Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Wisam Nabeel Ibrahim.
Immunotherapy is a type of cancer treatment that harnesses the power of the immune system to recognize and destroy cancer cells. The immune system is a complex network of cells, tissues, and organs that work together to protect the body from harmful pathogens and abnormal cells, including cancer cells. Normally, the immune system can recognize and destroy cancer cells, but sometimes cancer cells can evade the immune system and continue to grow and spread. Immunotherapy works by enhancing the ability of the immune system to recognize and attack cancer cells.
- cancer immunotherapy
- resistance
- immune checkpoint inhibitors
- tumor microenvironment
1. The Cancer Immune Cycle
This cycle was clearly described by Daniel S. Chen and Ira Mellman in 2013 [7][1]. The process that starts the cancer immunity cycle is the release of cancer-cell antigens as they are destroyed by chemotherapy. Antigen-presenting dendritic cells then capture these antigens and process them to create peptides that bind to the major histocompatibility complex (MHC) and are then presented to T cells. The CD4+ T-cell receptors can identify the peptide-MHC-II molecules. Subsequently, effector T cells are primed and turned on to react to the tumor antigens that have been presented. The T-cell receptor interacts with its corresponding antigen linked to MHC-I on the surface of cancer cells, causing activated T cells to migrate to the tumor location, infiltrate, and specifically bind to cancer cells. The stimulated T lymphocytes then destroy the cancer cells. Through the activation of a sequence of processes that result in cell death, T cells destroy cancer cells. The cycle is continued, and the anticancer response is amplified by the additional cancer-specific neoantigens that dying cancer cells produce. As the cycle continues, a growing number of tumor antigens are released upon cell death, enhancing the T cells’ immunological response. Furthermore, inhibitory checkpoint proteins, such as CTLA-4 and PD-L1, function at various stages of the cycle to lower the immune activity and, in this way, help to prevent autoimmune reactions. Understanding these stages can help researchers develop new immunotherapies that can enhance the cancer-immunity cycle and improve outcomes for cancer patients. Many immunotherapeutic drugs target specific stages of the cancer-immunity cycle, with the aim of triggering the cancer-immunity cycle without damaging normal cells. The process is demonstrated in Figure 1.
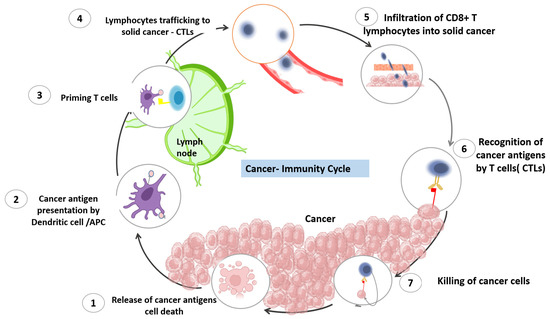
Figure 1. The cancer-immunity cycle. The cancer-immunity cycle is a series of steps through which the immune system passes in order to recognize and attack cancer cells. The cycle includes seven stages, starting with the release of cancer-cell antigens and ending with the destruction of cancer cells by T cells. The first stage involves the release of antigens from dying cancer cells. These antigens are then taken up by dendritic cells, which present them to T cells in the lymph nodes. In the second stage, activated T cells migrate to the tumor site and infiltrate the tumor. The third stage involves recognition of cancer cells by T cells, and the fourth stage involves the activation of T cells to recognize and kill cancer cells. In the fifth stage, T cells work to penetrate the tumor and release cytokines that recruit immune cells to the tumor site. In the sixth stage, immune cells, including macrophages and dendritic cells, infiltrate the tumor and destroy cancer cells. Finally, in the seventh stage, the immune system remembers the cancer cells, creating a lasting immune response that can protect against future cancer development.
2. Innate and Adaptive Immune Responses in the Tumor Microenvironment (TME)
The recognition of cancer cells by the immune system is a promising target in various cancer treatments aimed at eliminating cancer cells from both primary and metastatic sites. This intricate process involves a highly regulated interplay between cellular elements of the innate and adaptive immune systems. Innate immune cells initiate a series of responses, which are followed by adaptive immune responses. The process through which tumor antigens are recognized is illustrated in Figure 2.
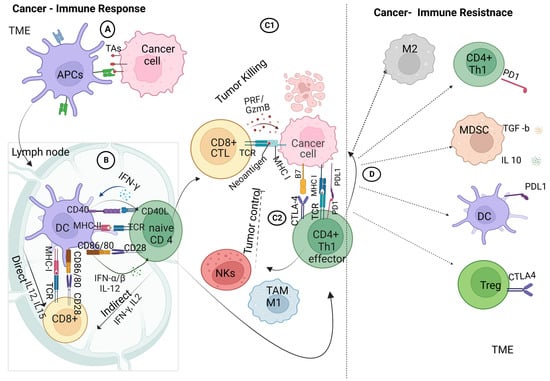
Figure 2. Crosstalk between cancer and immune cells: (A) innate adaptive immunity, in which the antigen-presenting cells within the tumor microenvironment take up the tumor-associated antigens and process them for antigen-presenting, priming, and activation immune response; (B) adaptive immune response, involving the crosstalk between the dendritic cells and T-cell receptors on naive CD4+ T cells and CD8+ T cells via the major histocompatibility complex class I/II in the presence of co-stimulatory molecules of CD86/80 on DCs and CD28 on CD4+/ CD8+ T cells, ending with the activation of effector T cells; (C1) interaction of effector cytotoxic T cells with tumor cells within the TME; (C2) interaction of effector cytotoxic T cells with tumor cells within the TME and the interaction of effector TH1 cells with tumor cells that trigger the recruitment of tumor-associated macrophages and natural killer cells; (D) immune-checkpoint interactions: activation of PD-1 tumor cells to interact with PD-L1 on effector TH1 lymphocytes, ending with the upregulation of immune suppressive cells; macrophages 2 (M2), regulatory T cells (Tregs), and myeloid-derived suppressor cells (MDSCs) produce IL10 and TGF-β1 with further immune-suppressive actions. Created with BioRender.com.
