Neuroblastoma is the most prevalent extracranial solid tumor in pediatric patients, originating from sympathetic nervous system cells. Metastasis can be observed in approximately 70% of individuals after diagnosis, and the prognosis is poor. The current care methods used, which include surgical removal as well as radio and chemotherapy, are largely unsuccessful, with high mortality and relapse rates. Marine cyanobacteria are a key source of physiologically active metabolites, which have recently received attention owing to their anticancer potential. Marine peptides possess several advantages over proteins or antibodies, including small size, simple manufacturing, cell membrane crossing capabilities, minimal drug–drug interactions, minimal changes in blood–brain barrier (BBB) integrity, selective targeting, chemical and biological diversities, and effects on liver and kidney functions.
- marine cyanopeptides
- apoptosis
- autophagy
- cell cycle arrest
- antimetastatic
1. Introduction
2. Marine Cyanobacterial Peptides
Cyanobacteria, which are among the oldest aquatic and photosynthetic oxygenic prokaryotes, are found worldwide. The presence of numerous bioactive secondary metabolites in cyanobacteria from various habitats, especially marine cyanobacteria, has recently been discovered. Bioactive compounds from aquatic cyanobacteria help them better adapt to a variety of complex, hypersaline, high-pressure, barren marine habitats by acting as chemical defenses. These cyanobacterial secondary metabolites exhibit a wide range of biological activities, including anti-tumor, antibacterial, enzyme inhibition, parasite resistance, anti-inflammatory, and other biological activities, in addition to having a significant impact on the growth and reproduction of cyanobacteria [13][63]. As a result, they received interest from scholars in various experimental fields, including medicinal chemistry, pharmacology, and marine chemical ecology [14][64]. Over 400 new natural compounds from marine cyanobacteria have been identified over the past decade thanks to the International Cooperative Biodiversity Group (ICGB) program [15][65]. Peptides and compounds containing peptides are the main secondary metabolites among these substances [16][66]. A total of 126 novel peptide compounds, mostly from the genera Lyngbya, Oscillatoria, and Symploca, were extracted from marine cyanobacteria by the end of 2016. Nevertheless, two new genera, Moorea and Okeania, previously recognized as the polyphyletic cyanobacterial genus Lyngbya, were identified by genome sequence analysis [17][18][67,68]. The majority of the cyclic peptides found in marine cyanobacteria are cyclic depsipeptides, which include 76 different molecules [19][69]. Two linear depsipeptides known as grassystatins A and B, have been isolated from the key Largo collected marine cyanobacterium Okeania lorea (formerly Lyngbya cf. confervoides). Veraguamides K and L, two linear bromine-containing depsipeptides isolated from the marine cyanobacterium cf. Oscillatoria margaritifera found in the Coiba Island National Park in Panama, are thought to have the structural characteristics of marine natural products [20][70]. The antimalarial bioassay-guided isolation of the marine cyanobacterium Moorea producens (formerly Lyngbya majuscula) yielded four lipopeptides: dragonamides A and B, carmabin A, and dragomabin [21][49]. Through the cytotoxicity-directed isolation of a marine cyanobacterium, the Symploca cf. hydnoides sample from Cetti Bay (Guam), seven novel cyclic hexadepsipeptides, known as veraguamides A–G, were discovered [22][23][71,72]. HT29 colorectal adenocarcinoma and HeLa cell lines exhibited moderate-to-mild cytotoxicity in response to these compounds [24][73]. Lyngbya majuscula has been proven to be a highly prolific species of cyanobacterium since a significant number of natural products with a wide range of structural characteristics have been isolated from it. The antimycobacterial cyclodepsipeptides known as pitipeptolides C–F were discovered from the marine cyanobacterium Lyngbya majuscule in the Piti Bomb Holes (Guam) [25][74]. Hoiamide A is an unusual cyclic depsipeptide that was isolated from the marine cyanobacteria Lyngbya majuscula and Phormidium gracile in Papua New Guinea. It is composed of an isoleucine moiety that has been modified by acetate and S-adenosyl methionine, a tri-heterocyclic fragment which contains two α-methylated thiazolines and one thiazole ring.3. Mechanistic Insights
3.1. Apoptosis
