, G.R. Effect of hydrophobicity of pharmaceuticals and personal care products for adsorption on activated carbon: Adsorption isotherms, kinetics and mechanism. Environ. Sci. Pollut. Res. 2017, 25, 20473–20485.
- Adeel, M.; Song, X.; Wang, Y.; Francis, D.; Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environ. Int. 2017, 99, 107–119.
- Kaur, H.; Hippargi, G.; Pophali, G.R.; Bansiwal, A. Biomimetic lipophilic activated carbon for enhanced removal of triclosan from water. J. Colloid Interface Sci. 2019, 535, 111–121.
- Zepon Tarpani, R.R.; Azapagic, A. Life cycle environmental impacts of advanced wastewater treatment techniques for removal of pharmaceuticals and personal care products (PPCPs). J. Environ. Manag. 2018, 215, 258–272.
- Valle-Sistac, J.; Molins-delgado, D.; Díaz, M.; Ibáñez, L.; Barceló, D.; Díaz-cruz, M.S. Determination of parabens and benzophenone-type UV fi lters in human placenta. First description of the existence of benzyl paraben and benzophenone-4. Environ. Int. 2016, 88, 243–249.
- Pietrzak, D.; Kmiecik, E.; Malina, G.; Wa, K. Fate of selected neonicotinoid insecticides in soil e water systems: Current state of the art and knowledge gaps. Chemosphere 2020, 255, 126981.
- Glinski, D.A.; Purucker, S.T.; Van Meter, R.J.; Black, M.C.; Henderson, W.M. Analysis of pesticides in surface water, stemflow, and throughfall in an agricultural area in South Georgia, USA. Chemosphere 2018, 209, 496–507.
- de Souza, R.M.; Seibert, D.; Quesada, H.B.; de Jesus Bassetti, F.; Fagundes-Klen, M.R.; Bergamasco, R. Occurrence, impacts and general aspects of pesticides in surface water: A review. Process Saf. Environ. Prot. 2020, 135, 22–37.
- Villamar-Ayala, C.A.; Carrera-Cevallos, J.V.; Espinoza-Montero, P.J.; Alejandra, C.; Carrera-Cevallos, J.V. Technology Fate, eco-toxicological characteristics, and treatment processes applied to water polluted with glyphosate: A critical review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1476–1514.
- Kaur, Y.; Bhatia, Y.; Chaudhary, S.; Chaudhary, G.R. Comparative performance of bare and functionalize ZnO nanoadsorbents for pesticide removal from aqueous solution. J. Mol. Liq. 2017, 234, 94–103.
- Franco, D.S.P.; Georgin, J.; Lima, E.C.; Silva, L.F.O. Advances made in removing paraquat herbicide by adsorption technology: A review. J. Water Process Eng. 2022, 49, 102988.
- Zavareh, S.; Farrokhzad, Z.; Darvishi, F. Modification of zeolite 4A for use as an adsorbent for glyphosate and as an antibacterial agent for water. Ecotoxicol. Environ. Saf. 2018, 155, 1–8.
- Ilyas, H.; van Hullebusch, E.D. Performance comparison of different constructed wetlands designs for the removal of personal care products. Int. J. Environ. Res. Public Health 2020, 17, 3091.
- Ahmad, J.; Naeem, S.; Ahmad, M.; Usman, A.R.A.; Al-wabel, M.I. A critical review on organic micropollutants contamination in wastewater and removal through carbon nanotubes. J. Environ. Manag. 2019, 246, 214–228.
- Adeel, M.; Yang, Y.S.; Wang, Y.Y.; Song, X.M.; Ahmad, M.A.; Rogers, H. Uptake and transformation of steroid estrogens as emerging contaminants influence plant development. Environ. Pollut. 2018, 243, 1487–1497.
- Diniz, V.; Gasparini Fernandes Cunha, D.; Rath, S. Adsorption of recalcitrant contaminants of emerging concern onto activated carbon: A laboratory and pilot-scale study. J. Environ. Manag. 2023, 325, 116489.
- Palmer, M.; Hatley, H. The role of surfactants in wastewater treatment: Impact, removal and future techniques: A critical review. Water Res. 2018, 147, 60–72.
- Praveena, S.M.; Cheema, M.S.; Guo, H.-R. Non-nutritive artificial sweeteners as an emerging contaminant in environment: A global review and risks perspectives. Ecotoxicol. Environ. Saf. 2019, 170, 699–707.
- Luo, J.; Zhang, Q.; Cao, M.; Wu, L.; Cao, J.; Fang, F.; Li, C. Ecotoxicity and environmental fates of newly recognized contaminants-artificial sweeteners: A review. Sci. Total Environ. 2019, 653, 1149–1160.
- Lapworth, D.J.; Baran, N.; Stuart, M.E.; Ward, R.S. Emerging organic contaminants in groundwater: A review of sources, fate and occurrence. Environ. Pollut. 2012, 163, 287–303.
- Kumar, K.S. Fluorinated Organic Chemicals: A Review. Res. J. Chem. Environ. 2005, 9, 50–79.
- Wang, Y.; Chang, W.; Wang, L.; Zhang, Y.; Zhang, Y.; Wang, M.; Wang, Y.; Li, P. A review of sources, multimedia distribution and health risks of novel fluorinated alternatives. Ecotoxicol. Environ. Saf. 2019, 182, 109402.
- Ivanković, T.; Hrenović, J. Surfactants in the environment. J. Surfactants Environ. 2010, 61, 95–110.
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141.
