Cardiovascular disease (CVD) is the leading cause of morbidity and mortality globally. In recent decades, clinical research has made significant advances, resulting in improved survival and recovery rates for patients with CVD. Despite this progress, there is substantial residual CVD risk and an unmet need for better treatment. The complex and multifaceted pathophysiological mechanisms underlying the development of CVD pose a challenge for researchers seeking effective therapeutic interventions. Consequently, exosomes have emerged as a new focus for CVD research because their role as intercellular communicators gives them the potential to act as noninvasive diagnostic biomarkers and therapeutic nanocarriers. In the heart and vasculature, cell types such as cardiomyocytes, endothelial cells, vascular smooth muscle, cardiac fibroblasts, inflammatory cells, and resident stem cells are involved in cardiac homeostasis via the release of exosomes. Exosomes encapsulate cell-type specific miRNAs, and this miRNA content fluctuates in response to the pathophysiological setting of the heart, indicating that the pathways affected by these differentially expressed miRNAs may be targets for new treatments.
- atherosclerosis
- cholesterol
- cardiovascular disease
1. Introduction
2. Overview of Exosomes
Intercellular communication among cells is vital in all living organisms for normal functioning [12][13]. Cell-to-cell communication occurs through direct contact in neighboring cells or via secretion of soluble factors, such as cytokines, hormones, and chemokines, which can extend interaction over distances [12][14]. Recent attention has focused on a means of intercellular communication via extracellular vesicles (EVs), cell-derived membrane-enclosed microvesicles of varying sizes secreted by most cell types [15][16]. EVs show promise in a number of medical applications and may be useful as non-invasive diagnostic biomarkers and therapeutic nanocarriers for the treatment of various diseases, including neurodegeneration, cardiovascular dysfunction, and cancer [17][18][19][20]. EVs can be categorized into four major classes, microvesicles, apoptotic bodies, virus-like particles, and exosomes, according to their size, subcellular origin, and content [21][22]. While the first three classes of EVs are formed by the outward budding of the plasma membrane, exosomes originate as intraluminal vesicles contained within multi-vesicular bodies (MVBs) (Figure 1) [23][24]. Successive extracellular secretion of these intraluminal vesicles by fusion with the plasma membrane releases exosomes with diameters of ~40 to 160 nanometers into the extracellular matrix [19][25][26].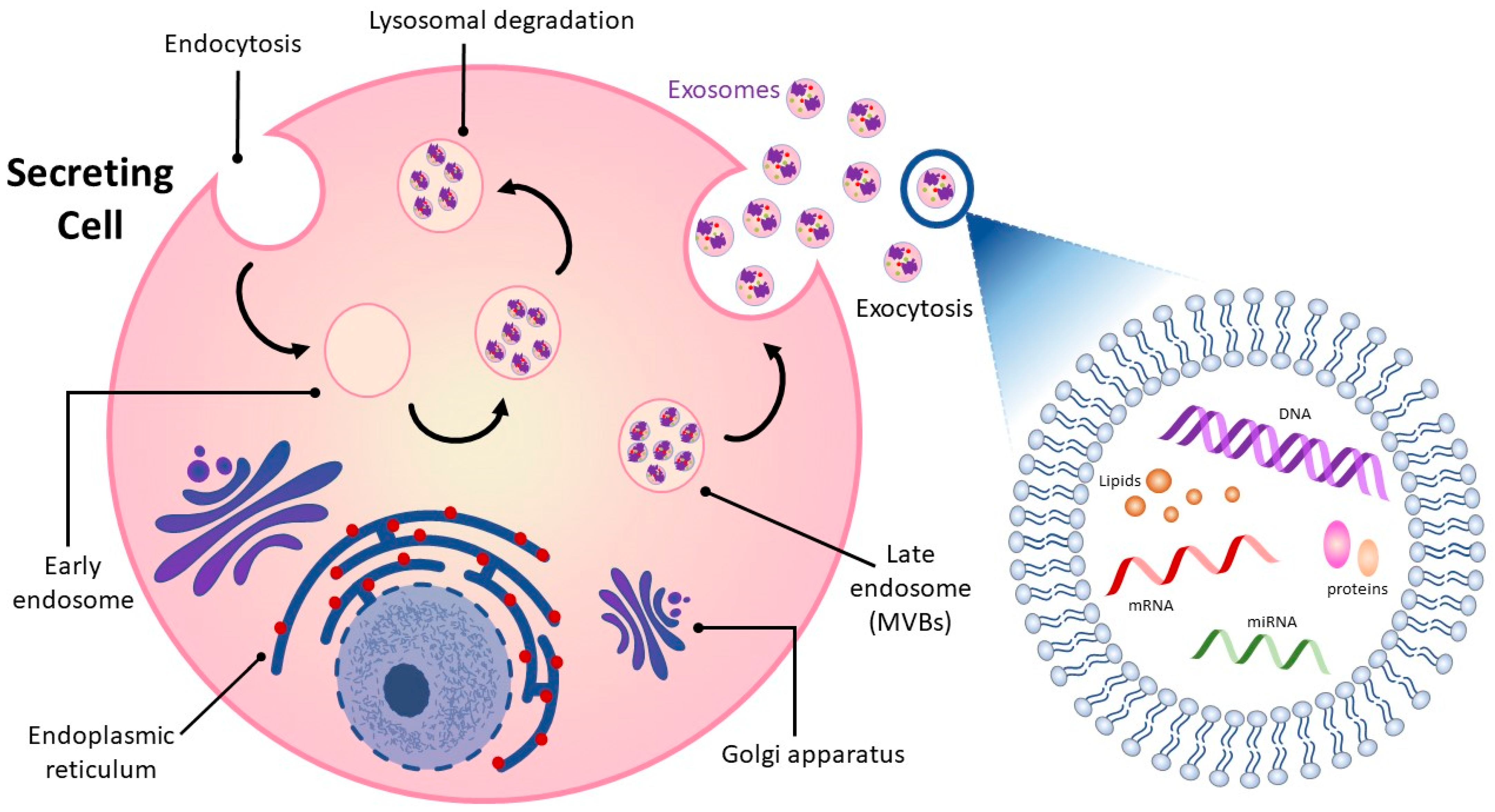
3. The Link between Cardiovascular Disease and Exosomes
3.1. Exosome Cargo and the Cardiovascular System
Exosomes are key factors in regulating CVD progression due to their role in the transport and exchange of signaling molecules [32][33][34][35]. A study by Peng et al. found that extracellular vesicles from atherosclerotic plaque with characteristics of exosomes may be able to spread atherosclerosis distally [36]. Extracellular vesicles were isolated from carotid atherosclerotic lesions of rats with knockout of the LDL receptor gene on a high-fat diet and injected into LDL receptor knockout mice on a normal diet, conditions under which they do not normally develop atherosclerosis. The extracellular vesicles from atherosclerotic tissue were taken up by endothelium in the carotids and promoted endothelial inflammation. In the heart and vasculature, cell types that interact to maintain homeostasis and are known to release exosomes include cardiomyocytes, endothelial and vascular smooth muscle cells, cardiac fibroblasts, inflammatory cells, and resident stem cells (Figure 2) [35][37][38][39]. Under both physiological and pathological conditions, exosomes are an integral mode of communication amongst these cell types (Table 1). Their rate of secretion and specific miRNA cargo can change in response to pathological states or drug treatments [40][41][42][43]. For example, in a mouse model, analysis of the miRNA content of exosomes derived from EPC found that the top ten miRNAs in abundance were all associated with atherosclerosis [44]. Administration of EPC-derived exosomes to atherosclerosis-prone hyperglycemic mice lowered oxidative stress and inflammation and reduced plaque area, indicating that EPC exosomes may improve endothelial function in diabetic atherosclerosis.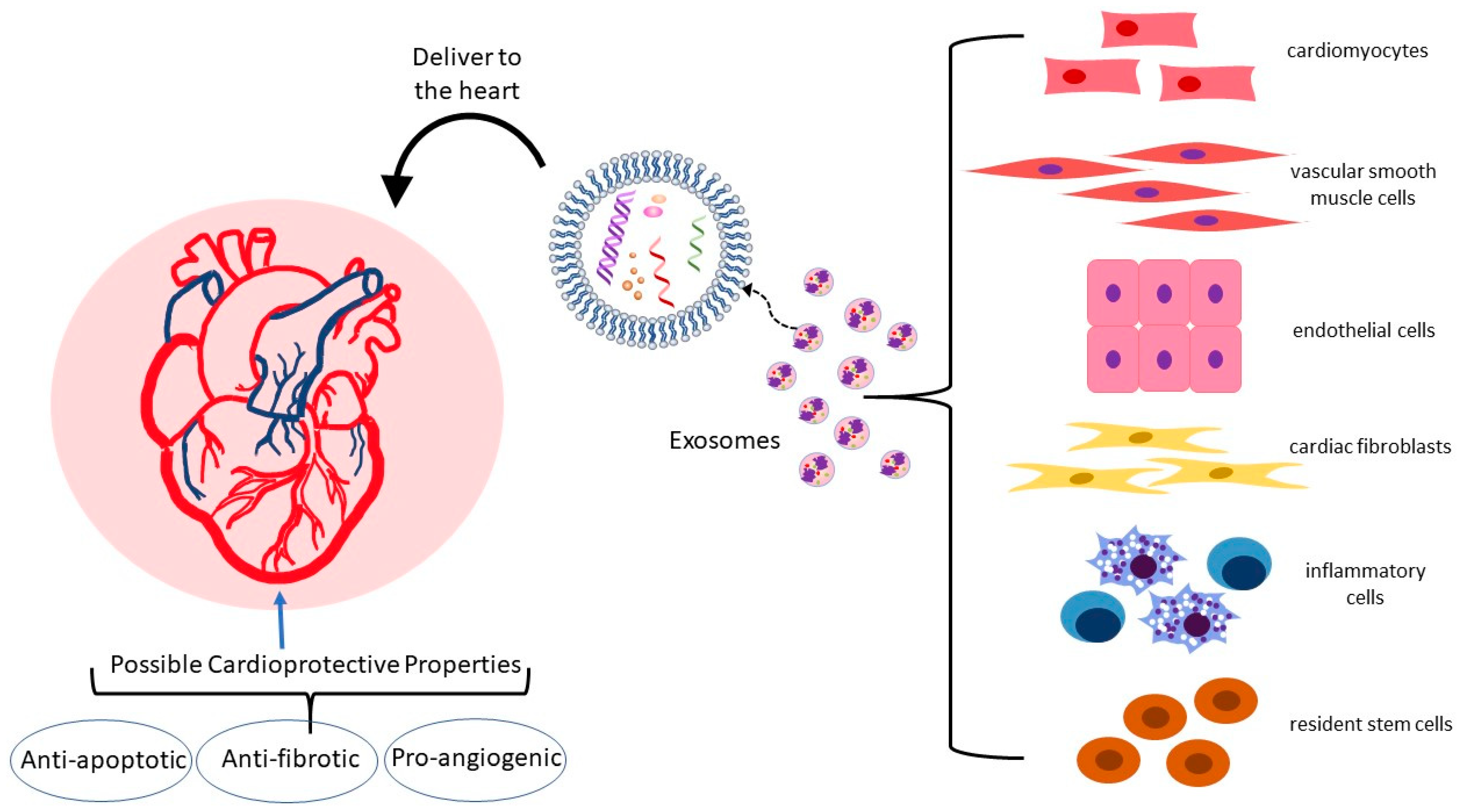
3.2. Exosomes in Atherosclerosis: Endothelial Dysfunction, Macrophage Recruitment, and Vascular Smooth Muscle Behavior
Endothelial dysfunction is an early step in the development of atherosclerosis. Activated endothelial cells upregulate expression of adhesion molecules ICAM-1, VCAM-1, P-selectin, and E-selectin, which act to recruit monocytes to the subendothelial layer of the artery. Important risk factors for atherosclerosis that cause activation of endothelium are an elevation in serum levels of oxidized LDL (ox-LDL) and an inflammatory environment of elevated cytokines and homocysteine [60][61][62][63]. Ox-LDL and homocysteine have been shown to induce the release of HSP-70-containing exosomes from aortic endothelial cells that can elicit immune inflammatory responses and selectively activate monocytes [64][65]. Monocyte activation leads to monocyte-endothelial cell adhesion and monocyte infiltration into the subendothelial space. Activated monocyte-derived macrophages in the arterial intima orchestrate multiple inflammatory atherosclerosis-promoting processes in plaque formation [66]. When activated, monocytes themselves can perpetuate their own adhesion to endothelium by producing exosomes that enter endothelial cells and activate NF-κB, thus causing endothelial production of adhesion molecules [67]. Exosomes isolated from murine bone marrow-derived macrophages (BMDM) and infused into atherosclerosis-prone ApoE-deficient Western diet-fed mice exhibit an ability to reduce atherosclerotic lesion necrosis and stabilize atheroma [68]. Cheng et al. showed, in a human cell culture model, that human umbilical vein endothelial cells cultured under inflammatory conditions were protected from apoptosis by exosomes from M2 polarized THP-1 macrophages and the effect was mediated by miR-221-3p [69]. VSMC proliferation, migration, and cytokine secretion contribute to atherogenesis [70]. VSMC behavior can be influenced by exosomes of macrophage origin. As far back