Apoptosis is an essential mechanism of cell death induced by cancer therapy. Therefore, identifying or developing anticancer agents capable of targeting apoptosis regulatory genes is a prerequisite for the advancement of unique anticancer therapies. As with most anticancer agents, there are a large number of marine-derived anticancer peptides with apoptotic activity in cancer cells [26][27][76,77]. The discharge of cytochrome-c (cyt c) activates caspases and triggers apoptosis [28][29][78,79]. Cyclolaxaphycins B and B3 increase caspase 3 in SH-SY5Y lines with IC50 of 1.8 and 0.8 µM, respectively (Figure 1) [30][61]. Coibamide A induces apoptosis in Neuro-2a cells (IC50 < 23 nM) through caspase-3,7 activation, cyt-c release and PARP cleavage. In U87-MG and SF-295 glioma cells, coibamide A triggered caspase-3/7 activation over a time-period associated with a loss of viability, although the activation profile for each cell line was different. Despite the fact that the MTT cell viability experiments showed that U87-MG cells were more sensitive than SF-295 cells to coibamide A-induced cell death, relatively large doses of coibamide A were required to cause the late activation of caspase-3/7 in these cells. Over a 96 h exposure period, researchers collected attached and detached coibamide A-treated cells and examined cell lysates for the expression of PARP1, a critical downstream target of caspase-dependent apoptosis, as well as a number of alternative cell death pathways [31][32][33][38,57,58].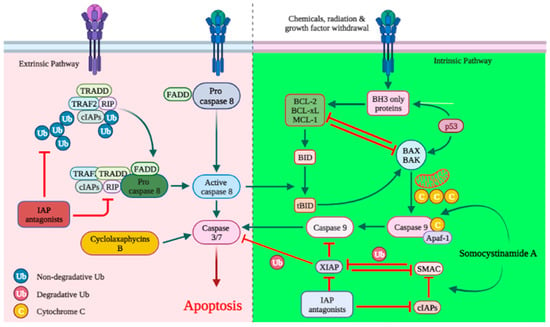
3.2. Cell Cycle Arrest
3.3. Sodium Channel Blocking Activity
3.4. Antimetastatic Activity
Microfilaments play an essential role in cell migration. The inhibition of actin polymerization disrupts microfilaments, reduces the cell motility, and slows the metastatic spread of neoplastic cells by G2/M phase arrest [45][46][90,91]. Microtubules and microtubule-associated proteins, which play a vital role in cell division, are essential constituents of the mitotic spindle. Microtubule dynamics is necessary for chromosomal movement throughout anaphase. A shift in the tubulin-microtubule balance alters the mitotic spindle, disrupting metaphase-anaphase progression of the cell cycle, resulting in cell death [47][48][92,93]. Microtubule-stabilizing compounds stimulate microtubule polymerization and, by binding to microtubules, target the cytoskeleton and spindle machinery of tumor cells, thus limiting mitosis [47][49][92,94]. Aurilide B-C, a cyclodepsipeptide isolated from Lyngbya majuscula, has been shown to destabilize microtubules in Neuro-2a cells with an IC50 of 0.01 and 0.05, μM, respectively [50][30]. STAT3 suppression induces apoptosis and inhibits metastasis in cancer cells. MMP2 and MMP9 are upregulated when the STAT3 pathway is activated, facilitating tumor invasion [51][97]. Apratoxin A is proposed to inhibit the phosphorylation of the signal transducer and activator of transcription (STAT) 3, causing metastasis in Neuro-2a cells with an IC50 of 1 µM [52][53][42,98]. Proteases are critical signaling molecules engaged in a variety of key processes such as apoptosis, metastasis and angiogenesis [54][55][56][99,100,101]. Serine proteases are highly expressed in NB [57][102]. Numerous cyanobacterial peptides have been shown to interfere with serine protease functions.3.5. Antiangiogenic Effect
VEGF and MMPs play an essential role in angiogenesis [58][59][104,105]. Angiogenesis is believed to be a fundamental prerequisite for the development, invasion, and metastasis of malignant NBs. Anti-angiogenic agents that inhibit neovascularization could represent a potential therapeutic strategy for NB [60][106]. The marine peptide coibamide A inhibits cancer cell migration by lowering the VEGFR2 and MMP-9 expressions [32][33][57,58].3.6. Autophagy
In the early stages of cancer, autophagy functions as a barrier to protect cells against damaging stimuli and malignant development [61][62][107,108]. The activation of mTOR inhibits autophagy induction and promotes tumor growth and metastasis (Figure 23). Therefore, the regulation of autophagy with mTOR inhibitors provides an anticancer effect [63][109]. AMPK activates the autophagy-initiating kinase Ulk1 and phosphorylates TSC2. TSC2 activation can inhibit the mTOR complex 1 (mTORC1), thus promoting autophagy [64][110].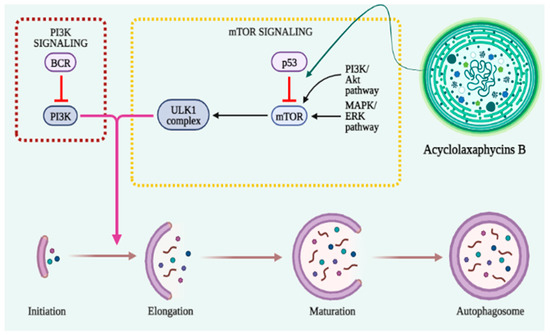
3.7. Unknown Mechanisms for Anticancer Activity
Several peptides including floridamide [65][55], guineamides B–C and G [66][67][32,33], hermitamides A–B [68][45], hoiamide A [69][36], jamaicamides A–C [70][46], and tiahuramides B–C [71][34], isolated from Lyngbya majuscula, bouillonamide [52][42], ulongamide A [52][42], isolated from Lyngbya bouillonii (now called Moorea bouillonii) [72][111]; wewakpeptin A–D [73][56] isolated from Lyngbya semiplena; dragonamides C and D [21][49], microcolin A–B, and desacetylmicrocolin B [74][50] isolated from Lyngbya polychroa all display significant cytotoxicity, though their specific modes of action have yet to be characterized. ReferencesReferences
