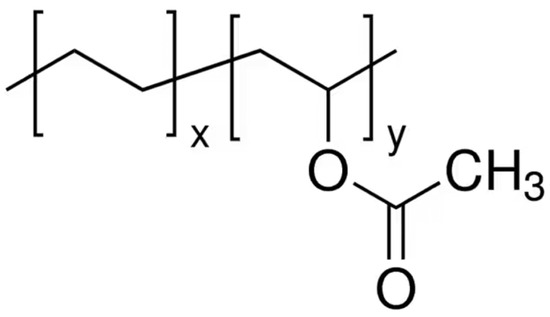
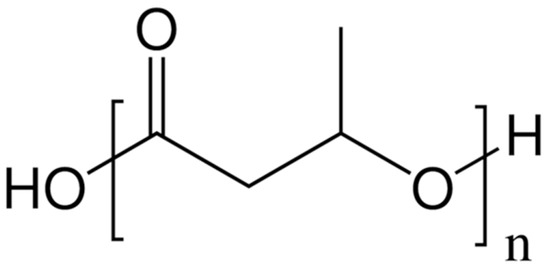
Aliphatic and aromatic polyesters of hydroxycarboxylic acids are characterized not only by biodegradability, but also by biocompatibility and inertness, which makes them suitable for use in different applications. Polyesters with high enzymatic hydrolysis capacity include poly(lactic acid), poly(ε-caprolactone), poly(butylene succinate) and poly(butylene adipate-co-terephthalate), poly(butylene succinate-co-adipate). At the same time, poly(lactic acid) is the most durable, widespread, and cheap polyester from this series. However, it has a number of drawbacks, such as high brittleness, narrow temperature-viscosity processing range, and limited biodegradability. Three main approaches are known for poly(lactic acid) modification: incorporation of dispersed particles or low molecular weight and oligomeric substances, copolymerization with other polymers, and blending with other polymers.
1. Introduction
Poly(lactic acid) or poly(lactide) (PLA) is an aliphatic semi-crystalline polyester obtained from renewable raw materials. As a rule, vegetable starch fermented under the action of microbiota from corn, cassava, sugar cane or sugar beet pulp serve as sources of lactic acid
[1]. It is also possible to obtain lactic acid by chemical means from acetaldehyde and prussic acid. By condensation reaction, a lactide dimer is obtained from oligomerized lactic acid, followed by polymerization of the cyclic lactide dimer with ring opening to polylactic acid
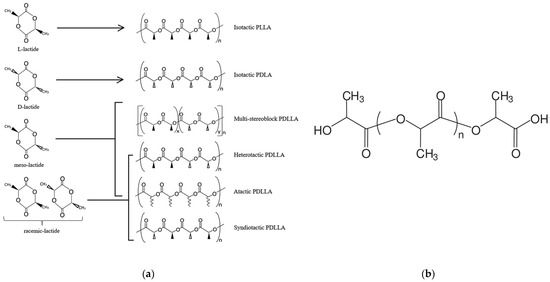
[2]. Due to the fact that lactic acid is able to exist in the form of two enantiomers (D and L), as a result of synthesis, it is possible to obtain poly(L-lactide) and poly(D-lactide), as well as their poly(D,L-lactide) stereocomplex (PDLLA). Most natural enzymes form L-lactide, however, at the stage of oligomerization and cyclization, while partial lactic acid racemization occurs when an isomer of the opposite configuration is formed from one isomer, for example, D-lactide or meso-lactide.
Depending on the ratio of the enantiomers used, various forms of PDLLA can be obtained. These different forms give materials with different degrees of crystallinity, thermal transitions, solubility and decomposition rate
[3]. Due to the high degree of stereoregularity, poly(L-lactide) is a brittle transparent polymer with a melting point of about 170–180 °C and a glass transition temperature of about 63 °C
[4]. Adding other stereoisomers into poly(L-lactide) reduces its melting point, degree of crystallinity, and rate of crystallization of the polymer
[5]. At the same time, the poly(D,L-lactide) stereocomplex (PDLLA) is two interwoven polymer chains, between which additional intermolecular interactions along polar groups are formed and a new highly ordered crystal structure arises. As a result, the melting point increases to 220–230 °C, the thermal resistance and resistance to hydrolytic degradation increase, and the water permeability decreases
[6]. The structure of the lactides and PLA is shown in
Figure 1.
Figure 1.
(
a
) The stereoisomers of lactic acid and the resulting polylactides [7]; ( b
) the chemical structure of poly(lactic acid).