as 2016, Niu et al. showed that extracellular vesicles derived from J774a.1 macrophage foam cells could enhance migration and adhesion of human aortic VSMC [71]. Ren et al. cultured VSMC in media with exosomes from ox-LDL-stimulated macrophages and found that these exosomes improved viability and invasive properties of the VSMC while suppressing apoptosis [72]. Of the overexpressed miRNA in the ox-LDL-stimulated macrophage exosomes, miR-186-5p was found to be responsible for these pro-atherogenic effects via inactivation of SHIP2, thus enhancing PI3K/AKT/mTOR signaling. The influence of VSMC-derived exosomes on atherosclerosis has been studied. Exosomes isolated from cultured primary human VSMC induced to undergo premature senescence and their properties were then analyzed [73]. The senescent VSMC produced more exosomes than the control VSMC. When T cells and monocytes were exposed to exosomes of senescent VSMC origin, they produced more pro-inflammatory cytokines than cells exposed to control VSMC exosomes.3.3. Exosomes in Apoptosis
A critical event in CVD progression, including myocardial infarction and heart failure, is the apoptosis and autophagy of cardiomyocytes [74]. Myocardial ischemia followed by therapeutic restoration of blood flow (ischemia-reperfusion) leads to cardiomyocyte damage and apoptosis in both the hypoxia stage and the rapid recovery stage [75][76]. Cardiac fibroblasts release exosomes that rescue cardiomyocytes from ischemia-reperfusion injury by protecting against apoptosis and pyroptosis (a form of programmed necrosis) [52][77][78]. Luo et al. showed that injection of rat cardiac fibroblast exosomes into the infarct border of the rat heart during hypoxia–reoxygenation (left anterior descending artery ligation and reperfusion) reduced infarct size. Comparison of expression levels of exosomal miRNAs showed miR-423-3p to be enriched in exosomes that limited infarct size and knockdown of this miRNA in cell culture companion studies confirmed its importance in maintaining cell viability and reducing apoptosis [79]. In studies using human exosomes, Qiao et al. isolated exosomes from conditioned media of cardiac cells from healthy volunteers and heart failure patients and performed intramyocardial injection of these exosomes into a mouse model of acute myocardial infarction induced by coronary vessel ligation. The study showed that the healthy volunteer exosomes had favorable effect on healing, apoptosis, and tissue preservation while exosomes of heart failure patients inhibited cardiomyocyte proliferation, inhibited angiogenesis, and worsened healing [80]. As in the case of exosomes from heart failure patients, exosomes from EPC of mice deficient in the anti-inflammatory and atheroprotective cytokine IL-10 also lose their protective effect in reducing infarct damage and, rather than inhibit apoptosis, these exosomes enhanced apoptosis [81][82].3.4. Exosomes in Hypertrophy
Pathologic cardiac hypertrophy occurs when the wall of the ventricle thickens, impairing systolic function [83]. It is often a result of chronic hypertension and can lead to ischemia and eventually to heart failure. Hallmarks of this process are enlargement of cardiomyocytes and fibrosis. Exosomes have been implicated in the regulation of cardiac hypertrophy via effects on the renin-angiotensin system. The mechanical stretch of cardiomyocytes has been found to release exosomes enriched with angiotensin II and its receptor content, contributing to increased vascular resistance and hypertrophy [84][85][86]. Constantin et al. found a potential benefit of exosomes from human mesenchymal stem cells in ameliorating hypertrophy in a model of human hypertrophic cardiomyocytes [87]. When hypertrophic cardiomyocytes were incubated with extracellular vesicles from subcutaneous adipose tissue stem cells or from bone marrow mesenchymal stem cells, cardiac markers for cardiomyocyte hypertrophy and inflammatory cytokine such as IL-1β, IL-4, IL-6, and TNF-α decreased [88]. Mao et al. engineered exosomes derived from human cardiosphere-derived cells to target cardiomyocytes [89]. They then infused these exosomes into a mouse model of myocardial hypertrophy and showed decreased perivascular and myocardial interstitial fibrosis of the left ventricle and decreased hypertrophy in treated mice versus sham mice and attributed the differences largely to effects of miRNA-148a, an miRNA with anti-proliferative properties. Ren et al.[1] evaluated the effect of bone marrow mesenchymal stem cell-derived exosomes from Sprague-Dawley rats on cultured H9c2 cells (rat embryonic cardiomyocytes) treated with angiotensin (Ang) II to induce hypertrophy and found that the exosomes increased viability of the Ang II-treated H9c2 cells while reducing apoptosis and inflammation. The bone marrow mesenchymal stem cell-derived exosomes acted, at least in part, by normalizing expression of Hippo-Yes-associated protein (YAP) signaling pathway proteins that had been altered by Ang II.3.5. Exosomes in Angiogenesis
The regulation of blood vessels involved in supporting the myocardium with oxygen and nutrients relies, in part, on communication between cardiomyocytes and myocardial vascular endothelial cells via exosomes [90]. Angiogenic capacity is of great importance in cardiac repair and regeneration post-ischemic injury. A number of cell types can release exosomes that deliver specific pro-angiogenic miRNAs. For example, mesenchymal stem cell exosomes isolated from human adipose tissue have been shown to promote angiogenesis in human umbilical vein endothelial cells by carrying miR-125a [91]. Exosomes extracted from cardiomyocytes after cardiomyocyte hypoxic preconditioning can protect cardiac microvascular endothelial cells from oxidative damage and promote angiogenesis in vitro and in vivo and this effect is mediated predominantly by miR-222 and miR-143 [92][93]. Gou et al. showed that purified exosomes from primary neonatal cardiomyocytes treated with H2O2 to induce injury were enriched in miR-19a-3p and this increase in miR-19a-3p was also true for human plasma in post-myocardial infarction patients [94]. They demonstrated uptake of exosomes from mouse neonatal cardiomyocytes by endothelial cells and showed that miR-19a-3p was anti-angiogenic and that neutralizing this miRNA promoted survival and proliferation of endothelial cells. They performed mouse studies in which they induced myocardial infarction via ligation of the left anterior descending coronary artery and then silencing of miR-19a-3p