- Chung, C. Neuroblastoma. Pediatr. Blood Cancer 2021, 68, e28473. [Google Scholar] [CrossRef]
- Lucas, J.T.; Wakefield, D.V.; Doubrovin, M.; Li, Y.; Santiago, T.; Federico, S.M.; Merchant, T.E.; Davidoff, A.M.; Krasin, M.J.; Shulkin, B.L.; et al. Risk factors associated with metastatic site failure in patients with high-risk neuroblastoma. Clin. Transl. Radiat. Oncol. 2022, 34, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Wang, W.; Liu, G.; Wang, X.; Xian, W.; McKeon, F.; Foster, J.; Zhou, J.; Zhang, R. Molecular targeting therapies for neuroblastoma: Progress and challenges. Med. Res. Rev. 2020, 41, 961–1021. [Google Scholar] [CrossRef] [PubMed]
- Fati, F.; Pulvirenti, R.; Paraboschi, I.; Martucciello, G. Surgical Approaches to Neuroblastoma: Review of the Operative Techniques. Children 2021, 8, 446. [Google Scholar] [CrossRef] [PubMed]
- Salemi, F.; Alam, W.; Hassani, M.S.; Hashemi, S.Z.; Jafari, A.A.; Mirmoeeni, S.M.S.; Arbab, M.; Mortazavizadeh, S.M.R.; Khan, H. Neuroblastoma: Essential genetic pathways and current therapeutic options. Eur. J. Pharmacol. 2022, 926, 175030. [Google Scholar] [CrossRef]
- Yun, C.W.; Kim, H.J.; Lee, S.H. Therapeutic Application of Diverse Marine-derived Natural Products in Cancer Therapy. Anticancer. Res. 2019, 39, 5261–5284. [Google Scholar] [CrossRef]
- Dayanidhi, D.L.; Thomas, B.C.; Osterberg, J.S.; Vuong, M.; Vargas, G.; Kwartler, S.K.; Schmaltz, E.; Dunphy-Daly, M.M.; Schultz, T.F.; Rittschof, D.; et al. Exploring the Diversity of the Marine Environment for New Anti-cancer Compounds. Front. Mar. Sci. 2021, 7, 614766. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospecting 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Cappello, E.; Nieri, P. From Life in the Sea to the Clinic: The Marine Drugs Approved and under Clinical Trial. Life 2021, 11, 1390. [Google Scholar] [CrossRef]
- Pereira, R.B.; Evdokimov, N.M.; Lefranc, F.; Valentão, P.; Kornienko, A.; Pereira, D.M.; Andrade, P.B.; Gomes, N.G.M. Marine-Derived Anticancer Agents: Clinical Benefits, Innovative Mechanisms, and New Targets. Mar. Drugs 2019, 17, 329. [Google Scholar] [CrossRef]
- Ahmed, I.; Asgher, M.; Sher, F.; Hussain, S.M.; Nazish, N.; Joshi, N.; Sharma, A.; Parra-Saldívar, R.; Bilal, M.; Iqbal, H.M.N. Exploring Marine as a Rich Source of Bioactive Peptides: Challenges and Opportunities from Marine Pharmacology. Mar. Drugs 2022, 20, 208. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Choi, M.-C.; Seo, C.H.; Park, Y. Therapeutic Properties and Biological Benefits of Marine-Derived Anticancer Peptides. Int. J. Mol. Sci. 2018, 19, 919. [Google Scholar] [CrossRef] [PubMed]
- Hachicha, R.; Elleuch, F.; Ben Hlima, H.; Dubessay, P.; de Baynast, H.; Delattre, C.; Pierre, G.; Hachicha, R.; Abdelkafi, S.; Michaud, P.; et al. Biomolecules from Microalgae and Cyanobacteria: Applications and Market Survey. Appl. Sci. 2022, 12, 1924. [Google Scholar] [CrossRef]
- Qamar, H.; Hussain, K.; Soni, A.; Khan, A.; Hussain, T.; Chénais, B. Cyanobacteria as Natural Therapeutics and Pharmaceutical Potential: Role in Antitumor Activity and as Nanovec-tors. Molecules 2021, 26, 247. [Google Scholar] [CrossRef] [PubMed]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of Current Potentials and Applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef]
- Ahmed, S.; Mirzaei, H.; Aschner, M.; Khan, A.; Al-Harrasi, A.; Khan, H. Marine peptides in breast cancer: Therapeutic and mechanistic understanding. Biomed. Pharmacother. 2021, 142, 112038. [Google Scholar] [CrossRef]
- Ahmed, S.; Hasan, M.M.; Aschner, M.; Mirzaei, H.; Alam, W.; Shah, S.M.M.; Khan, H. Therapeutic potential of marine peptides in glioblastoma: Mechanistic insights. Cell. Signal. 2021, 87, 110142. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, H.; Fakhri, S.; Aschner, M.; Cheang, W.S. Therapeutic potential of marine peptides in cervical and ovarian cancers. Mol. Cell. Biochem. 2021, 477, 605–619. [Google Scholar] [CrossRef]
- Ahmed, S.; Alam, W.; Jeandet, P.; Aschner, M.; Alsharif, K.F.; Saso, L.; Khan, H. Therapeutic Potential of Marine Peptides in Prostate Cancer: Mechanistic Insights. Mar. Drugs 2022, 20, 466. [Google Scholar] [CrossRef]
- Chiangjong, W.; Chutipongtanate, S.; Hongeng, S. Anticancer peptide: Physicochemical property, functional aspect and trend in clinical application (Review). Int. J. Oncol. 2020, 57, 678–696. [Google Scholar] [CrossRef]
- Kumla, D.; Sousa, M.E.; Vasconcelos, V.; Kijjoa, A. Chapter 2—Specialized metabolites from cyanobacteria and their biological activities. In The Pharmacological Poten-tial of Cyanobacteria; Lopes, G., Silva, M., Vasconcelos, V., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 21–54. [Google Scholar]