The complex of PLA properties depends primarily on the composition of enantiomers and molecular weight. In general, the physical and mechanical characteristics of PLA are similar to poly(ethylene terephthalate) or poly(styrene) having a high modulus of elasticity (2.7–16 GPa), that is, an ability to heat welding and good technological properties
[8]. However, because PLA has a high fragility, it is unsuitable for the manufacture of flexible polymeric products.
There are several ways to regulate the disadvantages of PLA, one of which is mixing with other polymers
[9][24]. Studying the relationship between the properties of PLA and different polymers allows us to develop polymeric materials with improved properties compared to individual polymers, and achieve synergy in the interaction of components that expands the scope of materials application.
2. Composites of Poly(lactic acid) with Poly(ε-caprolactone)
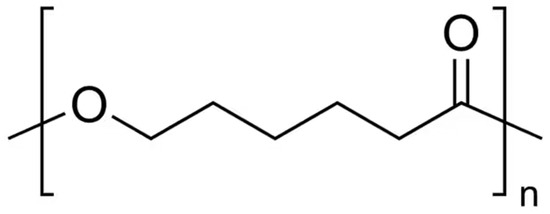
Poly(ε-caprolactone) (PCL) (
Figure 2) is a semi-crystalline aliphatic polyester, which is usually synthesized by ring-opening polymerization from caprolactone monomer, where divalent alcohols and tin (II) or tin salts serve as initiators
[1]. PCL has a high mobility of chain segments with low intermolecular interactions, which leads to a very low melting point (+60 °C) and glass transition point (−60 °C)
[10][25]. Due to its high flexibility, PCL demonstrates mechanical properties similar to those of other conventional non-biodegradable synthetic polymers (for example, poly(ethylene)). PCL is used to create long-term implants, drug delivery systems, and microcellular foams. Lower molecular weighted PCLs are used as plasticizers or compatibilizers for other biopolymers, as well as adhesives for the medical-pharmaceutical industry
[11][26].
Figure 2.
The chemical structure of poly(ε-caprolactone).
Since PCL refers to biodegradable, biocompatible, and non-toxic polymers, the direction of creating materials based on blends of PLA and PCL is actively developing. The main drawbacks of PCL include its low barrier properties and modulus of elasticity. Mixing with PLA partially allows us to improve these drawbacks of PCL. PLA gives PCL an increased strength and rigidity, while PCL provides high-impact strength due to its rubber-like characteristics
[12][13][27,28]. Further, a decrease in water vapor permeability has been proven when adding PLA to PCL
[14][29].
The blends of PLA and PCL have been studied by many researchers around the world. A number of works are devoted to the study of the phase structure of PLA and PCL blends. Considering the structure of such blends, separate researchers have pointed out the presence of interphase layers. N. Noroozi et al.
[15][30] studied the blends of PLA/PCL of various compositions. It was found that when the content of PCL is up to 30 wt.%, uniformly dispersed droplets of almost spherical PCL as a dispersed phase are formed in the blend. However, with an increase in the PCL content, the droplet size increases due to coalescence
[16][31]. A clear interface between the two phases indicates a high immiscibility of this system. With an increase in the PCL content above 50 wt.%, the morphology begins to change into a continuous structure. The morphology change is caused by a significant increase in the coalescence of PCL droplets
[17][32]. The images demonstrate a good level of dispersed phase distribution (PCL) in the PLA matrix. It is also noted that even when using different grades of PLA, with high and low molecular weight, the morphology of PCL droplets is similar. This morphology is also noted in other scientific studies
[18][19][33,34].
3. Composites of Poly(lactic acid) with Poly(glycolic acid)
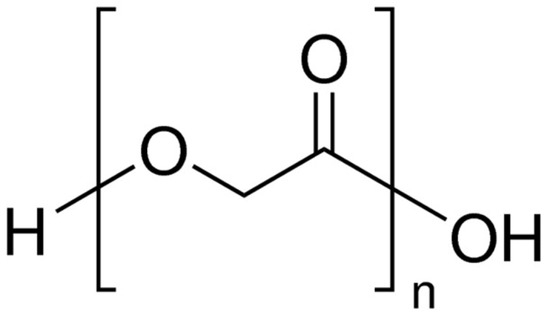
Poly(glycolic acid) (PGA) (
Figure 3) has a glass transition temperature of 35–40 °C, a melting point of 220–230 °C, and a density of 1.53 g/mL
[20][45]. PGA also has an increased degree of crystallinity, about 45–55%, which leads to insolubility in water. The solubility of this polyester varies from the molecular weight and the degree of crystallinity. Its high-molecular form is insoluble in almost all common organic solvents (acetone, dichloromethane, chloroform, ethyl acetate, tetrahydrofuran) with the exception of highly fluorinated organic compounds such as hexafluoroisopropanol
[21][46], while low-molecular oligomers are quite different in their physical properties and are more soluble. The mechanical properties of PGA are much higher than those of other biodegradable plastics and the most traditional plastics. It has a tensile strength of about 115 MPa and a Young’s modulus of about 7 GPa. The flexural strength and elastic modulus of PGA are about 222 MPa and 7.8 GPa, correspondingly. The molecular structure of PGA has a flat zigzag conformation, which may be the reason for high mechanical properties compared to other biopolymers
[22][47]. Poly(glycolic acid) is widely used in medicine, as well as in the fields of packaging and the oil and gas industry
[23][48].