and showed that silencing improved angiogenesis. The target of miR-19a-3p was found to be hypoxia-inducible factor (HIF)-1α, which is known to drive multiple genes involved in angiogenesis [95]. In a rat model of myocardial infarction induced by ligation of the left anterior descending coronary artery, exosomes derived from cardiac telocytes enhanced angiogenesis and reduced cardiac fibrosis [96]. Increased miR-126 and miR-199a in circulating exosomes in patients with coronary artery disease is associated with a reduced risk for major future adverse cardiovascular events [97]. Cultured endothelial cells exposed to these exosomes showed increased proliferation, migration, and tube-formation [98][99]. In the microenvironment induced by myocardial infarction, M1 macrophages predominate early, are inflammatory, and generate exosomes that transport miRNAs to endothelial cells that can reduce angiogenic potential and aggravate myocardial injury [100]. These proinflammatory exosomes are enriched in miR-155, which is known to inhibit pathways within endothelial cells, such as the Sirtuin 1, adenosine monophosphate-activated protein kinase (AMPK), and endothelial nitric oxide synthase which promote angiogenesis [101][102].3.6. Exosomes in Cardiac Fibrosis
Cardiac fibroblasts play an integral role in normal myocardial structure and physiology [103]. They produce an extracellular matrix and pro-fibrotic factors that can be reparative following injury [104]. However, when pathologically activated, they negatively affect cardiac function as well as myocardial compliance and stiffness [105][106][107][108]. Exosomes derived from different cell types and originating under normal or pathologic conditions can affect fibroblasts and modulate cardiac fibrosis. For instance, in a mouse model of diabetes, exosomes released from the heart after exercise on a treadmill reduced fibrosis via their higher content of miR-29b and miR-455, which acted by downregulating pro-fibrotic matrix metalloproteinase (MMP)-9 [109]. In humans with heart failure, the level of miR-217 in cardiac tissue is elevated and higher miR-217 correlates with poorer left ventricular ejection fraction [110][111]. In a mouse model of heart failure induced by thoracic aortic constriction, cardiomyocyte-derived exosomes enriched in miR-217 aggravated cardiac fibrosis via targeting of phosphatase and tensin homolog (PTEN) [111]. Yuan et al. also used the thoracic aortic constriction mouse model to look at cardiac remodeling under mechanical stress and found inhibition of excessive fibrosis in mice given an miR-378 mimic. Exosomes from cultured cardiomyocytes that had been subjected to mechanical stress harbored miR-378 and when cardiac fibroblasts that had also been subjected to mechanical stress were exposed to these exosomes, they showed reduced upregulation of collagen expression and lower MMP9 protein levels [112]. Anti-fibrotic effects on the cardiac fibroblasts were blocked with depletion of miR-378 from the stretched cardiomyocyte conditioned medium. Using murine cells in culture, Tang et al. showed that mechanical stress-treated cardiomyocytes can activate cardiac fibroblasts by delivering miR-494-3p in exosomes [113]. The cardiomyocyte exosomal miR-494-3p targets PTEN in the fibroblasts. The miR-494-3p enrichment of cardiomyocyte exosomes occurs due to stress-induced upregulation of the E3 ubiquitin ligase Peli1, a known participant in inflammatory processes, and is abrogated in cardiomyocytes that are deficient in Peli1. The pathological activation of cardiac fibroblasts is also influenced by exosomes derived from macrophages. In the diabetic microenvironment, exosomes released from macrophages exacerbate cardiac fibrosis [114].3.7. Exosomes in Myocardial Infarction
Exosomes are considered potential biomarkers for the diagnosis and monitoring of myocardial ischemia and infarction [115][116]. This is of value because of the limitations in sensitivity and specificity and delay in detectable change in the standard marker, troponin T [117]. Earlier diagnosis and confirmation of myocardial infarction allows faster intervention for heart muscle preservation [118]. Silvia-Palacios conducted a study in Mexico City in which exosomes were isolated from the plasma of 26 patients admitted to intensive care with acute ST-segment elevation myocardial infarct (STEMI) and compared to those of 26 healthy donors [119]. They found that ischemia prompted increased exosome release from the myocardial infarction patients. Both during the acute myocardial infarction and after reperfusion, miR-223-3p, an miRNA that targets inflammatory molecules, was found at high levels in patient exosomes. Chen et al. looked at differentially expressed miRNAs in plasma exosomes from patients with STEMI and non-STEMI versus healthy controls and proposed a set of 10 miRNAs that they found could discriminate between STEMI and non-STEMI, which has implications for prognosis and treatment planning [120].3.8. Adipose Tissue Exosomes
Obesity is often associated with atherosclerosis and the metabolic complications of obesity may implicate adipose-tissue (AT)-derived exosomes, although their explicit role in atherogenesis remains unclear [121][122]. Adipocyte-derived exosomes have been shown to deliver adipocyte-dominant transcripts into macrophages and promote AT macrophage activation [123][124]. Xie et al. showed that exosomes released from differently located and stressed ATs have distinct pro-atherosclerotic effects [121]. Barberio et al. characterized miRNAs from human adipose tissue-derived exosomes from obese and lean adolescents and found that six miRNAs contained in adipocyte exosomes targeted cholesterol efflux genes and were significantly associated with cholesterol efflux capacity [125]. They then exposed cultured THP-1 macrophages to adipocyte-derived exosomes and showed that obese subject exosomes significantly increased macrophage ox-LDL retention compared to lean subject exosomes. The findings of this study exemplify a mechanistic link between obesity and macrophage lipid handling. Liu et al. showed, in mice, that perivascular adipose tissue exosomes suppress macrophage foam cell transformation [126]. Treatment of a mouse macrophage cell line with the perivascular adipose tissue exosomes reduced ox-LDL uptake, likely due to decreased expression of ScR-A mRNA and promoted cholesterol efflux, likely due to enhanced expression of ABCA1 and ABCG1 mRNAs. They then conducted further study of the mechanisms involved and