- Singh, R.; Parihar, P.; Singh, M.; Bajguz, A.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Uncovering Potential Applications of Cyanobacteria and Algal Metabolites in Biology, Agriculture and Medicine: Current Status and Future Prospects. Front. Microbiol. 2017, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Macedo, M.W.F.S.; da Cunha, N.B.; Carneiro, J.A.; da Costa, R.A.; de Alencar, S.A.; Cardoso, M.H.; Franco, O.L.; Dias, S.C. Marine Organisms as a Rich Source of Biologically Active Peptides. Front. Mar. Sci. 2021, 8, 667764. [Google Scholar] [CrossRef]
- Zhang, J.-N.; Xia, Y.-X.; Zhang, H.-J. Natural Cyclopeptides as Anticancer Agents in the Last 20 Years. Int. J. Mol. Sci. 2021, 22, 3973. [Google Scholar] [CrossRef]
- Choi, J.-S.; Joo, S.H. Recent Trends in Cyclic Peptides as Therapeutic Agents and Biochemical Tools. Biomol. Ther. 2020, 28, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Sukmarini, L. Natural Bioactive Cyclopeptides from Microbes as Promising Anticancer Drug Leads: A Mini-review. Indones. J. Pharm. 2021, 32, 291–303. [Google Scholar] [CrossRef]
- Abdalla, M.A.; McGaw, L.J. Natural Cyclic Peptides as an Attractive Modality for Therapeutics: A Mini Review. Molecules 2018, 23, 2080. [Google Scholar] [CrossRef]
- Bera, S.; Mondal, D. Natural Cyclic Peptides as Clinical and Future Therapeutics. Curr. Org. Chem. 2019, 23, 38–75. [Google Scholar] [CrossRef]
- Sivanathan, S.; Scherkenbeck, J. Cyclodepsipeptides: A Rich Source of Biologically Active Compounds for Drug Research. Molecules 2014, 19, 12368–12420. [Google Scholar] [CrossRef]
- Han, B.; Gross, H.; Goeger, D.E.; Mooberry, S.L.; Gerwick, W.H. Aurilides B and C, Cancer Cell Toxins from a Papua New Guinea Collection of the Marine Cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2006, 69, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.L.; Nogle, L.M.; Media, J.; Valeriote, F.A.; Mooberry, S.L.; Gerwick, W.H. Desmethoxymajusculamide C, a Cyanobacterial Depsipeptide with Potent Cytotoxicity in Both Cyclic and Ring-Opened Forms. J. Nat. Prod. 2009, 72, 1011–1016. [Google Scholar] [CrossRef]
- Tan, L.T.; Sitachitta, N.; Gerwick, W.H. The Guineamides, Novel Cyclic Depsipeptides from a Papua New Guinea Collection of the Marine Cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2003, 66, 764–771. [Google Scholar] [CrossRef]
- Han, B. Wewakamide A and Guineamide G, Cyclic Depsipeptides from the Marine Cyanobacteria Lyngbya semiplena and Lyngbya majuscula. J. Microbiol. Biotechnol. 2011, 21, 930–936. [Google Scholar] [CrossRef]
- Levert, A.; Alvariño, R.; Bornancin, L.; Abou Mansour, E.; Burja, A.M.; Genevière, A.M.; Bonnard, I.; Alonso, E.; Botana, L.; Banaigs, B. Structures and activities of tiahuramides A–C, cyclic depsipeptides from a Tahitian collection of the marine cyano-bacterium Lyngbya majuscula. J. Nat. Prod. 2018, 81, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Nunnery, J.K.; Engene, N.; Esquenazi, E.; Byrum, T.; Dorrestein, P.C.; Gerwick, W.H. Palmyramide A, a Cyclic Depsipeptide from a Palmyra Atoll Collection of the Marine Cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Bose, S.; Banerjee, S.; Patra, J.; Malik, J.; Mandal, S.; Kilpatrick, K.; Das, G.; Kerry, R.; Fimognari, C.; et al. Marine Cyanobacteria and Microalgae Metabolites—A Rich Source of Potential Anticancer Drugs. Mar. Drugs 2020, 18, 476. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; McPhail, K.L.; Gross, H.; Goeger, D.E.; Mooberry, S.L.; Gerwick, W.H. Isolation and structure of five lyngbyabellin derivatives from a Papua New Guinea collection of the marine cyanobacterium Lyngbya majuscula. Tetrahedron 2005, 61, 11723–11729. [Google Scholar] [CrossRef]
- Medina, R.A.; Goeger, D.E.; Hills, P.; Mooberry, S.L.; Huang, N.; Romero, L.I.; Ortega-Barría, E.; Gerwick, W.H.; McPhail, K.L. Coibamide A, a potent antiproliferative cyclic depsipeptide from the Panamanian marine cyanobacterium Leptolyngbya sp. J. Am. Chem. Soc. 2008, 130, 6324–6325. [Google Scholar] [CrossRef]
- Thornburg, C.C.; Thimmaiah, M.; Shaala, L.A.; Hau, A.M.; Malmo, J.M.; Ishmael, J.E.; Youssef, D.T.A.; McPhail, K.L. Cyclic Depsipeptides, Grassypeptolides D and E and Ibu-epidemethoxylyngbyastatin 3, from a Red Sea Leptolyngbya Cyanobacterium. J. Nat. Prod. 2011, 74, 1677–1685. [Google Scholar] [CrossRef]
- Linington, R.G.; Edwards, D.J.; Shuman, C.F.; McPhail, K.L.; Matainaho, T.; Gerwick, W.H. Symplocamide A, a potent cytotoxin and chymotrypsin inhibitor from the marine Cyanobacterium Symploca sp. J. Nat. Prod. 2008, 71, 22–27. [Google Scholar] [CrossRef]
- Taori, K.; Paul, V.J.; Luesch, H. Structure and Activity of Largazole, a Potent Antiproliferative Agent from the Floridian Marine Cyanobacterium Symploca sp. J. Am. Chem. Soc. 2008, 130, 1806–1807. [Google Scholar] [CrossRef]
