C-X-C motif chemokine ligand 1 (CXCL1) is a member of the CXC chemokine subfamily and a ligand for CXCR2. Its main function in the immune system is the chemoattraction of neutrophils. Female breast cancer is the most commonly diagnosed cancer. In 2020, 2.26 million new cases of this cancer were diagnosed, accounting for 11.7% of all cancer diagnoses
- chemokine
- CXCL1
- breast cancer
1. Introduction
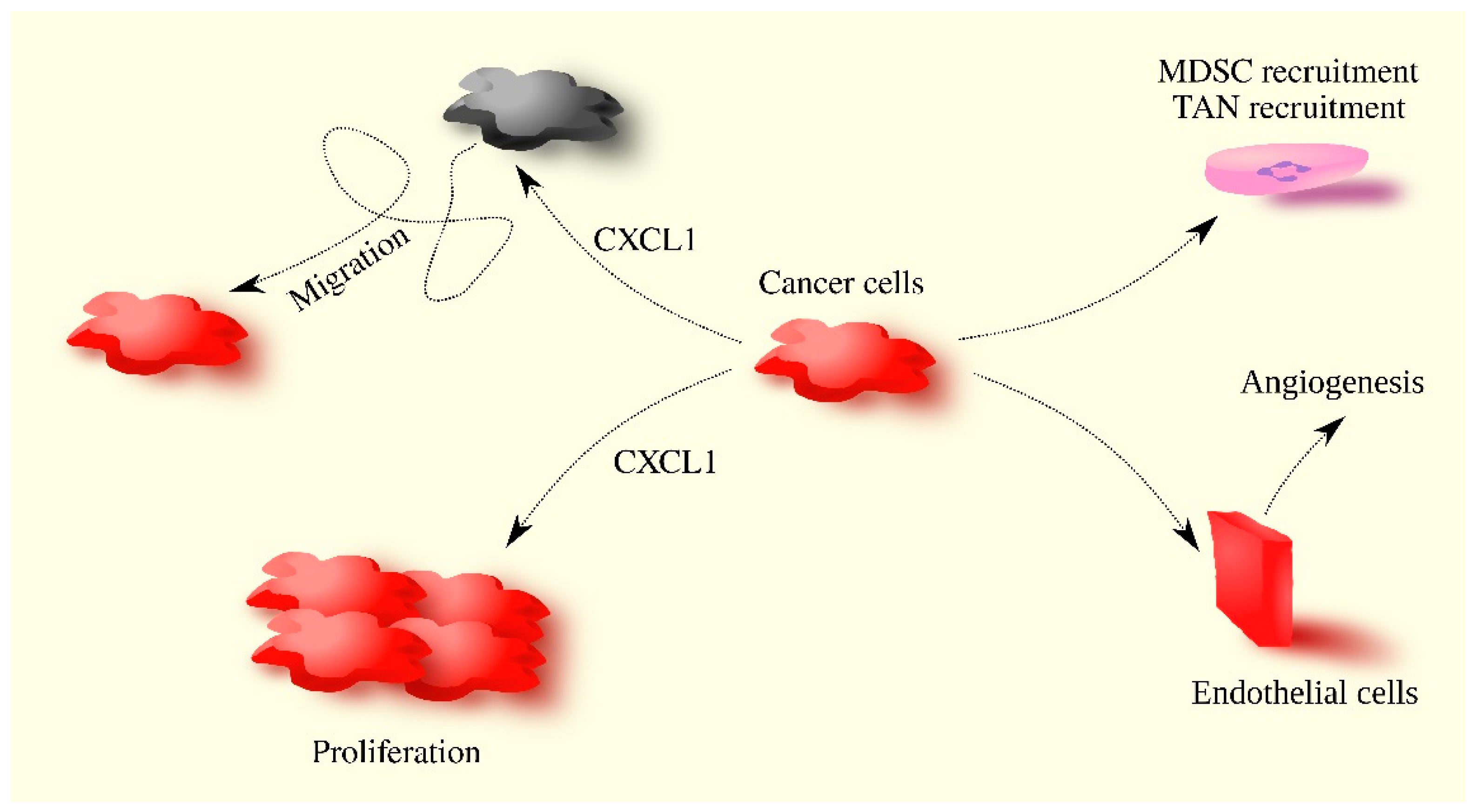
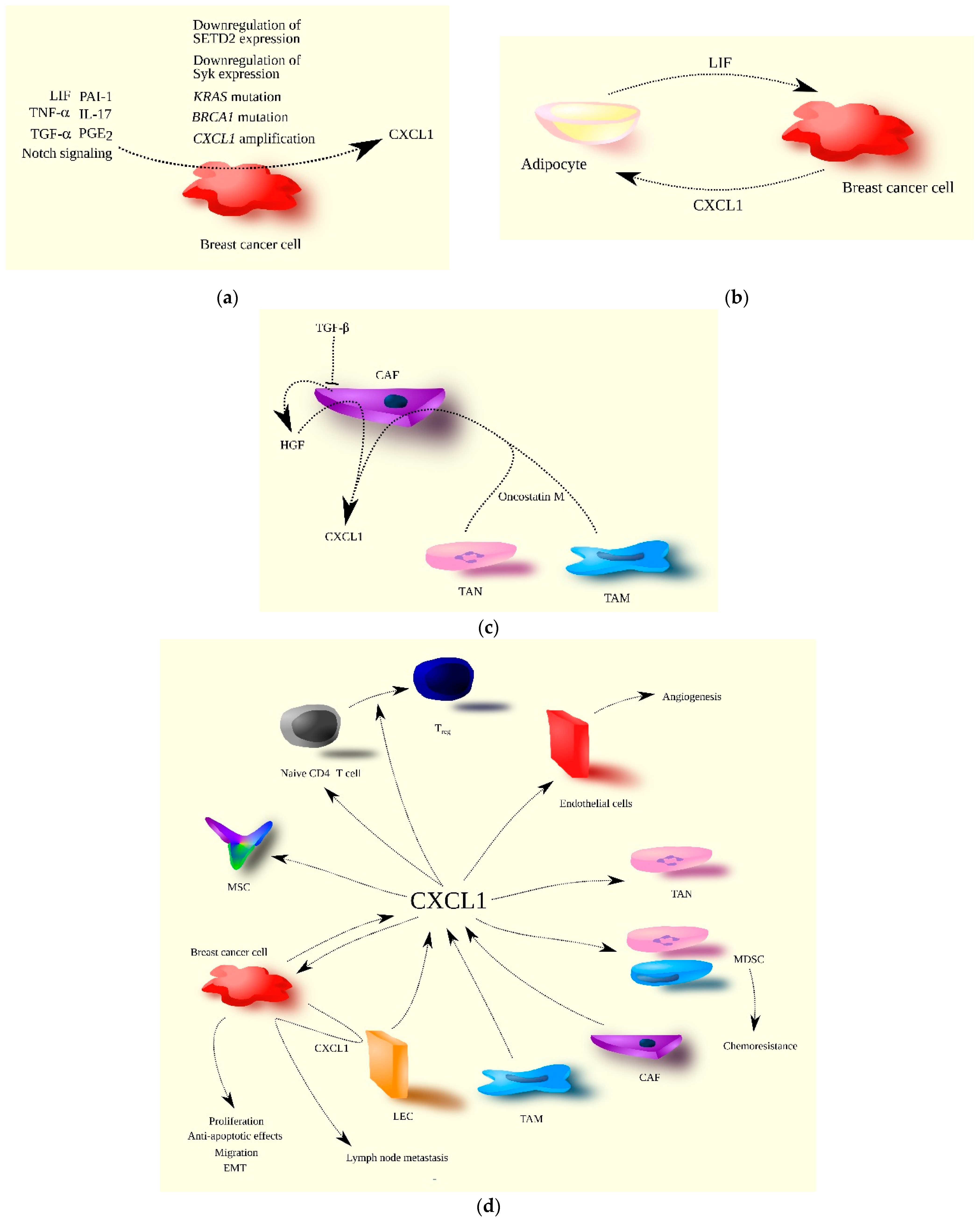
2. CXCL1 in Breast Cancer
-
Luminal A (ER+PR+HER2−);
-
Luminal B (ER+HER2− + PR− or Ki-67high);
-
Luminal HER2-positive (HER2+ + ER+ or PR+);
-
Non-luminal HER2-positive (ER−PR−HER2+);
-
Triple-negative breast cancer (ER−PR−HER2−).
|
Type of Cancer |
Expression Testing Method |
Impact on Survival at High CXCL1 Expression |
Number of Patients in the Study |
Notes |
Source |
|---|---|---|---|---|---|
|
Breast cancer: ERα-positive breast cancer |
qRT-PCR |
Worse prognosis |
48 |
RFS |
|
|
Breast cancer: ERα-positive breast cancer |
Microarray Kaplan–Meier Plotter database |
Better prognosis |
2061 |
RFS Analysis based on the Kaplan–Meier Plotter database |
|
|
Breast cancer |
IHC |
Worse prognosis |
655 |