found that the key miRNA mediating the upregulation of macrophage ABCA1 and ABCG1 by perivascular adipose tissue exosomes was miR-382-5p [126]. Wang et al. showed that in mice with myocardial infarction induced by ligation of the left anterior descending coronary artery, intravenous administration of adipose-derived stem cell exosomes reduced infarct area, inhibited apoptosis and improved cardiac recovery [2]. These myocardium-preserving results were found to be mediated via miR-205. Evidence is beginning to accumulate that exosomes from different cell types found within obese adipose tissue affects whole body metabolism, and, indirectly, atherosclerotic risk [127][128][129][130]. For example, macrophages that reside within adipose tissue produce exosomes that transfer to adipocytes, and, in a murine model, lean mice treated with adipose tissue macrophage exosomes from obese mice became insulin resistant [131]. Further evidence of the relationship among obesity, adipose tissue exosomes, and metabolism can be found in a human study showing that weight loss brought about after gastric bypass surgery changes the miRNA content of adipocyte-derived exosomes isolated from the peripheral blood [132]. One year after surgery, 10 miRNAs that target insulin signaling pathways were found to be altered and this correlated with improvements in insulin sensitivity with better glucose homeostasis.4. The Application of Exosomes in Treatment of Heart Disease
There is great interest in using the unique properties of exosomes to treat and prevent CVD [3]. As discussed in researchers' full paper, exosomes can affect key signaling pathways that are disrupted in the ischemic state and during myocardial infarction and they can change the proteome of the heart [4] [5]. Exosomes may be used as vehicles for delivery of drugs, lipids, proteins, miRNAs and mRNAs to the heart and blood vessels. Advantages of exosomes in transporting these therapeutics include their biocompatibility, low immunogenicity and low toxicity as well as their ability to move through biological fluids [6]. Further, they can be manipulated to target specific cell types by changing their surface markers [7]. Initiatives to apply exosomes in human CVD treatment are slow to move forward, but several interventional studies have been initiated [8]. Acknowledgments: Thank Ms. Lynn Drucker and Mr. Robert Buescher.| Cell/Tissue Source of Exosomes | Reference | CVD Impact | Key Cargo Involved |
|---|---|---|---|
| Carotid atherosclerotic tissue | [36] | Endothelial inflammation in rats | Whole exosomes |
| Endothelial progenitor cells (EPC) | [44] | ↓ oxidative stress and inflammation and ↓ plaque area in hyperglycemic mouse model | Whole exosomes |
| Cardiomyocyte | [49] | Protects against apoptosis, remodeling and cardiac hypertrophy in diabetic mice | Heat shock protein (HSP)-20, |
| Cardiomyocyte | [50][51] | Atherogenic via induction of inflammatory mediators | HSP-60 |
| Blood | [56][57] | Anti-inflammatory, atheroprotective effects on macrophages via IL-10 release, facilitation of cholesterol efflux | HSP-27 |
| Blood | [59] | Delivery of free fatty acids to cardiac endothelium and myocytes for energy | CD36 |
| Arterial endothelium | [64][65] | Monocyte activation and adhesion | HSP-70 |
| Macrophage foam cells | [71][72] | Enhanced adhesion and suppressed apoptosis of vascular smooth muscle cells | MiR-186-5p |
| Cardiac fibroblasts | [77][78][79] | ↓ infarct size in rat ischemia-reperfusion injury, limited apoptosis | MiR-423-3p |
| Human mesenchymal stem cells | [88] | ↓ cardiac markers for cardiomyocyte hypertrophy and ↓ inflammatory cytokine levels | Whole exosomes |
| Human cardiospheres | [89] | ↓ left ventricular fibrosis and hypertrophy in a mouse cardiac hypertrophy model | MiRNA-148a |
| Human adipose tissue mesenchymal stem cells | [91] | Promote angiogenesis | MiR-125a |
| Cardiomyocyte after hypoxic preconditioning | [92][93] | Promote angiogenesis and protect microvascular endothelium from oxidative damage | MiR-222 and miR-143 |
| Primary neonatal cardiomyocytes | [94][95] | Anti-angiogenic | MiR-19a-3p |
| Blood | [97][98][99] | ↑ proliferation, migration and tube-formation in endothelial cells | MiR-126 and miR-199a |
| Inflammatory M1 macrophages | [101][102] | Anti-angiogenic | MiR-155 |
| Heart after exercise | [109] | ↓ fibrosis | MiR-29b and miR-455 |
| Cardiomyocyte | [111] | ↑ fibrosis and hypertrophy | MiR-217 |
| Cardiomyocytes after mechanical stress | [112] | ↓ fibrosis | MiR-378 |
| Cardiomyocytes after mechanical stress | [113] | ↑ fibrosis | MiR-494-3p |
| Perivascular adipose tissue | [126] | Atheroprotective, ↑ macrophage cholesterol efflux, ABCA1, and ABCG1 | MiR-382-5p |
| Obese mouse adipose tissue macrophages | [131] | Confer insulin resistance on lean mice | Whole exosomes |
References
- Li, Z.; Lin, L.; Wu, H.; Yan, L.; Wang, H.; Yang, H.; Li, H. Global, Regional, and National Death, and Disability-Adjusted Life-Years (DALYs) for Cardiovascular Disease in 2017 and Trends and Risk Analysis From 1990 to 2017 Using the Global Burden of Disease Study and Implications for Prevention. Front. Public Health 2021, 9, 559751.
- Campbell, N.R.C.; Ordunez, P.; Giraldo, G.; Rodriguez Morales, Y.A.; Lombardi, C.; Khan, T.; Padwal, R.; Tsuyuki, R.T.; Varghese, C. WHO HEARTS: A Global Program to Reduce Cardiovascular Disease Burden: Experience Implementing in the Americas and Opportunities in Canada. Can. J. Cardiol. 2021, 37, 744–755.
- Salvatore, F.P.; Spada, A.; Fortunato, F.; Vrontis, D.; Fiore, M. Identification of Health Expenditures Determinants: A Model to Manage the Economic Burden of Cardiovascular Disease. Int. J. Environ. Res. Public Health 2021, 18, 4652.
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596.
- De Araújo, J.M.; Eufrosino de Alencar Rodrigues, R.; da Costa Pereira de Arruda Neta, A.; Leite Lima Ferreira, F.E.; Lira Formiga Cavalcanti de Lima, R.; Pinheiro de Toledo Vianna, R.; Vasconcelos Leitão Moreira, L.; Moreira da Silva Neto, J.; Moreira, P.V.L. The direct and indirect costs of cardiovascular diseases in Brazil. PLoS ONE 2022, 17, e0278891.
- Braunwald, E. Control of residual dyslipidaemic risk. Eur. Heart J. 2022, 43, 3824–3825.
- van Rosendael, S.E.; van den Hoogen, I.J.; Lin, F.Y.; Andreini, D.; Al-Mallah, M.H.; Budoff, M.J.; Cademartiri, F.; Chinnaiyan, K.; Choi, J.H.; Conte, E.; et al. Clinical and Coronary Plaque Predictors of Atherosclerotic Nonresponse to Statin Therapy. JACC Cardiovasc. Imaging 2022, S1936-878X(22)00655-6.