- Tan, L.T.; Okino, T.; Gerwick, W.H. Bouillonamide: A Mixed Polyketide–Peptide Cytotoxin from the Marine Cyanobacterium Moorea bouillonii. Mar. Drugs 2013, 11, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. Lipopeptides: From self-assembly to bioactivity. Chem. Commun. 2015, 51, 8574–8583. [Google Scholar] [CrossRef]
- Barreca, M.; Spanò, V.; Montalbano, A.; Cueto, M.; Marrero, A.R.D.; Deniz, I.; Erdoğan, A.; Bilela, L.L.; Moulin, C.; Taffin-De-Givenchy, E.; et al. Marine Anticancer Agents: An Overview with a Particular Focus on Their Chemical Classes. Mar. Drugs 2020, 18, 619. [Google Scholar] [CrossRef]
- Tan, L.T.; Okino, T.; Gerwick, W.H. Hermitamides A and B, Toxic Malyngamide-Type Natural Products from the Marine Cya-nobacterium Lyngbya majuscula. J. Nat. Prod. 2000, 63, 952–955. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.J.; Marquez, B.L.; Nogle, L.M.; McPhail, K.; Goeger, D.E.; Roberts, M.A.; Gerwick, W.H. Structure and Biosynthesis of the Jamaicamides, New Mixed Polyketide-Peptide Neurotoxins from the Marine Cyanobacterium Lyngbya majuscula. Chem. Biol. 2004, 11, 817–833. [Google Scholar] [CrossRef] [PubMed]
- Gross, H.; McPhail, K.L.; Goeger, D.E.; Valeriote, F.A.; Gerwick, W.H. Two cytotoxic stereoisomers of malyngamide C, 8-epi-malyngamide C and 8-O-acetyl-8-epi-malyngamide C, from the marine cyanobacterium Lyngbya majuscula. Phytochemistry 2010, 71, 1729–1735. [Google Scholar] [CrossRef]
- Wrasidlo, W.; Mielgo, A.; Torres, V.A.; Barbero, S.; Stoletov, K.; Suyama, T.L.; Klemke, R.L.; Gerwick, W.H.; Carson, D.A.; Stupack, D.G. The marine lipopeptide somocystinamide A triggers apoptosis via caspase 8. Proc. Natl. Acad. Sci. USA 2008, 105, 2313–2318. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; Ross, C.; Paul, V.J.; Matthew, S.; Luesch, H. Dragonamides C and D, Linear Lipopeptides from the Marine Cyanobacterium Brown Lyngbya Polychroa. J. Nat. Prod. 2008, 71, 887–890. [Google Scholar] [CrossRef]
- Meickle, T.; Matthew, S.; Ross, C.; Luesch, H.; Paul, V. Bioassay-guided isolation and identification of desacetylmicrocolin B from Lyngbya cf. polychroa. Planta Med. 2009, 75, 1427–1430. [Google Scholar] [CrossRef]
- Roxin, Á.; Zheng, G. Flexible or fixed: A comparative review of linear and cyclic cancer-targeting peptides. Futur. Med. Chem. 2012, 4, 1601–1618. [Google Scholar] [CrossRef]
- Linington, R.G.; Clark, B.R.; Trimble, E.E.; Almanza, A.; Ureña, L.-D.; Kyle, D.E.; Gerwick, W.H. Antimalarial Peptides from Marine Cyanobacteria: Isolation and Structural Elucidation of Gallinamide A. J. Nat. Prod. 2008, 72, 14–17. [Google Scholar] [CrossRef]
- Jones, M.R.; Pinto, E.; Torres, M.A.; Dörr, F.; Mazur-Marzec, H.; Szubert, K.; Tartaglione, L.; Dell’Aversano, C.; Miles, C.O.; Beach, D.G.; et al. CyanoMetDB, a comprehensive public database of secondary metabolites from cyanobacteria. Water Res. 2021, 196, 117017. [Google Scholar] [CrossRef]
- Sato, S.-I.; Murata, A.; Orihara, T.; Shirakawa, T.; Suenaga, K.; Kigoshi, H.; Uesugi, M. Marine Natural Product Aurilide Activates the OPA1-Mediated Apoptosis by Binding to Prohibitin. Chem. Biol. 2011, 18, 131–139. [Google Scholar] [CrossRef]
- Sabry, O.M.; Goeger, D.E.; Gerwick, W.H. Biologically active new metabolites from a Florida collection of Moorea producens. Nat. Prod. Res. 2017, 31, 555–561. [Google Scholar] [CrossRef]
- Han, B.; Goeger, D.; Maier, C.S.; Gerwick, W.H. The Wewakpeptins, Cyclic Depsipeptides from a Papua New Guinea Collection of the Marine Cyanobacterium Lyngbya semiplena. J. Org. Chem. 2005, 70, 3133–3139. [Google Scholar] [CrossRef] [PubMed]
- Hau, A.M.; Greenwood, J.A.; Löhr, C.V.; Serrill, J.D.; Proteau, P.J.; Ganley, I.G.; McPhail, K.L.; Ishmael, J.E. Coibamide A induces mTOR-independent autophagy and cell death in human glioblastoma cells. PLoS ONE 2013, 8, e65250. [Google Scholar] [CrossRef] [PubMed]
- Serrill, J.D.; Wan, X.; Hau, A.M.; Jang, H.S.; Coleman, D.J.; Indra, A.K.; Alani, A.W.G.; McPhail, K.L.; Ishmael, J.E. Coibamide A, a natural lariat depsipeptide, inhibits VEGFA/VEGFR2 expression and suppresses tumor growth in glioblastoma xenografts. Investig. New Drugs 2015, 34, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, X.; Zhou, Y.; Zhang, K.; Lu, Y.; Liu, J.; Huang, Y.; Wang, G.; Jiang, S.; Zhou, G. Suppression of Musashi-2 by the small compound largazole exerts inhibitory effects on malignant cells. Int. J. Oncol. 2020, 56, 1274–1283. [Google Scholar] [CrossRef]
- Al-Awadhi, F.H.; Salvador-Reyes, L.A.; Elsadek, L.A.; Ratnayake, R.; Chen, Q.Y.; Luesch, H. Largazole is a Brain-Penetrant Class I HDAC Inhibitor with Extended Applicability to Glioblastoma and CNS Diseases. ACS Chem. Neurosci. 2020, 11, 1937–1943. [Google Scholar] [CrossRef]