When tumor cells die due to treatment or limited blood supply, tumor antigens (TAs) are released. Dendritic cells (DCs), a specific subset of innate immune cells, engulf and process these TAs from the oncogenic mass. These cells act as antigen-presenting cells (APCs), forming a bridge between innate and adaptive immune cells [13][2].
Next, the DCs move to the lymph nodes, where they present TAs via major histocompatibility complex class II (MHC-II), which binds with receptors on naive CD4 T cells. Co-stimulation occurs when the CD80/86 (B7) on the DCs binds with the CD28 ligand on CD4+ T cells. The autocrine signaling of interleukin-2 (IL-2) is required to trigger and activate the effector-T-cell response, followed by the clonal expansion and differentiation of effector and memory CD4+ T cells [7][1]. Similarly, naive CD8+ T cells are activated by DCs through the MHC-I complex, with a similar co-stimulation response, leading to the clonal expansion of cytotoxic CD8+ T lymphocytes.
Stimulated CD4+ and CD8+ T lymphocytes may respond to immunogenic signals, such as pro-inflammatory cytokines, which are released from dead tumor or immune cells. Furthermore, DCs express CXCL9 and CXCL10 chemokines to recruit more infiltrating T lymphocytes (ITLs) to the tumor tissue, allowing crosstalk between cancer and immune cells [14][3].
Infiltrating cytotoxic T cells (CTLs) initiate the immune attack on cancer cells by releasing granzymes, perforin, interferon γ (IFN-γ), and tumor necrosis factor α (TNF-α) [15][4]. Furthermore, CD4+ cells recognize tumor cells through the interaction of the T-cell receptor with the tumor antigen that is associated with the MHC-I complex on tumor cells in the presence of costimulatory molecules (CTLA4/CD4+ T cells–B7 tumor cells) [16][5]. Effector CD4+ T cells then provoke tumor-associated macrophages (TAMs/M1) and natural killer cells (NK) in the tumor microenvironment, leading to the mediation of local antitumor immunity with the release of pro-inflammatory molecules [17][6]. Additionally, the activated NK cells release cytokines targeting tumor cells, leading to subsequent apoptosis.
However, tumor cells modulate the immune response by highly expressing the programmed cell death ligand I (PD-L1) on their surfaces upon interaction with immune cells. The PD-L1 interacts with programmed cell death protein 1 (PD-1) on T cells, leading to the downregulation of T-cell activation and triggering suppressive T-regulatory cells (Tregs) [18][7]. Furthermore, the membrane-bound transforming growth factor-β1 (TGF-β1) on myeloid-derived suppressor cells (MDSCs) mediates the suppression of NK cells and upregulates more Tregs [19,20][8][9]. These modulations are key elements in the initiation of cancer resistance to the immune response.
References
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10.
- Vesely, M.D.; Kershaw, M.H.; Schreiber, R.D.; Smyth, M.J. Natural innate and adaptive immunity to cancer. Annu. Rev. Immunol. 2011, 29, 235–271.
- Vilgelm, A.E.; Richmond, A. Chemokines Modulate Immune Surveillance in Tumorigenesis, Metastasis, and Response to Immunotherapy. Front. Immunol. 2019, 10, 333.
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br. J. Cancer 2021, 124, 359–367.
- Oh, D.Y.; Fong, L. Cytotoxic CD4(+) T cells in cancer: Expanding the immune effector toolbox. Immunity 2021, 54, 2701–2711.
- Dallavalasa, S.; Beeraka, N.M.; Basavaraju, C.G.; Tulimilli, S.V.; Sadhu, S.P.; Rajesh, K.; Aliev, G.; Madhunapantula, S.V. The Role of Tumor Associated Macrophages (TAMs) in Cancer Progression, Chemoresistance, Angiogenesis and Metastasis—Current Status. Curr. Med. Chem. 2021, 28, 8203–8236.
- Demaria, O.; Cornen, S.; Daëron, M.; Morel, Y.; Medzhitov, R.; Vivier, E. Harnessing innate immunity in cancer therapy. Nature 2019, 574, 45–56.
- Li, H.; Han, Y.; Guo, Q.; Zhang, M.; Cao, X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J. Immunol. 2009, 182, 240–249.
- Bruger, A.M.; Dorhoi, A.; Esendagli, G.; Barczyk-Kahlert, K.; van der Bruggen, P.; Lipoldova, M.; Perecko, T.; Santibanez, J.; Saraiva, M.; Van Ginderachter, J.A.; et al. How to measure the immunosuppressive activity of MDSC: Assays, problems and potential solutions. Cancer Immunol. Immunother. 2019, 68, 631–644.
More