- Zhang, Y.; Hu, Y.W.; Zheng, L.; Wang, Q. Characteristics and Roles of Exosomes in Cardiovascular Disease. DNA Cell. Biol. 2017, 36, 202–211.
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420.
- Zheng, D.; Huo, M.; Li, B.; Wang, W.; Piao, H.; Wang, Y.; Zhu, Z.; Li, D.; Wang, T.; Liu, K. The Role of Exosomes and Exosomal MicroRNA in Cardiovascular Disease. Front. Cell Dev. Biol. 2021, 8, 616161.
- Small, E.M.; Olson, E.N. Pervasive roles of microRNAs in cardiovascular biology. Nature 2011, 469, 336–342.
- Chang, W.; Wang, J. Exosomes and their noncoding RNA cargo are emerging as new modulators for diabetes mellitus. Cells 2019, 8, 853.
- Yoon, Y.J.; Kim, O.Y.; Gho, Y.S. Extracellular vesicles as emerging intercellular communicasomes. BMB Rep. 2014, 47, 531–539.
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nature Cell Biol. 2019, 21, 9–17.
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228.
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383.
- Lee, J.H.; Song, J.; Kim, I.G.; You, G.; Kim, H.; Ahn, J.H.; Mok, H. Exosome-Mediated Delivery of Transforming Growth Factor-β Receptor 1 Kinase Inhibitors and Toll-Like Receptor 7/8 Agonists for Combination Therapy of Tumors. Acta Biomater. 2022, 141, 354–363.
- Gao, P.; Li, X.; Du, X.; Liu, S.; Xu, Y. Diagnostic and Therapeutic Potential of Exosomes in Neurodegenerative Diseases. Front. Aging Neurosci. 2021, 13, 790863.
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19.
- Xiong, F.; Mao, R.; Zhao, R.; Zhang, L.; Tan, K.; Liu, C.; Wang, S.; Xu, M.; Li, Y.; Zhang, T. Plasma Exosomal S1PR5 and CARNS1 as Potential Non-invasive Screening Biomarkers of Coronary Heart Disease. Front. Cardiovasc. Med. 2022, 9, 845673.
- Zaborowski, M.P.; Balaj, L.; Breakefield, X.O.; Lai, C.P. Extracellular Vesicles: Composition, Biological Relevance, and Methods of Study. Bioscience 2015, 65, 783–797.
- Brennan, K.; Martin, K.; Fitzgerald, S.P.; O’sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039.
- Urbanelli, L.; Magini, A.; Buratta, S.; Brozzi, A.; Sagini, K.; Polchi, A.; Tancini, B.; Emiliani, C. Signaling pathways in exosomes biogenesis, secretion and fate. Genes 2013, 4, 152–170.
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct. Target. Ther. 2020, 5, 145.
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977.
- Saad, M.G.; Beyenal, H.; Dong, W.J. Exosomes as Powerful Engines in Cancer: Isolation, Characterization and Detection Techniques. Biosensors 2021, 11, 518.
- Record, M.; Carayon, K.; Poirot, M.; Silvente-Poirot, S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim. Biophys. Acta 2014, 1841, 108–120.
- Turchinovich, A.; Drapkina, O.; Tonevitsky, A. Transcriptome of extracellular vesicles: State-of-the-art. Front. Immunol. 2019, 10, 202.
- Willms, E.; Johansson, H.J.; Mäger, I.; Lee, Y.; Blomberg, K.E.; Sadik, M.; Alaarg, A.; Smith, C.I.; Lehtiö, J.; El Andaloussi, S.; et al. Cells release subpopulations of exosomes with distinct molecular and biological properties. Sci. Rep. 2016, 6, 22519.
- Chen, Y.; Zhao, Y.; Yin, Y.; Jia, X.; Mao, L. Mechanism of cargo sorting into small extracellular vesicles. Bioengineered 2021, 12, 8186–8201.
- Colombo, M.; Raposo, G.; Thery, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289.
- Fu, L.; Wu, S.S. Advances in studies on exosomes and microvesicles as markers of cardiovascular disease. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2622–2629.
- Guo, D.; Xu, Y.; Ding, J.; Dong, J.; Jia, N.; Li, Y.; Zhang, M. Roles and Clinical Applications of Exosomes in Cardiovascular Disease. Biomed. Res. Int. 2020, 2020, 5424281.
- Barile, L.; Moccetti, T.; Marbán, E.; Vassalli, G. Roles of exosomes in cardioprotection. Eur. Heart J. 2017, 38, 1372–1379.
- Sahoo, S.; Losordo, D.W. Exosomes and cardiac repair after myocardial infarction. Circ. Res. 2014, 114, 333–344.
- Peng, M.; Sun, R.; Hong, Y.; Wang, J.; Xie, Y.; Zhang, X.; Li, J.; Guo, H.; Xu, P.; Li, Y.; et al. Extracellular vesicles carrying proinflammatory factors may spread atherosclerosis to remote locations. Cell. Mol. Life Sci. 2022, 79, 430.
- Henning, R.J. Cardiovascular Exosomes and MicroRNAs in Cardiovascular Physiology and Pathophysiology. J. Cardiovasc. Transl. Res. 2021, 14, 195–212.
- Sluijter, J.; Verhage, V.; Deddens, J.; van den Akker, F.; Doevendans, P. Microvesicles and exosomes for intracardiac communication. Cardiovasc. Res. 2014, 102, 302–311.
- Pironti, G.; Strachan, R.T.; Abraham, D.; Mon-Wei, Y.U.S.; Chen, M.; Chen, W.; Hanada, K.; Mao, L.; Watson, L.J.; Rockman, H.A. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 2015, 24, 2120–2130.
- Davidson, S.M.; Riquelme, J.A.; Takov, K.; Vicencio, J.M.; Boi-Doku, C.; Khoo, V.; Doreth, C.; Radenkovic, D.; Lavandero, S.; Yellon., D.M. Cardioprotection mediated by exosomes is impaired in the setting of type II diabetes but can be rescued by the use of non-diabetic exosomes in vitro. J. Cell. Mol. Med. 2018, 22, 141–151.
- Xu, M.Y.; Ye, Z.S.; Song, X.T.; Huang, R.C. Differences in the cargos and functions of exosomes derived from six cardiac cell types: A systematic review. Stem Cell Res. Ther. 2019, 10, 194.
- Malik, Z.; Kott, K.; Poe, A.; Kuo, T.; Chen, L.; Ferrara, K.; Knowlton, A. Cardiac myocyte exosomes: Stability, HSP60, and proteomics. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H954–H965.
- Cambier, L.; Giani, J.F.; Liu, W.; Ijichi, T.; Echavez, A.K.; Valle, J.; Marbán, E. Angiotensin II-induced end-organ damage in mice is attenuated by human exosomes and by an exosomal Y RNA fragment. Hypertension 2018, 72, 370–380.
- Bai, S.; Yin, Q.; Dong, T.; Dai, F.; Qin, Y.; Ye, L.; Du, J.; Zhang, Q.; Chen, H.; Shen, B. Endothelial progenitor cell-derived exosomes ameliorate endothelial dysfunction in a mouse model of diabetes. Biomed. Pharmacother. 2020, 131, 110756.
- Feng, Y.; Huang, W.; Meng, W.; Jegga, A.G.; Wang, Y.; Cai, W.; Kim, H.W.; Pasha, Z.; Wen, Z.; Rao, F.; et al. Heat shock improves Sca-1+ stem cell survival and directs ischemic cardiomyocytes toward a prosurvival phenotype via exosomal transfer: A critical role for HSF1/miR-34a/HSP70 pathway. Stem Cells 2014, 32, 462–472.
- Yu, D.W.; Ge, P.P.; Liu, A.L.; Yu, X.Y.; Liu, T.T. HSP20-mediated cardiomyocyte exosomes improve cardiac function in mice with myocardial infarction by activating Akt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4873–4881.