- Alvariño, R.; Alonso, E.; Bornancin, L.; Bonnard, I.; Inguimbert, N.; Banaigs, B.; Botana, L. Biological Activities of Cyclic and Acyclic B-Type Laxaphycins in SH-SY5Y Human Neuroblastoma Cells. Mar. Drugs 2020, 18, 364. [Google Scholar] [CrossRef]
- Nogle, L.M.; Gerwick, W.H. Somocystinamide A, a Novel Cytotoxic Disulfide Dimer from a Fijian Marine Cyanobacterial Mixed Assemblage. Org. Lett. 2002, 4, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W.; Mahmood, N.A.; Hyde, E.G. Natural toxins from cyanobacteria (blue-green algae). In Marine Toxins; ACS Publications: Washington, DC, USA, 1990; pp. 87–106. [Google Scholar]
- Martins, J.; Vasconcelos, V. Cyanobactins from Cyanobacteria: Current Genetic and Chemical State of Knowledge. Mar. Drugs 2015, 13, 6910–6946. [Google Scholar] [CrossRef] [PubMed]
- Suffness, M.; Cragg, G.G.; Grever, M.M.; Grifo, F.F.; Johnson, G.; Mead, J.A.R.; Schepartz, S.S.; Venditti, J.J.; Wolpert, M. The National Cooperative Natural Products Drug Discovery Group (NCNPDDG) and International Cooperative Biodiversity Group (ICBG) Programs. Int. J. Pharmacogn. 1995, 33, 5–16. [Google Scholar] [CrossRef]
- Tan, L.T. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry 2007, 68, 954–979. [Google Scholar] [CrossRef]
- Engene, N.; Rottacker, E.C.; Kaštovský, J.; Byrum, T.; Choi, H.; Ellisman, M.H.; Komárek, J.; Gerwick, W.H. Moorea producens gen. nov., sp. nov. and Moorea bouillonii comb. nov., tropical marine cyanobacteria rich in bio-active secondary metabolites. Int. J. Syst. Evol. Microbiol. 2012, 62 Pt 5, 1171. [Google Scholar] [CrossRef] [PubMed]
- Engene, N.; Paul, V.J.; Byrum, T.; Gerwick, W.H.; Thor, A.; Ellisman, M.H. Five chemically rich species of tropical marine cyanobacteria of the genus O keania gen. nov. (O scillatoriales, C yanoprokaryota). J. Phycol. 2013, 49, 1095–1106. [Google Scholar] [CrossRef]
- Lee, Y.; Phat, C.; Hong, S.-C. Structural diversity of marine cyclic peptides and their molecular mechanisms for anticancer, antibacterial, antifungal, and other clinical applications. Peptides 2017, 95, 94–105. [Google Scholar] [CrossRef]
- Mevers, E.; Liu, W.-T.; Engene, N.; Mohimani, H.; Byrum, T.; Pevzner, P.A.; Dorrestein, P.C.; Spadafora, C.; Gerwick, W.H. Cytotoxic Veraguamides, Alkynyl Bromide-Containing Cyclic Depsipeptides from the Marine Cyanobacterium cf. Oscillatoria margaritifera. J. Nat. Prod. 2011, 74, 928–936. [Google Scholar] [CrossRef]
- Mi, Y.; Zhang, J.; He, S.; Yan, X. New Peptides Isolated from Marine Cyanobacteria, an Overview over the Past Decade. Mar. Drugs 2017, 15, 132. [Google Scholar] [CrossRef] [PubMed]
- Nikapitiya, C. Bioactive Secondary Metabolites from Marine Microbes for Drug Discovery. Adv. Food Nutr. Res. 2012, 65, 363–387. [Google Scholar] [CrossRef]
- Salvador, L.A.; Biggs, J.S.; Paul, V.J.; Luesch, H. Veraguamides A− G, cyclic hexadepsipeptides from a dolastatin 16-producing cyanobacterium Symploca cf. hydnoides from Guam. J. Nat. Prod. 2011, 74, 917–927. [Google Scholar] [CrossRef]
- Montaser, R.; Paul, V.J.; Luesch, H. Pitipeptolides C–F, antimycobacterial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula from Guam. Phytochemistry 2011, 72, 2068–2074. [Google Scholar] [CrossRef]
- Pereira, A.; Cao, Z.; Murray, T.F.; Gerwick, W.H. Hoiamide a, a sodium channel activator of unusual architecture from a consortium of two papua new Guinea cyanobacteria. Chem. Biol. 2009, 16, 893–906. [Google Scholar] [CrossRef]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and Molecular Targeting Therapy in Cancer. BioMed. Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Shi, Y. Caspase activation, inhibition, and reactivation: A mechanistic view. Protein Sci. Publ. Protein Soc. 2004, 13, 1979–1987. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, X. Cytochrome C-Mediated Apoptosis. Annu. Rev. Biochem. 2004, 73, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Stupack, D.G.; Teitz, T.; Potter, M.D.; Mikolon, D.; Houghton, P.J.; Kidd, V.J.; Lahti, J.M.; Cheresh, D.A. Potentiation of neuroblastoma metastasis by loss of caspase-8. Nature 2006, 439, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Tummers, B.; Green, D.R. Caspase-8: Regulating life and death. Immunol. Rev. 2017, 277, 76–89. [Google Scholar] [CrossRef]
- Herkenne, S.; Scorrano, L. OPA1, a new mitochondrial target in cancer therapy. Aging 2020, 12, 20931–20933. [Google Scholar] [CrossRef]
- MacArthur, I.; Bei, Y.; Garcia, H.D.; Ortiz, M.V.; Toedling, J.; Klironomos, F.; Rolff, J.; Eggert, A.; Schulte, J.H.; Kentsis, A.; et al. Prohibitin promotes dedifferentiation and is a potential therapeutic target in neuroblastoma. JCI Insight. 2019, 4, e127130. [Google Scholar] [CrossRef] [PubMed]