OS, nuclear CXCL1 expression, at cytoplasmic CXCL1 expression there was only a trend (p = 0.08) |
|
|
Breast cancer |
Microarray UALCAN/TCGA database |
Better prognosis |
1066 |
OS, Analysis based on UALCAN |
|
|
Breast cancer |
Microarray |
Worse prognosis |
121 |
OS |
|
|
Breast cancer |
Microarray Kaplan–Meier Plotter database |
Better prognosis |
3951/RFS 1402/OS |
OS, RFS analysis based on the Kaplan–Meier Plotter database |
|
|
Breast cancer |
Microarray Kaplan–Meier Plotter database |
No significant impact on prognosis |
1402 |
OS, trend of worse prognosis at high CXCL1 (p = 0.064), based on the Kaplan–Meier Plotter database |
|
|
Breast cancer |
ELISA |
Worse prognosis |
61 |
OS, PFS circulating level of CXCL1 |
|
|
Breast cancer: basal breast cancer |
Microarray Kaplan–Meier Plotter database |
Worse prognosis |
54 |
OS, based on the Kaplan–Meier Plotter database |
|
|
Breast cancer |
Microarray Finak microarray database |
Worse prognosis |
53 |
RFS stromal CXCL1 expression based on Finak microarray database |
ELISA—enzyme-linked immunosorbent assay; IHC—immunohistochemistry; OS—overall survival; PFS—progression-free survival; RFS—relapse-free survival; qRT-PCR—quantitative real-time polymerase chain reaction. Red color—worse prognosis, Blue color—better prognosis.