- Gupta, S.; Knowlton, A.A. HSP60 trafficking in adult cardiac myocytes: Role of the exosomal pathway. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H3052–H3056.
- Zininga, T.; Ramatsui, L.; Shonhai, A. Heat Shock Proteins as Immunomodulants. Molecules 2018, 23, 2846.
- Wang, X.; Gu, H.; Huang, W.; Peng, J.; Li, Y.; Yang, L.; Qin, D.; Essandoh, K.; Wang, Y.; Peng, T.; et al. Hsp20-Mediated Activation of Exosome Biogenesis in Cardiomyocytes Improves Cardiac Function and Angiogenesis in Diabetic Mice. Diabetes 2016, 65, 3111–3128.
- Kim, S.C.; Stice, J.P.; Chen, L.; Jung, J.S.; Gupta, S.; Wang, Y.; Baumgarten, G.; Trial, J.; Knowlton, A.A. Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ. Res. 2009, 105, 1186–1195.
- Duan, Y.; Tang, H.; Mitchell-Silbaugh, K.; Fang, X.; Han, Z.; Ouyang, K. Heat Shock Protein 60 in Cardiovascular Physiology and Diseases. Front. Mol. Biosci. 2020, 7, 73.
- Vicencio, J.M.; Yellon, D.M.; Sivaraman, V.; Das, D.; Boi-Doku, C.; Arjun, S.; Zheng, Y.; Riquelme, J.A.; Kearney, J.; Sharma, V.; et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1525–1536.
- Song, N.; Ma, J.; Meng, X.W.; Liu, H.; Wang, H.; Song, S.Y.; Chen, Q.C.; Liu, H.Y.; Zhang, J.; Peng, K.; et al. Heat Shock Protein 70 Protects the Heart from Ischemia/Reperfusion Injury through Inhibition of p38 MAPK Signaling. Oxid. Med. Cell. Longev. 2020, 2020, 3908641.
- Shi, C.; Ulke-Lemée, A.; Deng, J.; Batulan, Z.; O’Brien, E.R. Characterization of heat shock protein 27 in extracellular vesicles: A potential anti-inflammatory therapy. FASEB J. 2019, 33, 1617–1630.
- Pulakazhi Venu, V.K.; Adijiang, A.; Seibert, T.; Chen, Y.-X.; Shi, C.; Batulan, Z.; O’Brien, E.R. Heat shock protein 27-derived atheroprotection involves reverse cholesterol transport that is dependent on GM-CSF to maintain ABCA1 and ABCG1 expression in ApoE−/− mice. FASEB J. 2017, 31, 2364–2379.
- Seibert, T.A.; Hibbert, B.; Chen, Y.X.; Rayner, K.; Simard, T.; Hu, T.; Cuerrier, C.M.; Zhao, X.; de Belleroche, J.; Chow, B.J.; et al. Serum heat shock protein 27 levels represent a potential therapeutic target for atherosclerosis: Observations from a human cohort and treatment of female mice. J. Am. Coll. Cardiol. 2013, 62, 1446–1454.
- Shi, C.; Alvarez-Olmedo, D.; Zhang, Y.; Pattar, B.S.B.; O’Brien, E.R. The Heat Shock Protein 27 Immune Complex Enhances Exosomal Cholesterol Efflux. Biomedicines 2020, 8, 290.
- Boilard, E. Extracellular vesicles and their content in bioactive lipid mediators: More than a sack of microRNA. J. Lipid Res. 2018, 59, 2037–2046.
- Garcia, N.A.; Gonzalez-King, H.; Grueso, E.; Sanchez, R.; Martinez-Romero, A.; Javega, B.; O’Connor, J.E.; Simons, P.J.; Handberg, A.; Sepulveda, P. Circulating exosomes deliver free fatty acids from the bloodstream to cardiac cells: Possible role of CD36. PLoS ONE 2019, 14, e0217546.
- Tsuda, K. Plasma homocysteine levels and endothelial dysfunction in cerebro- and cardiovascular diseases in the metabolic syndrome. Am. J. Hypertens. 2015, 28, 1489.
- Liao, J.K. Linking Endothelial Dysfunction with Endothelial Cell Activation. J. Clin. Investig. 2013, 123, 540–541.
- Lüscher, T.F. Inflammation: The new cardiovascular risk factor. Eur. Heart J. 2018, 39, 3483–3487.
- Li, Y.; Wang, B. Circular RNA circCHFR downregulation protects against oxidized low-density lipoprotein-induced endothelial injury via regulation of microRNA-15b-5p/growth arrest and DNA damage inducible gamma. Bioengineered 2022, 13, 4481–4492.
- Zhan, R.; Leng, X.; Liu, X.; Wang, X.; Gong, J.; Yan, L.; Wang, L.; Wang, Y.; Wang, X.; Qian, L.J. Heat shock protein 70 is secreted from endothelial cells by a non-classical pathway involving exosomes. Biochem. Biophys. Res. Commun. 2009, 387, 229–233.
- Xie, F.; Zhan, R.; Yan, L.C.; Gong, J.B.; Zhao, Y.; Ma, J.; Qian, L.J. Diet-induced elevation of circulating HSP70 may trigger cell adhesion and promote the development of atherosclerosis in rats. Cell Stress Chaperones 2016, 21, 907–914.
- Bäck, M.; Yurdagul, A., Jr.; Tabas, I.; Öörni, K.; Kovanen, P.T. Inflammation and its resolution in atherosclerosis: Mediators and therapeutic opportunities. Nat. Rev. Cardiol. 2019, 16, 389–406.
- Tang, N.; Sun, B.; Gupta, A.; Rempel, H.; Pulliam, L. Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-κB in endothelial cells. FASEB J. 2016, 30, 3097–3106.
- Bouchareychas, L.; Duong, P.; Covarrubias, S.; Alsop, E.; Phu, T.A.; Chung, A.; Gomes, M.; Wong, D.; Meechoovet, B.; Capili, A.; et al. Macrophage Exosomes Resolve Atherosclerosis by Regulating Hematopoiesis and Inflammation via MicroRNA Cargo. Cell Rep. 2020, 32, 107881.
- Cheng, X.; Zhou, H.; Zhou, Y.; Song, C. M2 Macrophage-Derived Exosomes Inhibit Apoptosis of HUVEC Cell through Regulating miR-221-3p Expression. Biomed. Res. Int. 2022, 2022, 1609244.
- Grootaert, M.O.J.; Bennett, M.R. Vascular smooth muscle cells in atherosclerosis: Time for a re-assessment. Cardiovasc. Res. 2021, 117, 2326–2339.
- Niu, C.; Wang, X.; Zhao, M.; Cai, T.; Liu, P.; Li, J.; Willard, B.; Zu, L.; Zhou, E.; Li, Y.; et al. Macrophage Foam Cell-Derived Extracellular Vesicles Promote Vascular Smooth Muscle Cell Migration and Adhesion. J. Am. Heart Assoc. 2016, 5, e004099.
- Ren, L.; Chen, S.; Yao, D.; Yan, H. OxLDL-stimulated macrophage exosomes promote proatherogenic vascular smooth muscle cell viability and invasion via delivering miR-186-5p then inactivating SHIP2 mediated PI3K/AKT/mTOR pathway. Mol. Immunol. 2022, 146, 27–37.
- Głuchowska, A.; Cysewski, D.; Baj-Krzyworzeka, M.; Szatanek, R.; Węglarczyk, K.; Podszywałow-Bartnicka, P.; Sunderland, P.; Kozłowska, E.; Śliwińska, M.A.; Dąbrowski, M.; et al. Unbiased proteomic analysis of extracellular vesicles secreted by senescent human vascular smooth muscle cells reveals their ability to modulate immune cell functions. Geroscience 2022, 44, 2863–2884.
- Wencker, D.; Chandra, M.; Nguyen, K.; Miao, W.; Garantziotis, S.; Factor, S.M.; Shirani, J.; Amstrong, R.C.; Kitsis, R.N. A mechanistic role for cardiac myocyte apoptosis in heart failure. J. Clin. Investig. 2003, 111, 1497–1504.