Figure 3.
The chemical structure of poly(glycolic acid).
The presence of hydrolysable ester bonds in the structure of macromolecules makes the polymer capable of enzymatic hydrolysis during composting. According to the studies, the duration of biodegradation for PGA is 1.5–3 months
[24][49]. Therefore, PGA has better mechanical properties and faster degradation than PLA that provides appealing to develop materials based on PLA/PGA blends.
Due to the widespread use of PGA for medical purposes, special attention is paid to nonwovens based on PLA/PGA blends. Y. You et al.
[25][50] have obtained the matrices from ultrafine PLA/PGA fibers by an electrospinning method.
By varying the ratio of PLA/PGA in the blend, it is possible to control the rate of biodegradation
[26][51], as well as the mechanical properties of the materials obtained by an electrospinning method
[27][52]. An acceptable level of physical and mechanical characteristics of PLA/PGA nonwovens allows us to create natural soft tissues, in particular the stretching of blood vessels on their basis. PLA/PGA scaffolds have shown effectiveness in the proliferation and stability of epithelial cells in the intestinal epithelium, and in the treatment of short bowel syndrome
[28][53]. In addition, such materials have shown an increase in the ability to pass the blood-brain barrier
[29][54].
4. Composites of Poly(lactic acid) with Poly(butylene adipate-co-terephthalate)
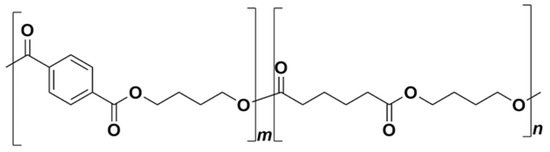
Poly(butylene adipate-co-terephthalate) (PBAT) is a biodegradable statistical copolymer from the class of polyesters, in particular the copolymer of adipic acid, 1,4-butanediol, and terephthalic acid (from dimethyl terephthalate). The structural formula of the PBAT monomer units is shown in
Figure 4.
Figure 4.
The chemical structure of poly(butylene adipate-co-terephthalate).
In terms of technological and operational properties, PBAT is similar to polyethylene; however, its properties can vary in a wide range depending on the composition and molecular weight of the copolymer (a number of adipic acid and terephthalic acid units)
[30][57]. A modulus of elasticity increases, and an elongation at break decreases with an increase in the content of terephthalic units
[31][58]. It has been defined that a tensile strength of PBAT increases while the elongation at break decreases with an increase in molecular weight
[32][33][59,60]. On average, PBAT is characterized by the following parameters: a tensile strength of 20–22 MPa, an elongation at break of 600–700%, a bending strength of 7–8 MPa, and a modulus of elasticity at a bending of 120–130 MPa.
The monomeric composition of PBAT has a significant effect on the rate of biological degradation (mineralization) of the material. It has been shown that the decomposition rate of PBAT under industrial compost at 60 °C depends on the content of terephthalic acid units and aromatic sequences
[34][61]. With an increase in the proportion of terephthalic acid units, a rate of biological decomposition decreases. Longer aromatic oligomers can be biodegradable in the compost conditions only at elevated temperatures.
The statistical structure of PBAT causes an absence of structural ordering, which affects a low degree of crystallinity up to its absence. A flexible-chain nature of the polymer and a low degree of crystallinity, in turn, causes a low modulus of elasticity and stiffness, but high flexibility. Precisely, the flexibility of this polymer makes it promising for mixing with other rigid polymers, for example, with PLA, to give flexibility while maintaining a complete biodegradability of the blends
[35][62].
In addition, PBAT is used for the lamination of cardboard and paper products used as a part of the flexible films for developing highly filled composites with nano- and micro-sized particles, including biobased fillers. The most well-known commercial trademarks of the materials based on PLA/PBAT blends are Ecoflex and Ecovio, both produced by BASF Corporation.
A number of works consider the problems of combining PLA and PBAT
[36][63], as well as a mutual influence of the polymers on the structure and properties of the blends on their basis. Y.Y. Liu et al.