References
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971.
- Egeblad, M.; Nakasone, E.S.; Werb, Z. Tumors as organs: Complex tissues that interface with the entire organism. Develop. Cell 2010, 18, 884–901.
- Do, H.T.T.; Lee, C.H.; Cho, J. Chemokines and their Receptors: Multifaceted Roles in Cancer Progression and Potential Value as Cancer Prognostic Markers. Cancers 2020, 12, 287.
- Richmond, A.; Lawson, D.H.; Nixon, D.W.; Chawla, R.K. Characterization of autostimulatory and transforming growth factors from human melanoma cells. Cancer Res. 1985, 45, 6390–6394.
- Ludwig, A.; Petersen, F.; Zahn, S.; Götze, O.; Schröder, J.M.; Flad, H.D.; Brandt, E. The CXC-chemokine neutrophil-activating peptide-2 induces two distinct optima of neutrophil chemotaxis by differential interaction with interleukin-8 receptors CXCR-1 and CXCR-2. Blood 1997, 90, 4588–4597.
- Szabo, M.C.; Soo, K.S.; Zlotnik, A.; Schall, T.J. Chemokine class differences in binding to the Duffy antigen-erythrocyte chemokine receptor. J. Biol. Chem. 1995, 270, 25348–25351.
- Damaj, B.B.; McColl, S.R.; Mahana, W.; Crouch, M.F.; Naccache, P.H. Physical association of Gi2α with interleukin-8 receptors. J. Biol. Chem. 1996, 271, 12783–12789.
- Kuwano, Y.; Adler, M.; Zhang, H.; Groisman, A.; Ley, K. Gαi2 and Gαi3 differentially regulate arrest from flow and chemotaxis in mouse neutrophils. J. Immunol. 2016, 196, 3828–3833.
- Raman, D.; Neel, N.F.; Sai, J.; Mernaugh, R.L.; Ham, A.J.; Richmond, A.J. Characterization of chemokine receptor CXCR2 interacting proteins using a proteomics approach to define the CXCR2 “chemosynapse”. Methods Enzymol. 2009, 460, 315–330.
- Kawanishi, H.; Matsui, Y.; Ito, M.; Watanabe, J.; Takahashi, T.; Nishizawa, K.; Nishiyama, H.; Kamoto, T.; Mikami, Y.; Tanaka, Y.; et al. Secreted CXCL1 is a potential mediator and marker of the tumor invasion of bladder cancer. Clin. Cancer Res. 2008, 14, 2579–2587.
- Lee, C.H.; Syu, S.H.; Liu, K.J.; Chu, P.Y.; Yang, W.C.; Lin, P.; Shieh, W.Y. Interleukin-1 beta transactivates epidermal growth factor receptor via the CXCL1-CXCR2 axis in oral cancer. Oncotarget 2015, 6, 38866–38880.
- Grist, J.J.; Marro, B.S.; Skinner, D.D.; Syage, A.R.; Worne, C.; Doty, D.J.; Fujinami, R.S.; Lane, T.E. Induced CNS expression of CXCL1 augments neurologic disease in a murine model of multiple sclerosis via enhanced neutrophil recruitment. Eur. J. Immunol. 2018, 48, 1199–1210.
- Michael, B.D.; Bricio-Moreno, L.; Sorensen, E.W.; Miyabe, Y.; Lian, J.; Solomon, T.; Kurt-Jones, E.A.; Luster, A.D. Astrocyte- and Neuron-Derived CXCL1 Drives Neutrophil Transmigration and Blood-Brain Barrier Permeability in Viral Encephalitis. Cell Rep. 2020, 32, 108150.
- Cortés-Vieyra, R.; Rosales, C.; Uribe-Querol, E. Neutrophil Functions in Periodontal Homeostasis. J. Immunol. Res. 2016, 2016, 1396106.
- Roh, Y.S.; Zhang, B.; Loomba, R.; Seki, E. TLR2 and TLR9 contribute to alcohol-mediated liver injury through induction of CXCL1 and neutrophil infiltration. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G30–G41.
- Sander, L.E.; Sackett, S.D.; Dierssen, U.; Beraza, N.; Linke, R.P.; Müller, M.; Blander, J.M.; Tacke, F.; Trautwein, C. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J. Exp. Med. 2010, 207, 1453–1464.