- Hausenloy, D.J.; Yellon, D.M. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013, 123, 92–100.
- Lopez-Neblina, F.; Toledo, A.H.; Toledo-Pereyra, L.H. Molecular biology of apoptosis in ischemia and reperfusion. J. Investig. Surg. 2005, 18, 335–350.
- Abrial, M.; Da Silva, C.C.; Pillot, B.; Augeul, L.; Ivanes, F.; Teixeira, G.; Cartier, R.; Angoulvant, D.; Ovize, M.; Ferrera, R. Cardiac fibroblasts protect cardiomyocytes against lethal ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2014, 68, 56–65.
- Liu, N.; Xie, L.; Xiao, P.; Chen, X.; Kong, W.; Lou, Q.; Chen, F.; Lu, X. Cardiac fibroblasts secrete exosome microRNA to suppress cardiomyocyte pyroptosis in myocardial ischemia/reperfusion injury. Mol. Cell. Biochem. 2022, 477, 1249–1260.
- Luo, H.; Li, X.; Li, T.; Zhao, L.; He, J.; Zha, L.; Qi, Q.; Yu, Z. microRNA-423-3p exosomes derived from cardiac fibroblasts mediates the cardioprotective effects of ischaemic post-conditioning. Cardiovasc. Res. 2019, 115, 1189–1204.
- Qiao, L.; Hu, S.; Liu, S.; Zhang, H.; Ma, H.; Huang, K.; Li, Z.; Su, T.; Vandergriff, A.; Tang, J.; et al. microRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J. Clin. Investig. 2019, 129, 2237–2250.
- Yue, Y.; Garikipati, V.N.S.; Verma, S.K.; Goukassian, D.A.; Kishore, R. Interleukin-10 Deficiency Impairs Reparative Properties of Bone Marrow-Derived Endothelial Progenitor Cell Exosomes. Tissue Eng. Part A 2017, 23, 1241–1250.
- Yue, Y.; Wang, C.; Benedict, C.; Huang, G.; Truongcao, M.; Roy, R.; Cimini, M.; Garikipati, V.N.S.; Cheng, Z.; Koch, W.J.; et al. Interleukin-10 Deficiency Alters Endothelial Progenitor Cell-Derived Exosome Reparative Effect on Myocardial Repair via Integrin-Linked Kinase Enrichment. Circ. Res. 2020, 126, 315–329.
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262.
- Bhullar, S.K.; Dhalla, N.S. Angiotensin II-Induced Signal Transduction Mechanisms for Cardiac Hypertrophy. Cells 2022, 11, 3336.
- Tian, C.; Gao, L.; Zimmerman, M.C.; Zucker, I.H. Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H928–H939.
- Lyu, L.; Wang, H.; Li, B.; Qin, Q.; Qi, L.; Nagarkatti, M.; Nagarkatti, P.; Janicki, J.S.; Wang, X.L.; Cui, T. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J. Mol. Cell. Cardiol. 2015, 89, 268–279.
- Constantin, A.; Comarița, I.K.; Alexandru, N.; Filippi, A.; Bojin, F.; Gherghiceanu, M.; Vîlcu, A.; Nemecz, M.; Niculescu, L.S.; Păunescu, V.; et al. Stem cell-derived extracellular vesicles reduce the expression of molecules involved in cardiac hypertrophy-In a model of human-induced pluripotent stem cell-derived cardiomyocytes. Front. Pharmacol. 2022, 13, 1003684.
- Ren, Y.; Wu, Y.; He, W.; Tian, Y.; Zhao, X. Exosomes secreted from bone marrow mesenchymal stem cells suppress cardiomyocyte hypertrophy through Hippo-YAP pathway in heart failure. Genet. Mol. Biol. 2023, 46, e20220221.
- Mao, L.; Li, Y.D.; Chen, R.L.; Li, G.; Zhou, X.X.; Song, F.; Wu, C.; Hu, Y.; Hong, Y.X.; Dang, X.; et al. Heart-targeting exosomes from human cardiosphere-derived cells improve the therapeutic effect on cardiac hypertrophy. J. Nanobiotechnol. 2022, 20, 435.
- Nguyen, B.Y.; Azam, T.; Wang, X. Cellular signaling cross-talk between different cardiac cell populations: An insight into the role of exosomes in the heart diseases and therapy. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1213–H1234.
- Liang, X.; Zhang, L.; Wang, S.; Han, Q.; Zhao, R.C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell Sci. 2016, 129, 2182–2189.
- Wang, Y.; Zhao, R.; Liu, W.; Wang, Z.; Rong, J.; Long, X.; Liu, Z.; Ge, J.; Shi, B. Exosomal circHIPK3 Released from Hypoxia-Pretreated Cardiomyocytes Regulates Oxidative Damage in Cardiac Microvascular Endothelial Cells via the miR-29a/IGF-1 Pathway. Oxid. Med. Cell. Longev. 2019, 2019, 7954657.
- Ribeiro-Rodrigues, T.M.; Laundos, T.L.; Pereira-Carvalho, R.; Batista-Almeida, D.; Pereira, R.; Coelho-Santos, V.; Silva, A.P.; Fernandes, R.; Zuzarte, M.; Enguita, F.J.; et al. Exosomes secreted by cardiomyocytes subjected to ischaemia promote cardiac angiogenesis. Cardiovasc. Res. 2017, 113, 1338–1350.
- Gou, L.; Xue, C.; Tang, X.; Fang, Z. Inhibition of Exo-miR-19a-3p derived from cardiomyocytes promotes angiogenesis and improves heart function in mice with myocardial infarction via targeting HIF-1α. Aging 2020, 12, 23609–23618.
- Zimna, A.; Kurpisz, M. Hypoxia-inducible Factor-1 in physiological and pathophysiological angiogenesis: Applications and therapies. Biomed. Res. Int. 2015, 2015, 549412.
- Yang, J.; Li, Y.; Xue, F.; Liu, W.; Zhang, S. Exosomes derived from cardiac telocytes exert positive effects on endothelial cells. Am. J. Transl. Res. 2017, 9, 5375–5387.
- Jansen, F.; Yang, X.; Proebsting, S.; Hoelscher, M.; Przybilla, D.; Baumann, K.; Schmitz, T.; Dolf, A.; Endl, E.; Franklin, B.S.; et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J. Am. Heart Assoc. 2014, 3, e001249.
- Shatseva, T.; Lee, D.Y.; Deng, Z.; Yang, B.B. MicroRNA miR-199a-3p regulates cell proliferation and survival by targeting caveolin-2. J. Cell Sci. 2011, 124, 2826–2836.
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 2008, 15, 261–267.
- Liu, S.; Chen, J.; Shi, J.; Zhou, W.; Wang, L.; Fang, W.; Zhong, Y.; Chen, X.; Chen, Y.; Sabri, A.; et al. M1-like macrophage-derived exosomes suppress angiogenesis and exacerbate cardiac dysfunction in a myocardial infarction microenvironment. Basic Res. Cardiol. 2020, 115, 22.
- Wang, C.; Zhang, C.; Liu, L.; Axx, X.; Chen, B.; Li, Y.; Du, J. Macrophage-derived mir-155-containing exosomes suppress fibroblast proliferation and promote fibroblast inflammation during cardiac injury. Mol. Ther. 2017, 25, 192–204.
- Wu, S.; Zou, M.H. AMPK, Mitochondrial Function, and Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 4987.
- Ivey, M.J.; Tallquist, M.D. Defining the Cardiac Fibroblast. Circ. J. 2016, 80, 2269–2276.
- Kurose, H. Cardiac Fibrosis and Fibroblasts. Cells 2021, 10, 1716.
- Aghajanian, H.; Kimura, T.; Rurik, J.G.; Hancock, A.S.; Leibowitz, M.S.; Li, L.; Scholler, J.; Monslow, J.; Lo, A.; Han, W.; et al. Targeting cardiac fibrosis with engineered T cells. Nature 2019, 573, 430–433.
- Lam, C.S.P.; Voors, A.A.; de Boer, R.A.; Solomon, S.D.; van Veldhuisen, D.J. Heart failure with preserved ejection fraction: From mechanisms to therapies. Eur. Heart J. 2018, 39, 2780–2792.