- Silva, A.B.; Bastos, A.S.; Justino, C.I.L.; Duarte, A.C.; Rocha-santos, T.A.P. Microplastics in the environment: Challenges in analytical chemistry—A review. Anal. Chim. Acta 2018, 1017, 1–19.
- Avio, C.G.; Gorbi, S.; Regoli, F. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 2017, 128, 2–11.
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020, 18, 807–828.
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374.
- Zhou, Y.; Wang, T.; Zhi, D.; Guo, B.; Zhou, Y. Applications of nanoscale zero-valent iron and its composites to the removal of antibiotics: A review. J. Mater. Sci. 2019, 54, 12171–12188.
- Abuwatfa, W.H.; Al-Muqbel, D.; Al-Othman, A.; Halalsheh, N.; Tawalbeh, M. Insights into the removal of microplastics from water using biochar in the era of COVID-19: A mini review. Case Stud. Chem. Environ. Eng. 2021, 4, 100151.
- Shi, X.; Zhang, X.; Gao, W.; Zhang, Y.; He, D. Removal of microplastics from water by magnetic nano-Fe3O4. Sci. Total Environ. 2022, 802, 149838.
- León, G.R.; Aldás, M.B.; Guerrero, V.H.; Landázuri, A.C.; Almeida-Naranjo, C.E. Caffeine and irgasan removal from water using bamboo, laurel and moringa residues impregnated with commercial TiO2 nanoparticles. MRS Adv. 2019, 4, 3553–3567.
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156.
- Almeida-Naranjo, C.E.; Frutos, M.; Tejedor, J.; Cuestas, J.; Valenzuela, F.; Rivadeneira, M.I.; Villamar, C.A.; Guerrero, V.H. Caffeine adsorptive performance and compatibility characteristics (Eisenia foetida Savigny) of agro-industrial residues potentially suitable for vermifilter beds. Sci. Total Environ. 2021, 801, 149666.
- Ghauch, A.; Tuqan, A.M.; Kibbi, N. Ibuprofen removal by heated persulfate in aqueous solution: A kinetics study. Chem. Eng. J. 2012, 197, 483–492.
- Deng, Y.; Ok, Y.S.; Mohan, D.; Pittman, C.U.; Dou, X. Carbamazepine removal from water by carbon dot-modified magnetic carbon nanotubes. Environ. Res. 2018, 169, 434–444.
- Jiang, N.; Erdős, M.; Moultos, O.A.; Shang, R.; Vlugt, T.J.H.; Heijman, S.G.J.; Rietveld, L.C. The adsorption mechanisms of organic micropollutants on high-silica zeolites causing S-shaped adsorption isotherms: An experimental and Monte Carlo simulation study. Chem. Eng. J. 2020, 389, 123968.
- Boudrahem, N.; Delpeux-ouldriane, S.; Khenniche, L. Single and mixture adsorption of clofibric acid, tetracycline and paracetamol onto Activated carbon developed from cotton cloth residue. Process Saf. Environ. Prot. 2017, 111, 544–559.
- Duan, W.; Wang, N.; Xiao, W.; Zhao, Y.; Zheng, Y. Ciprofloxacin adsorption onto different micro-structured tourmaline, halloysite and biotite. J. Mol. Liq. 2018, 269, 874–881.
- Peng, J.; Wang, X.; Yin, F.; Xu, G. Characterizing the removal routes of seven pharmaceuticals in the activated sludge process. Sci. Total Environ. 2019, 650, 2437–2445.
- Kim, H.; Hwang, Y.S.; Sharma, V.K. Adsorption of antibiotics and iopromide onto single-walled and multi-walled carbon nanotubes. Chem. Eng. J. 2014, 255, 23–27.
- Akhtar, J.; Aishah, N.; Amin, S.; Shahzad, K. A review on removal of pharmaceuticals from water by adsorption. Desalin. Water Treat. 2015, 57, 12842–12860.
- Lozano-Morales, V.; Gardi, I.; Nir, S.; Undabeytia, T. Removal of pharmaceuticals from water by clay-cationic starch sorbents. J. Clean. Prod. 2018, 190, 703–711.
- Käfferlein, H.U.; Göen, T.; Angerer, J. Musk xylene: Analysis, occurrence, kinetics, and toxicology. Crit. Rev. Toxicol. 1998, 28, 431–476.
- Meffe, R.; Bustamante, I. De Science of the Total Environment Emerging organic contaminants in surface water and groundwater: A fi rst overview of the situation in Italy. Sci. Total Environ. 2014, 481, 280–295.
- Shoiful, A.; Ueda, Y.; Nugroho, R.; Honda, K. Degradation of organochlorine pesticides (OCPs) in water by iron (Fe)-based materials. J. Water Process Eng. 2016, 11, 110–117.
- Man, Y.B.; Chow, K.L.; Cheng, Z.; Kang, Y.; Wong, M.H. Profiles and removal efficiency of organochlorine pesticides with emphasis on DDTs and HCHs by two different sewage treatment works. Environ. Technol. Innov. 2018, 9, 220–231.
- Honorio, J.F.; Veit, M.T.; Regina, C.; Tavares, G. Alternative adsorbents applied to the removal of natural hormones from pig farming effluents and characterization of the biofertilizer. Environ. Sci. Pollut. Res. 2018, 26, 28429–28435.
- Akhbarizadeh, R.; Dobaradaran, S.; Schmidt, T.C.; Nabipour, I.; Spitz, J. Worldwide bottled water angmuire of emerging contaminants: A review of the recent scientific literature. J. Hazard. Mater. 2020, 392, 122271.
More