- Semenzato, M.; Cogliati, S.; Scorrano, L. Prohibitin(g) Cancer: Aurilide and Killing by Opa1-Dependent Cristae Remodeling. Chem. Biol. 2011, 18, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Pilgrim, A.; Cuya, S.; Chen, D.; Schnepp, R. Abstract 3657: Defining the role of the RNA-binding protein MSI2 in neuroblastoma. Cancer Res. 2019, 79 (Suppl. 13), 3657. [Google Scholar] [CrossRef]
- Phimmachanh, M.; Han, J.Z.R.; O’Donnell, Y.E.I.; Latham, S.L.; Croucher, D.R. Histone Deacetylases and Histone Deacetylase Inhibitors in Neuroblastoma. Front. Cell Dev. Biol. 2020, 8, 578770. [Google Scholar] [CrossRef] [PubMed]
- Visconti, R.; Della Monica, R.; Grieco, D. Cell cycle checkpoint in cancer: A therapeutically targetable double-edged sword. J. Exp. Clin. Cancer Res. 2016, 35, 153. [Google Scholar] [CrossRef]
- Angus, M.; Ruben, P. Voltage gated sodium channels in cancer and their potential mechanisms of action. Channels 2019, 13, 400–409. [Google Scholar] [CrossRef]
- Djamgoz, M.B.A.; Fraser, S.P.; Brackenbury, W.J. In Vivo Evidence for Voltage-Gated Sodium Channel Expression in Carcinomas and Potentiation of Metastasis. Cancers 2019, 11, 1675. [Google Scholar] [CrossRef]
- Jiang, X.; Qin, Y.; Kun, L.; Zhou, Y. The Significant Role of the Microfilament System in Tumors. Front. Oncol. 2021, 11, 620390. [Google Scholar] [CrossRef]
- Fife, C.M.; A McCarroll, J.; Kavallaris, M. Movers and shakers: Cell cytoskeleton in cancer metastasis. Br. J. Pharmacol. 2014, 171, 5507–5523. [Google Scholar] [CrossRef]
- Fanale, D.; Bronte, G.; Passiglia, F.; Calò, V.; Castiglia, M.; Di Piazza, F.; Barraco, N.; Cangemi, A.; Catarella, M.T.; Insalaco, L.; et al. Stabilizing versus destabilizing the microtubules: A double-edge sword for an effective cancer treatment option? Anal. Cell. Pathol. 2015, 2015, 690916. [Google Scholar] [CrossRef]
- Mukhtar, E.; Adhami, V.M.; Mukhtar, H. Targeting Microtubules by Natural Agents for Cancer Therapy. Mol. Cancer Ther. 2014, 13, 275–284. [Google Scholar] [CrossRef]
- Stanton, R.A.; Gernert, K.M.; Nettles, J.H.; Aneja, R. Drugs that target dynamic microtubules: A new molecular perspective. Med. Res. Rev. 2011, 31, 443–481. [Google Scholar] [CrossRef] [PubMed]
- Michels, J.; Johnson, P.W.; Packham, G. Mcl-1. Int. J. Biochem. Cell Biol. 2005, 37, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Eastman, A. Microtubule destabilising agents: Far more than just antimitotic anticancer drugs. Br. J. Clin. Pharmacol. 2016, 83, 255–268. [Google Scholar] [CrossRef]
- Lee, H.; Jeong, A.J.; Ye, S.-K. Highlighted STAT3 as a potential drug target for cancer therapy. BMB Rep. 2019, 52, 415–423. [Google Scholar] [CrossRef]
- Liu, Y.; Law, B.K.; Luesch, H. Apratoxin A Reversibly Inhibits the Secretory Pathway by Preventing Cotranslational Translocation. Mol. Pharmacol. 2009, 76, 91. [Google Scholar] [CrossRef] [PubMed]
- Shukla, D.; Mandal, T.; Saha, P.; Kumar, D.; Kumar, S.; Srivastava, A.K. Chapter 14—Tumor-suppressive proteases revisited: Role in inhibiting tumor progression and metastasis. In Cancer-Leading Proteases; Gupta, S.P., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 391–416. [Google Scholar]
- Rakashanda, S.; Amin, S. Proteases as Targets in Anticancer Therapy Using Their Inhibitors. J. Life Sci. 2013, 5, 133–138. [Google Scholar] [CrossRef]
- Schrader, K.; Huai, J.; Jöckel, L.; Oberle, C.; Borner, C. Non-caspase proteases: Triggers or amplifiers of apoptosis? Cell. Mol. Life Sci. 2010, 67, 1607–1618. [Google Scholar] [CrossRef]
- D’Angelo, V.; Pecoraro, G.; Indolfi, P.; Iannotta, A.; Donofrio, V.; Errico, M.E.; Indolfi, C.; Ramaglia, M.; Lombardi, A.; Di Martino, M.; et al. Expression and localization of serine protease Htra1 in neuroblastoma: Correlation with cellular differentiation grade. J. Neuro-Oncol. 2014, 117, 287–294. [Google Scholar] [CrossRef]
- Stolze, S.C.; Meltzer, M.; Ehrmann, M.; Kaiser, M. Solid phase total synthesis of the 3-amino-6-hydroxy-2-piperidone (Ahp) cyclodepsipeptide and protease inhibitor Symplocamide A. Chem. Commun. 2009, 46, 8857–8859. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.R.; Karmakar, S.; Banik, N.L.; Ray, S.K. Targeting Angiogenesis for Controlling Neuroblastoma. J. Oncol. 2012, 2012, 782020. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.M.; Hanif, E.A.M.; Chin, S.-F. Is targeting autophagy mechanism in cancer a good approach? The possible double-edge sword effect. Cell Biosci. 2021, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Dominguez, R.; Perez-Medina, M.; Lopez-Gonzalez, J.S.; Galicia-Velasco, M.; Aguilar-Cazares, D. The Double-Edge Sword of Autophagy in Cancer: From Tumor Suppression to Pro-tumor Activity. Front. Oncol. 2020, 10, 578418. [Google Scholar] [CrossRef]