- Addison, C.L.; Daniel, T.O.; Burdick, M.D.; Liu, H.; Ehlert, J.E.; Xue, Y.Y.; Buechi, L.; Walz, A.; Richmond, A.; Strieter, R.M. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J. Immunol. 2000, 165, 5269–5277.
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Lomma, C.; Chan, A.; Chih, H.; Reid, C.; Peter, W. Male Breast Cancer in Australia. Asia Pac. J. Clin. Oncol. 2021, 17, e57–e62.
- Onitilo, A.A.; Engel, J.M.; Greenlee, R.T.; Mukesh, B.N. Breast cancer subtypes based on ER/PR and Her2 expression: Comparison of clinicopathologic features and survival. Clin. Med. Res. 2009, 7, 4–13.
- Yersal, O.; Barutca, S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412–424.
- Johansson, A.L.V.; Trewin, C.B.; Hjerkind, K.V.; Ellingjord-Dale, M.; Johannesen, T.B.; Ursin, G. Breast cancer-specific survival by clinical subtype after 7 years follow-up of young and elderly women in a nationwide cohort. Int. J. Cancer 2019, 144, 1251–1261.
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast cancer. Lancet 2021, 397, 1750–1769.
- Harbeck, N. Insights into biology of luminal HER2 vs. enriched HER2 subtypes: Therapeutic implications. Breast 2015, 24 (Suppl. 2), S44–S48.
- Bertucci, F.; Finetti, P.; Cervera, N.; Esterni, B.; Hermitte, F.; Viens, P.; Birnbaum, D. How basal are triple-negative breast cancers? Int. J. Cancer 2008, 123, 236–240.
- Rojas, K.; Stuckey, A. Breast Cancer Epidemiology and Risk Factors. Clin. Obstet. Gynecol. 2016, 59, 651–672.
- Yi, S.; Zhou, W. Tumorigenesis-related key genes in adolescents and young adults with HR(+)/HER2(−) breast cancer. Int. J. Clin. Exp. Pathol. 2020, 13, 2701–2709.
- Bièche, I.; Chavey, C.; Andrieu, C.; Busson, M.; Vacher, S.; Le Corre, L.; Guinebretière, J.M.; Burlinchon, S.; Lidereau, R.; Lazennec, G. CXC chemokines located in the 4q21 region are up-regulated in breast cancer. Endocr. Relat. Cancer 2007, 14, 1039–1052.
- Chen, E.; Qin, X.; Peng, K.; Xu, X.; Li, W.; Cheng, X.; Tang, C.; Cui, Y.; Wang, Z.; Liu, T. Identification of Potential Therapeutic Targets Among CXC Chemokines in Breast Tumor Microenvironment Using Integrative Bioinformatics Analysis. Cell Physiol. Biochem. 2018, 45, 1731–1746.
- Li, Y.; Liang, M.; Lin, Y.; Lv, J.; Chen, M.; Zhou, P.; Fu, F.; Wang, C. Transcriptional Expressions of CXCL9/10/12/13 as Prognosis Factors in Breast Cancer. J. Oncol. 2020, 2020, 4270957.
- Wang, F.; Yuan, C.; Wu, H.Z.; Liu, B.; Yang, Y.F. Bioinformatics, Molecular Docking and Experiments In Vitro Analyze the Prognostic Value of CXC Chemokines in Breast Cancer. Front. Oncol. 2021, 11, 665080.
- Abrahamsson, A.; Rzepecka, A.; Dabrosin, C. Equal Pro-inflammatory Profiles of CCLs, CXCLs, and Matrix Metalloproteinases in the Extracellular Microenvironment In Vivo in Human Dense Breast Tissue and Breast Cancer. Front. Immunol. 2018, 8, 1994.
- Wang, N.; Liu, W.; Zheng, Y.; Wang, S.; Yang, B.; Li, M.; Song, J.; Zhang, F.; Zhang, X.; Wang, Q.; et al. CXCL1 derived from tumor-associated macrophages promotes breast cancer metastasis via activating NF-κB/SOX4 signaling. Cell Death Dis. 2018, 9, 880.
- Ignacio, R.M.C.; Gibbs, C.R.; Lee, E.S.; Son, D.S. The TGFα-EGFR-Akt signaling axis plays a role in enhancing proinflammatory chemokines in triple-negative breast cancer cells. Oncotarget 2018, 9, 29286–29303.
- Lerebours, F.; Vacher, S.; Andrieu, C.; Espie, M.; Marty, M.; Lidereau, R.; Bieche, I. NF-kappa B genes have a major role in inflammatory breast cancer. BMC Cancer 2008, 8, 41.
- Zou, A.; Lambert, D.; Yeh, H.; Yasukawa, K.; Behbod, F.; Fan, F.; Cheng, N. Elevated CXCL1 expression in breast cancer stroma predicts poor prognosis and is inversely associated with expression of TGF-β signaling proteins. BMC Cancer 2014, 14, 781.