[37][64] have reported that the solubility parameters (δ) of poly(L-lactide) (PLLA) and PBAT are 19.70 and 19.83 J
0.5/cm
1.5, respectively. The proximity of these values suggests that these two polymers are potentially miscible. According to the results of the study, the highest miscibility has been demonstrated for the blend with 25 wt.% of PBAT. However, in subsequent studies, the researchers have pointed out a limited solubility of PBAT in the PLA matrix when added up to 2.5 wt.%
[36][38][63,65]. Above this amount, it is possible to distinguish droplets (domains) separated by phases, the so-called “sea island” morphology
[39][66].
5. Composites of Poly(lactic acid) with Poly(ethylene-co-vinyl acetate)
The copolymer of poly(ethylene-co-vinyl acetate) (EVA) belongs to the class of poly(olefins) and is obtained as a result of copolymerization of poly(ethylene-co-vinyl acetate) monomer (
Figure 5). The properties of EVA vary in a wide range depending on the number of vinyl acetate groups in the chain.
Figure 5.
The chemical structure of poly(ethylene-co-vinyl acetate).
The vinyl acetate content has two fundamental effects affecting the properties of EVA copolymers. The first effect is the destruction of crystalline regions formed by poly(ethylene) segments of the copolymer. Low- and medium-density poly(ethylenes) obtained by volumetric method under high pressure usually have a degree of crystallinity in the range of 40–65%. The degree of crystallinity of poly(ethylene-co-vinyl acetate) gradually decreases as the content of VA units increases, the copolymer with 40–50 wt.% of VA units becomes completely amorphous. The second most important effect of the vinyl acetate content is related with their polar nature. Thus, with an increase in the vinyl acetate content, a polarity of the copolymer changes. Although an increase in polarity is not as important as a decrease in crystallinity, it also leads to certain patterns of behaviour of the material
[40][78]. The molecular structure of EVA determines a wide variety of applications of this copolymer.
6. Composites of Poly(lactic acid) with Poly(3-hydroxybutyrate)
Poly(3-hydroxybutyrate) (PHB) was the first isolated and characterized among poly(hydroxyalkanoates)
[41][94]. It is formed as a carbon reserve in a wide range of producing bacterial strains and is produced industrially by bacterial fermentation
[42][95].
Figure 6 shows the typical chemical structure of poly(3-hydroxybutyrate). PHB is characterized by the presence of a methyl functional group (-CH
3) and a group of ester bonds (−COOR); these functional groups are responsible for the thermoplasticity of materials, hydrophobicity, high crystallinity, and brittleness.
Figure 6.
The chemical structure of poly(3-hydroxybutyrate).
PHB is semi-crystalline polymer due to its linear-chain structure containing both amorphous and crystalline phases. Typical values of the degree of crystallinity range from 50 to 80%
[43][96]. A higher the degree of crystallinity induces more rigidity and durability. The degree of crystallinity can also influence hardness, the modulus of elasticity, density, transparency and a pattern of cold drawing or ductile flows. One of the problems associated with PHB is its narrow temperature range of processing, particularly a small difference between malting and decomposition temperatures, as a result of which this material is subjected to thermal decomposition at temperatures around the melting point. The addition of PLA to PHB provides an increase in the thermal stability of the latter.
PHB has better barrier properties compared to both polyethylene terephthalate (PET) and polyvinyl chloride (PVC); however, it is more rigid and less flexible than traditional synthetic polymers. PHB is characterised by a high ability to biodegrade when the material is in contact with decomposing microorganisms in biologically active media, such as soils, fresh water, and industrial composting conditions, which make it an environmentally friendly alternative to synthetic polymers.
The creation of blends based on PLA and PHB makes it possible to level the disadvantages of each of the polymers. M.K. Patel et al.
[44][97] have described the effect of PHB on barrier properties of PLA in the blends. The materials were obtained from PLA/PHB blends with glycerin triacetate (GTA) as a plasticizer and chitin nanocrystals (CNA) as a structural modifier (increasing the crystallinity). The blend of 75PLA/25PHB showed the formation of many small spherulites of PHB with the highest crystallinity of PHB among the studied composites and was chosen as a matrix for nanocomposites with CAN.
GTA has a negative effect on thermal properties. CNA acts as a nucleating agent for the PHB phase and lead to the formation of well-dispersed PHB spherulites in the PLA phase and a significant increase in the crystallization rate and degree of crystallinity. The nanocomposite with the highest crystallinity is characterized by significantly reduced permeability to oxygen and carbon dioxide and increased mechanical properties compared to the non-filled blend. A significant improvement in crystallinity and the formation of homogeneous spherulites was reflected in the excellent barrier characteristics of nanocomposites.