- Hua, H.; Kong, Q.; Zhang, H.; Wang, J.; Luo, T.; Jiang, Y. Targeting mTOR for cancer therapy. J. Hematol. Oncol. 2019, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Park, R.; Kim, H.; Namkoong, S.; Jo, D.; Huh, Y.H.; Jang, I.-S.; Lee, J.I.; Park, J. AMPK contributes to autophagosome maturation and lysosomal fusion. Sci. Rep. 2018, 8, 12637. [Google Scholar] [CrossRef]
- McGregor, G.B.; Sendall, B.C. Cyanobacterial diversity and taxonomic uncertainty: Polyphasic pathways to improved resolution. In Advances in Phytoplankton Ecology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 7–45. [Google Scholar] [CrossRef]
- Ruiz-Torres, V.; Encinar, J.A.; Herranz-López, M.; Pérez-Sánchez, A.; Galiano, V.; Barrajón-Catalán, E.; Micol, V. An Updated Review on Marine Anticancer Compounds: The Use of Virtual Screening for the Discovery of Small-Molecule Cancer Drugs. Molecules 2017, 22, 1037. [Google Scholar] [CrossRef]
- Deng, C.; Pan, B.; O’Connor, O.A. Brentuximab VedotinDrug Update of Brentuximab. Clin. Cancer Res. 2013, 19, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Natsume, T.; Watanabe, J.-I.; Horiuchi, T.; Kobayashi, M. Combination effect of TZT-1027 (Soblidotin) with other anticancer drugs. Anticancer. Res. 2006, 26, 1145–1151. [Google Scholar] [PubMed]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; El-Wahed, A.A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [PubMed]
- Ebbinghaus, S.; Hersh, E.; Cunningham, C.C.; O’Day, S.; McDermott, D.; Stephenson, J.; Richards, D.A.; Eckardt, J.; Haider, O.L.; Hammond, L.A. Phase II study of synthadotin (SYN-D.; ILX651) administered daily for 5 consecutive days once every 3 weeks (qdx5q3w) in patients (Pts) with inoperable locally advanced or metastatic melanoma. J. Clin. Oncol. 2004, 22 (Suppl. 14), 7530. [Google Scholar] [CrossRef]
- Aesoy, R.; Herfindal, L. Chapter 3—Cyanobacterial anticancer compounds in clinical use: Lessons from the dolastatins and cryp-tophycins. In The Pharmacological Potential of Cyanobacteria; Lopes, G., Silva, M., Vasconcelos, V., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 55–79. [Google Scholar]
- Feling, R.H.; Buchanan, G.O.; Mincer, T.J.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Salinosporamide A: A highly cytotoxic proteasome inhibitor from a novel microbial source, a marine bacterium of the new genus Salinospora. Angew. Chem. Int. Ed. 2003, 42, 355–357. [Google Scholar] [CrossRef]
- DuBois, S.G.; Macy, M.E.; Henderson, T.O. High-Risk and Relapsed Neuroblastoma: Toward More Cures and Better Outcomes. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 768–780. [Google Scholar] [CrossRef]
- Robles-Bañuelos, B.; Durán-Riveroll, L.M.; Rangel-López, E.; Pérez-López, H.I.; González-Maya, L. Marine Cyanobacteria as Sources of Lead Anticancer Compounds: A Review of Families of Metabolites with Cytotoxic, Antiproliferative, and Antineoplastic Effects. Molecules 2022, 27, 4814. [Google Scholar] [CrossRef]
- Wang, E.; Sorolla, M.A.; Krishnan, P.D.G.; Sorolla, A. From Seabed to Bedside: A Review on Promising Marine Anticancer Compounds. Biomolecules 2020, 10, 248. [Google Scholar] [CrossRef]
- Rotter, A.; Barbier, M.; Bertoni, F.; Bones, A.M.; Cancela, M.L.; Carlsson, J.; Carvalho, M.F.; Cegłowska, M.; Chirivella-Martorell, J.; Dalay, M.C.; et al. The Essentials of Marine Biotechnology. Front. Mar. Sci. 2021, 8, 629629. [Google Scholar] [CrossRef]
- Lath, A.; Santal, A.R.; Kaur, N.; Kumari, P.; Singh, N.P. Anti-cancer peptides: Their current trends in the development of peptide-based therapy and anti-tumor drugs. Biotechnol. Genet. Eng. Rev. 2022, 1–40. [Google Scholar] [CrossRef]
- Lamers, C. Overcoming the shortcomings of peptide-based therapeutics. Futur. Drug Discov. 2022, 4, FDD75. [Google Scholar] [CrossRef]
- Luan, X.; Wu, Y.; Shen, Y.-W.; Zhang, H.; Zhou, Y.-D.; Chen, H.-Z.; Nagle, D.G.; Zhang, W.-D. Cytotoxic and antitumor peptides as novel chemotherapeutics. Nat. Prod. Rep. 2020, 38, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Kurrikoff, K.; Aphkhazava, D.; Langel, Ü. The future of peptides in cancer treatment. Curr. Opin. Pharmacol. 2019, 47, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Günday Türeli, N. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef] [PubMed]
- Haumann, R.; Videira, J.C.; Kaspers, G.J.L.; van Vuurden, D.G.; Hulleman, E. Overview of Current Drug Delivery Methods Across the Blood–Brain Barrier for the Treatment of Primary Brain Tumors. CNS Drugs 2020, 34, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Islam, Y.; Leach, A.G.; Smith, J.; Pluchino, S.; Coxon, C.; Sivakumaran, M.; Downing, J.E.; A Fatokun, A.; Teixidò, M.; Ehtezazi, T. Peptide based drug delivery systems to the brain. Nano Express 2020, 1, 012002. [Google Scholar] [CrossRef]