- Divella, R.; Daniele, A.; Savino, E.; Palma, F.; Bellizzi, A.; Giotta, F.; Simone, G.; Lioce, M.; Quaranta, M.; Paradiso, A.; et al. Circulating levels of transforming growth factor-βeta (TGF-β) and chemokine (C-X-C motif) ligand-1 (CXCL1) as predictors of distant seeding of circulating tumor cells in patients with metastatic breast cancer. Anticancer Res. 2013, 33, 1491–1497.
- Fujisawa, N.; Sakao, Y.; Hayashi, S.; Hadden, W.A., 3rd; Harmon, C.L.; Miller, E.J. alpha-Chemokine growth factors for adenocarcinomas; a synthetic peptide inhibitor for alpha-chemokines inhibits the growth of adenocarcinoma cell lines. J. Cancer Res. Clin. Oncol. 2000, 126, 19–26.
- Li, J.; Sidell, N. Growth-related oncogene produced in human breast cancer cells and regulated by Syk protein-tyrosine kinase. Int. J. Cancer 2005, 117, 14–20.
- Wang, Y.; Tu, L.; Du, C.; Xie, X.; Liu, Y.; Wang, J.; Li, Z.; Jiang, M.; Cao, D.; Yan, X.; et al. CXCR2 is a novel cancer stem-like cell marker for triple-negative breast cancer. OncoTargets Ther. 2018, 11, 5559–5567.
- Acharyya, S.; Oskarsson, T.; Vanharanta, S.; Malladi, S.; Kim, J.; Morris, P.G.; Manova-Todorova, K.; Leversha, M.; Hogg, N.; Seshan, V.E.; et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell 2012, 150, 165–178.
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404.
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 2013, 6, pl1.
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis Oncol. 2017, 2017, PO.17.00073.
- Ding, L.; Bailey, M.H.; Porta-Pardo, E.; Thorsson, V.; Colaprico, A.; Bertrand, D.; Gibbs, D.L.; Weerasinghe, A.; Huang, K.L.; Tokheim, C.; et al. Perspective on Oncogenic Processes at the End of the Beginning of Cancer Genomics. Cell 2018, 173, 305–320.e10.
- Ellrott, K.; Bailey, M.H.; Saksena, G.; Covington, K.R.; Kandoth, C.; Stewart, C.; Hess, J.; Ma, S.; Chiotti, K.E.; McLellan, M.; et al. Scalable Open Science Approach for Mutation Calling of Tumor Exomes Using Multiple Genomic Pipelines. Cell Syst. 2018, 6, 271–281.e7.
- Gao, Q.; Liang, W.W.; Foltz, S.M.; Mutharasu, G.; Jayasinghe, R.G.; Cao, S.; Liao, W.W.; Reynolds, S.M.; Wyczalkowski, M.A.; Yao, L.; et al. Driver Fusions and Their Implications in the Development and Treatment of Human Cancers. Cell Rep. 2018, 23, 227–238.e3.
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304.e6.
- Taylor, A.M.; Shih, J.; Ha, G.; Gao, G.F.; Zhang, X.; Berger, A.C.; Schumacher, S.E.; Wang, C.; Hu, H.; Liu, J.; et al. Genomic and Functional Approaches to Understanding Cancer Aneuploidy. Cancer Cell 2018, 33, 676–689.e3.
- Tkocz, D.; Crawford, N.T.; Buckley, N.E.; Berry, F.B.; Kennedy, R.D.; Gorski, J.J.; Harkin, D.P.; Mullan, P.B. BRCA1 and GATA3 corepress FOXC1 to inhibit the pathogenesis of basal-like breast cancers. Oncogene 2012, 31, 3667–3678.
- Gibson, J.T.; Orlandella, R.M.; Turbitt, W.J.; Behring, M.; Manne, U.; Sorge, R.E.; Norian, L.A. Obesity-Associated Myeloid-Derived Suppressor Cells Promote Apoptosis of Tumor-Infiltrating CD8 T Cells and Immunotherapy Resistance in Breast Cancer. Front. Immunol. 2020, 11, 590794.
- Pease, N.A.; Shephard, M.S.; Sertorio, M.; Waltz, S.E.; Vinnedge, L.M.P. DEK Expression in Breast Cancer Cells Leads to the Alternative Activation of Tumor Associated Macrophages. Cancers 2020, 12, 1936.
- Zhou, Y.; Zheng, X.; Xu, B.; Deng, H.; Chen, L.; Jiang, J. Histone methyltransferase SETD2 inhibits tumor growth via suppressing CXCL1-mediated activation of cell cycle in lung adenocarcinoma. Aging 2020, 12, 25189–25206.
- Al Sarakbi, W.; Sasi, W.; Jiang, W.G.; Roberts, T.; Newbold, R.F.; Mokbel, K. The mRNA expression of SETD2 in human breast cancer: Correlation with clinico-pathological parameters. BMC Cancer 2009, 9, 290.
- Vazquez-Martin, A.; Colomer, R.; Menendez, J.A. Protein array technology to detect HER2 (erbB-2)-induced ‘cytokine signature’ in breast cancer. Eur. J. Cancer 2007, 43, 1117–1124.