- Kong, P.; Christia, P.; Frangogiannis, N.G. The pathogenesis of cardiac fibrosis. Cell. Mol. Life Sci. 2014, 71, 549–574.
- Sullivan, K.E.; Deems Black, L. The role of cardiac fibroblasts in extracellular matrix-mediated signaling during normal and pathological cardiac development. J. Biomech. Eng. 2013, 135, 71001.
- Chaturvedi, P.; Kalani, A.; Medina, I.; Familtseva, A.; Tyagi, S.C. Cardiosome mediated regulation of MMP9 in diabetic heart: Role of mir29b and mir455 in exercise. J. Cell. Mol. Med. 2015, 19, 2153–2161.
- Li, H.; Fan, J.; Yin, Z.; Wang, F.; Chen, C.; Wang, D.W. Identification of cardiac-related circulating microRNA profile in human chronic heart failure. Oncotarget 2016, 7, 33–45.
- Nie, X.; Fan, J.; Li, H.; Yin, Z.; Zhao, Y.; Dai, B.; Dong, N.; Chen, C.; Wang, D.W. miR-217 Promotes Cardiac Hypertrophy and Dysfunction by Targeting PTEN. Mol. Ther. Nucleic Acids 2018, 12, 254–266.
- Yuan, J.; Liu, H.; Gao, W.; Zhang, L.; Ye, Y.; Yuan, L.; Ding, Z.; Wu, J.; Kang, L.; Zhang, X.; et al. MicroRNA-378 suppresses myocardial fibrosis through a paracrine mechanism at the early stage of cardiac hypertrophy following mechanical stress. Theranostics 2018, 8, 2565–2582.
- Tang, C.; Hou, Y.X.; Shi, P.X.; Zhu, C.H.; Lu, X.; Wang, X.L.; Que, L.L.; Zhu, G.Q.; Liu, L.; Chen, Q.; et al. Cardiomyocyte-specific Peli1 contributes to the pressure overload-induced cardiac fibrosis through miR-494-3p-dependent exosomal communication. FASEB J. 2023, 37, e22699.
- Govindappa, P.K.; Patil, M.; Garikipati, V.; Verma, S.K.; Saheera, S.; Narasimhan, G.; Zhu, W.; Kishore, R.; Zhang, J.; Krishnamurthy, P. Targeting exosome-associated human antigen R attenuates fibrosis and inflammation in diabetic heart. FASEB J. 2020, 34, 2238–2251.
- Kanno, S.; Sakamoto, T.; Fukuta, M.; Kato, H.; Aoki, Y. Stability of exosomes in the postmortem serum and preliminary study on exosomal miRNA expression profiling in serum from myocardial infarction cadavers. Int. J. Legal Med. 2022, 9, 5375–5387.
- Kuwabara, Y.; Ono, K.; Horie, T.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Baba, O.; Kojima, Y.; Shizuta, S.; et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ. Cardiovasc. Genet. 2011, 4, 446–454.
- Hammarsten, O.; Mair, J.; Möckel, M.; Lindahl, B.; Jaffe, A.S. Possible mechanisms behind cardiac troponin elevations. Biomarkers 2018, 23, 725–734.
- Chaulin, A.M. The Metabolic Pathway of Cardiac Troponins Release: Mechanisms and Diagnostic Role. Cardiol Res. 2022, 13, 190–205.
- Silva-Palacios, A.; Arroyo-Campuzano, M.; Flores-García, M.; Patlán, M.; Hernández-Díazcouder, A.; Alcántara, D.; Ramírez-Camacho, I.; Arana-Hidalgo, D.; Soria-Castro, E.; Sánchez, F.; et al. Citicoline Modifies the Expression of Specific miRNAs Related to Cardioprotection in Patients with ST-Segment Elevation Myocardial Infarction Subjected to Coronary Angioplasty. Pharmaceuticals 2022, 15, 925.
- Chen, X.; Huang, F.; Liu, Y.; Liu, S.; Tan, G. Exosomal miR-152-5p and miR-3681-5p function as potential biomarkers for ST-segment elevation myocardial infarction. Clinics 2022, 77, 100038.
- Xie, Z.; Wang, X.; Liu, X.; Du, H.; Sun, C.; Shao, X.; Tian, J.; Gu, X.; Wang, H.; Tian, J.; et al. Adipose-Derived Exosomes Exert Proatherogenic Effects by Regulating Macrophage Foam Cell Formation and Polarization. J. Am. Heart Assoc. 2018, 7, e007442.
- Ferrante, S.C.; Nadler, E.P.; Pillai, D.K.; Hubal, M.J.; Wang, Z.; Wang, J.M.; Gordish-Dressman, H.; Koeck, E.; Sevilla, S.; Wiles, A.A.; et al. Adipocyte-derived exosomal miRNAs: A novel mechanism for obesity-related disease. Pediatr. Res. 2015, 77, 447–454.
- Ogawa, R.; Tanaka, C.; Sato, M.; Nagasaki, H.; Sugimura, K.; Okumura, K.; Nakagawa, Y.; Aoki, N. Adipocyte-derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem. Biophys. Res. Commun. 2010, 398, 723–729.
- Zhang, Y.; Mei, H.; Chang, X.; Chen, F.; Zhu, Y.; Han, X. Adipocyte-derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR-155. J. Mol. Cell Biol. 2016, 8, 505–517.
- Barberio, M.D.; Kasselman, L.J.; Playford, M.P.; Epstein, S.B.; Renna, H.A.; Goldberg, M.; DeLeon, J.; Voloshyna, I.; Barlev, A.; Salama, M.; et al. Cholesterol efflux alterations in adolescent obesity: Role of adipose-derived extracellular vesical microRNAs. J. Transl. Med. 2019, 17, 232.
- Liu, Y.; Sun, Y.; Lin, X.; Zhang, D.; Hu, C.; Liu, J.; Zhu, Y.; Gao, A.; Han, H.; Chai, M.; et al. Perivascular adipose-derived exosomes reduce macrophage foam cell formation through miR-382-5p and the BMP4-PPARγ-ABCA1/ABCG1 pathways. Vascul. Pharmacol. 2022, 143, 106968.
- Kita, S.; Maeda, N.; Shimomura, I. Interorgan communication by exosomes, adipose tissue, and adiponectin in metabolic syndrome. J. Clin. Investig. 2019, 129, 4041–4049.
- Phu, T.A.; Ng, M.; Vu, N.K.; Bouchareychas, L.; Raffai, R.L. IL-4 polarized human macrophage exosomes control cardiometabolic inflammation and diabetes in obesity. Mol. Ther. 2022, 30, 2274–2297.
- Liu, T.; Sun, Y.C.; Cheng, P.; Shao, H.G. Adipose tissue macrophage-derived exosomal miR-29a regulates obesity-associated insulin resistance. Biochem. Biophys. Res. Commun. 2019, 515, 352–358.
- Liu, X.; Chu, H.; Ji, Y.; Bosnjak, Z.; Ao, H.; Li, T. Which BMI for Diabetes Patients is Better? From the View of the Adipose Tissue Macrophage-Derived Exosome. Diabetes Metab. Syndr. Obes. 2022, 15, 141–153.
- Ying, W.; Riopel, M.; Bandyopadhyay, G.; Dong, Y.; Birmingham, A.; Seo, J.B.; Ofrecio, J.M.; Wollam, J.; Hernandez-Carretero, A.; Fu, W.; et al. Adipose Tissue Macrophage-Derived Exosomal miRNAs Can Modulate In Vivo and In Vitro Insulin Sensitivity. Cell 2017, 17, 1372–1784.e12.
- Hubal, M.J.; Nadler, E.P.; Ferrante, S.C.; Barberio, M.D.; Suh, J.H.; Wang, J.; Dohm, G.L.; Pories, W.J.; Mietus-Snyder, M.; Freishtat, R.J. Circulating adipocyte-derived exosomal MicroRNAs associated with decreased insulin resistance after gastric bypass. Obesity 2017, 25, 102–110.