- Franklin, D.A.; Sharick, J.T.; Ericsson-Gonzalez, P.I.; Sanchez, V.; Dean, P.T.; Opalenik, S.R.; Cairo, S.; Judde, J.G.; Lewis, M.T.; Chang, J.C.; et al. MEK activation modulates glycolysis and supports suppressive myeloid cells in TNBC. JCI Insight 2020, 5, e134290.
- Wang, D.; Wang, H.; Brown, J.; Daikoku, T.; Ning, W.; Shi, Q.; Richmond, A.; Strieter, R.; Dey, S.K.; DuBois, R.N. CXCL1 induced by prostaglandin E2 promotes angiogenesis in colorectal cancer. J. Exp. Med. 2006, 203, 941–951.
- Varikuti, S.; Oghumu, S.; Elbaz, M.; Volpedo, G.; Ahirwar, D.K.; Alarcon, P.C.; Sperling, R.H.; Moretti, E.; Pioso, M.S.; Kimble, J.; et al. STAT1 gene deficient mice develop accelerated breast cancer growth and metastasis which is reduced by IL-17 blockade. Oncoimmunology 2017, 6, e1361088.
- Ma, K.; Yang, L.; Shen, R.; Kong, B.; Chen, W.; Liang, J.; Tang, G.; Zhang, B. Th17 cells regulate the production of CXCL1 in breast cancer. Int. Immunopharmacol. 2018, 56, 320–329.
- Novitskiy, S.V.; Pickup, M.W.; Gorska, A.E.; Owens, P.; Chytil, A.; Aakre, M.; Wu, H.; Shyr, Y.; Moses, H.L. TGF-β receptor II loss promotes mammary carcinoma progression by Th17 dependent mechanisms. Cancer Discov. 2011, 1, 430–441.
- Wei, C.Y.; Tan, Q.X.; Zhu, X.; Qin, Q.H.; Zhu, F.B.; Mo, Q.G.; Yang, W.P. Expression of CDKN1A/p21 and TGFBR2 in breast cancer and their prognostic significance. Int. J. Clin. Exp. Pathol. 2015, 8, 14619–14629.
- Zhou, C.; He, X.; Tong, C.; Li, H.; Xie, C.; Wu, Y.; Wang, L.; Yan, X.; Luo, D.; Tang, Y.; et al. Cancer-associated adipocytes promote the invasion and metastasis in breast cancer through LIF/CXCLs positive feedback loop. Int. J. Biol. Sci. 2022, 18, 1363–1380.
- Jing, Y.; Kovacs, K.; Kurisetty, V.; Jiang, Z.; Tsinoremas, N.; Merchan, J.R. Role of plasminogen activator inhibitor-1 in urokinase’s paradoxical in vivo tumor suppressing or promoting effects. Mol. Cancer Res. 2012, 10, 1271–1281.
- Han, L.; Korangath, P.; Nguyen, N.K.; Diehl, A.; Cho, S.; Teo, W.W.; Cope, L.; Gessler, M.; Romer, L.; Sukumar, S. HEYL Regulates Neoangiogenesis Through Overexpression in Both Breast Tumor Epithelium and Endothelium. Front. Oncol. 2021, 10, 581459.
- Bierie, B.; Chung, C.H.; Parker, J.S.; Stover, D.G.; Cheng, N.; Chytil, A.; Aakre, M.; Shyr, Y.; Moses, H.L. Abrogation of TGF-beta signaling enhances chemokine production and correlates with prognosis in human breast cancer. J. Clin. Investig. 2009, 119, 1571–1582.
- Erez, N.; Glanz, S.; Raz, Y.; Avivi, C.; Barshack, I. Cancer associated fibroblasts express pro-inflammatory factors in human breast and ovarian tumors. Biochem. Biophys. Res. Commun. 2013, 437, 397–402.
- Fang, W.B.; Mafuvadze, B.; Yao, M.; Zou, A.; Portsche, M.; Cheng, N. TGF-β Negatively Regulates CXCL1 Chemokine Expression in Mammary Fibroblasts through Enhancement of Smad2/3 and Suppression of HGF/c-Met Signaling Mechanisms. PLoS ONE 2015, 10, e0135063.
- Bernard, S.; Myers, M.; Fang, W.B.; Zinda, B.; Smart, C.; Lambert, D.; Zou, A.; Fan, F.; Cheng, N. CXCL1 Derived from Mammary Fibroblasts Promotes Progression of Mammary Lesions to Invasive Carcinoma through CXCR2 Dependent Mechanisms. J. Mammary Gland Biol. Neoplasia 2018, 23, 249–267.
- Camp, J.T.; Elloumi, F.; Roman-Perez, E.; Rein, J.; Stewart, D.A.; Harrell, J.C.; Perou, C.M.; Troester, M.A. Interactions with fibroblasts are distinct in Basal-like and luminal breast cancers. Mol. Cancer Res. 2011, 9, 3–13.
- Queen, M.M.; Ryan, R.E.; Holzer, R.G.; Keller-Peck, C.R.; Jorcyk, C.L. Breast cancer cells stimulate neutrophils to produce oncostatin M: Potential implications for tumor progression. Cancer Res. 2005, 65, 8896–8904.
- Araujo, A.M.; Abaurrea, A.; Azcoaga, P.; López-Velazco, J.I.; Manzano, S.; Rodriguez, J.; Rezola, R.; Egia-Mendikute, L.; Valdés-Mora, F.; Flores, J.M.; et al. Stromal oncostatin M cytokine promotes breast cancer progression by reprogramming the tumor microenvironment. J. Clin. Investig. 2022, 132, e148667.
- Wang, N.; Zheng, Y.; Gu, J.; Cai, Y.; Wang, S.; Zhang, F.; Chen, J.; Situ, H.; Lin, Y.; Wang, Z. Network-pharmacology-based validation of TAMS/CXCL-1 as key mediator of XIAOPI formula preventing breast cancer development and metastasis. Sci. Rep. 2017, 7, 14513.
- Wang, S.; Liu, X.; Huang, R.; Zheng, Y.; Wang, N.; Yang, B.; Situ, H.; Lin, Y.; Wang, Z. XIAOPI Formula Inhibits Breast Cancer Stem Cells via Suppressing Tumor-Associated Macrophages/C-X-C Motif Chemokine Ligand 1 Pathway. Front. Pharmacol. 2019, 10, 1371.
- Li, J.; Wang, S.; Wang, N.; Zheng, Y.; Yang, B.; Wang, X.; Zhang, J.; Pan, B.; Wang, Z. Aiduqing formula inhibits breast cancer metastasis by suppressing TAM/CXCL1-induced Treg differentiation and infiltration. Cell Commun. Signal 2021, 19, 89.
- Wang, Y.; Liu, J.; Jiang, Q.; Deng, J.; Xu, F.; Chen, X.; Cheng, F.; Zhang, Y.; Yao, Y.; Xia, Z.; et al. Human Adipose-Derived Mesenchymal Stem Cell-Secreted CXCL1 and CXCL8 Facilitate Breast Tumor Growth by Promoting Angiogenesis. Stem Cells 2017, 35, 2060–2070.
- Ryan, D.; Sinha, A.; Bogan, D.; Davies, J.; Koziol, J.; ElShamy, W.M. A niche that triggers aggressiveness within BRCA1-IRIS overexpressing triple negative tumors is supported by reciprocal interactions with the microenvironment. Oncotarget 2017, 8, 103182–103206.
- Bhat, K.; Sarkissyan, M.; Wu, Y.; Vadgama, J.V. GROα overexpression drives cell migration and invasion in triple negative breast cancer cells. Oncol. Rep. 2017, 38, 21–30.
- Ciummo, S.L.; D’Antonio, L.; Sorrentino, C.; Fieni, C.; Lanuti, P.; Stassi, G.; Todaro, M.; Di Carlo, E. The C-X-C Motif Chemokine Ligand 1 Sustains Breast Cancer Stem Cell Self-Renewal and Promotes Tumor Progression and Immune Escape Programs. Front. Cell Develop. Biol. 2021, 9, 689286.
- Xu, H.; Lin, F.; Wang, Z.; Yang, L.; Meng, J.; Ou, Z.; Shao, Z.; Di, G.; Yang, G. CXCR2 promotes breast cancer metastasis and chemoresistance via suppression of AKT1 and activation of COX2. Cancer Lett. 2018, 412, 69–80.
- Youngs, S.J.; Ali, S.A.; Taub, D.D.; Rees, R.C. Chemokines induce migrational responses in human breast carcinoma cell lines. Int. J. Cancer 1997, 71, 257–266.
- Yang, C.; Yu, H.; Chen, R.; Tao, K.; Jian, L.; Peng, M.; Li, X.; Liu, M.; Liu, S. CXCL1 stimulates migration and invasion in ER-negative breast cancer cells via activation of the ERK/MMP2/9 signaling axis. Int. J. Oncol. 2019, 55, 684–696.
- Strell, C.; Lang, K.; Niggemann, B.; Zaenker, K.S.; Entschladen, F. Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Exp. Cell Res. 2010, 316, 138–148.
- Zhu, H.; Gu, Y.; Xue, Y.; Yuan, M.; Cao, X.; Liu, Q. CXCR2+ MDSCs promote breast cancer progression by inducing EMT and activated T cell exhaustion. Oncotarget 2017, 8, 114554–114567.
- Oliveira-Ferrer, L.; Milde-Langosch, K.; Eylmann, K.; Rossberg, M.; Müller, V.; Schmalfeldt, B.; Witzel, I.; Wellbrock, J.; Fiedler, W. Mechanisms of Tumor-Lymphatic Interactions in Invasive Breast and Prostate Carcinoma. Int. J. Mol. Sci. 2020, 21, 602.
- Grassi, F.; Piacentini, A.; Cristino, S.; Toneguzzi, S.; Cavallo, C.; Facchini, A.; Lisignoli, G. Human osteoclasts express different CXC chemokines depending on cell culture substrate: Molecular and immunocytochemical evidence of high levels of CXCL10 and CXCL12. Histochem. Cell Biol. 2003, 120, 391–400.
- Hardaway, A.L.; Herroon, M.K.; Rajagurubandara, E.; Podgorski, I. Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer. Clin. Exp. Metastasis 2015, 32, 353–368.
- Lee, Y.C.; Gajdosik, M.S.; Josic, D.; Clifton, J.G.; Logothetis, C.; Yu-Lee, L.Y.; Gallick, G.E.; Maity, S.N.; Lin, S.H. Secretome analysis of an osteogenic prostate tumor identifies complex signaling networks mediating cross-talk of cancer and stromal cells within the tumor microenvironment. Mol. Cell Proteom. 2015, 14, 471–483.
- Sharma, B.; Nannuru, K.C.; Saxena, S.; Varney, M.L.; Singh, R.K. CXCR2: A Novel Mediator of Mammary Tumor Bone Metastasis. Int. J. Mol. Sci. 2019, 20, 1237.
- Bendre, M.S.; Margulies, A.G.; Walser, B.; Akel, N.S.; Bhattacharrya, S.; Skinner, R.A.; Swain, F.; Ramani, V.; Mohammad, K.S.; Wessner, L.L.; et al. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res. 2005, 65, 11001–11009.
- Xing, F.; Liu, Y.; Sharma, S.; Wu, K.; Chan, M.D.; Lo, H.W.; Carpenter, R.L.; Metheny-Barlow, L.J.; Zhou, X.; Qasem, S.A.; et al. Activation of the c-Met Pathway Mobilizes an Inflammatory Network in the Brain Microenvironment to Promote Brain Metastasis of Breast Cancer. Cancer Res. 2016, 76, 4970–4980.
- Strell, C.; Niggemann, B.; Voss, M.J.; Powe, D.G.; Zänker, K.S.; Entschladen, F. Norepinephrine promotes the β1-integrin-mediated adhesion of MDA-MB-231 cells to vascular endothelium by the induction of a GROα release. Mol. Cancer Res. 2012, 10, 197–207.
- Han, B.; Alonso-Valenteen, F.; Wang, Z.; Deng, N.; Lee, T.Y.; Gao, B.; Zhang, Y.; Xu, Y.; Zhang, X.; Billet, S.; et al. A chemokine regulatory loop induces cholesterol synthesis in lung-colonizing triple-negative breast cancer cells to fuel metastatic growth. Mol. Ther. 2022, 30, 672–687.
- SenGupta, S.; Hein, L.E.; Xu, Y.; Zhang, J.; Konwerski, J.R.; Li, Y.; Johnson, C.; Cai, D.; Smith, J.L.; Parent, C.A. Triple-Negative Breast Cancer Cells Recruit Neutrophils by Secreting TGF-β and CXCR2 Ligands. Front. Immunol. 2021, 12, 659996.
- Senst, C.; Nazari-Shafti, T.; Kruger, S.; Höner Zu Bentrup, K.; Dupin, C.L.; Chaffin, A.E.; Srivastav, S.K.; Wörner, P.M.; Abdel-Mageed, A.B.; Alt, E.U.; et al. Prospective dual role of mesenchymal stem cells in breast tumor microenvironment. Breast Cancer Res. Treat. 2013, 137, 69–79.
- Zheng, Y.; Wang, N.; Wang, S.; Yang, B.; Situ, H.; Zhong, L.; Lin, Y.; Wang, Z. XIAOPI formula inhibits the pre-metastatic niche formation in breast cancer via suppressing TAMs/CXCL1 signaling. Cell Commun. Signal 2020, 18, 48.
- Sprung, C.N.; Yang, Y.; Forrester, H.B.; Li, J.; Zaitseva, M.; Cann, L.; Restall, T.; Anderson, R.L.; Crosbie, J.C.; Rogers, P.A. Genome-wide transcription responses to synchrotron microbeam radiotherapy. Radiat. Res. 2012, 178, 249–259.
- Wang, P.; Song, D.; Wan, D.; Li, L.; Mei, W.; Li, X.; Han, L.; Zhu, X.; Yang, L.; Cai, Y.; et al. Ginsenoside panaxatriol reverses TNBC paclitaxel resistance by inhibiting the IRAK1/NF-κB and ERK pathways. PeerJ 2020, 8, e9281.
- Dalmases, A.; González, I.; Menendez, S.; Arpí, O.; Corominas, J.M.; Servitja, S.; Tusquets, I.; Chamizo, C.; Rincón, R.; Espinosa, L.; et al. Deficiency in p53 is required for doxorubicin induced transcriptional activation of NF-κB target genes in human breast cancer. Oncotarget 2014, 5, 196–210.
- Kluger, H.M.; Chelouche Lev, D.; Kluger, Y.; McCarthy, M.M.; Kiriakova, G.; Camp, R.L.; Rimm, D.L.; Price, J.E. Using a xenograft model of human breast cancer metastasis to find genes associated with clinically aggressive disease. Cancer Res. 2005, 65, 5578–5587.
