Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by Massimo Torreggiani.
The definition of hypertensive disorders of pregnancy (HDP) encompasses a spectrum of conditions that extends from gestational hypertension to preeclampsia (PE), eclampsia, and to hemolysis, elevated liver enzymes, and low platelet-count syndrome (HELLP). Other anomalies are non-uniformly aggregated in this family of diseases; this is the case for isolated or pregnancy-induced proteinuria (in normotensive pregnancies) and fetal growth restriction (FGR), which have, however, been demonstrated to predict the risk of developing PE during gestation as well as the risk of adverse short- and long-term maternal-fetal outcomes, including the development of chronic kidney disease (CKD)
- pregnancy
- chronic kidney disease
- preeclampsia
- hypertension
1. Hypertensive Disorders of Pregnancy
The incidence of hypertensive disorders during pregnancy has increased in the last three decades, reaching 18,080,000 cases/year (a 10.92% increase), with a higher rate in South Asia and sub-Saharan Africa, in contrast to Australasia, Oceania, and Central Europe, where the lowest incidence is found, according to Wang and his colleagues in their population-based study [3][1]. Despite the growing number of cases, the number of deaths, estimated at 27,830 per year, has decreased significantly (a 30.05% reduction over 30 years, from 1990 to 2019); however, hypertensive disorders of pregnancy (HDP)-related mortality and morbidity remain unacceptably high [3][1].
There are two peaks in the age distribution of HDP, corresponding to the extremes of reproductive age [3][1]. The highest risk of all adverse pregnancy outcomes (including low birth weight, preterm delivery, and PE) recorded in younger age groups is influenced by several factors associated with teenage pregnancy, including low educational level, low income, malnutrition, precarious health before gestation, and marital status, factors that also reflect a continued lack of concern for young women’s health [4,5][2][3].
The other extreme of the spectrum is represented by advanced maternal age; the increase that is observed can largely be explained by hormonal changes, together with an increased prevalence of obesity, chronic diseases (mainly diabetes and hypertension), and, at least in Western countries, with recourse to medically assisted reproduction techniques [6][4].
2. Definitions
2.1. Hypertension in Pregnancy
Gestational or pregnancy-induced hypertension, which affects up to 10% of pregnant women, is recognized by all societies as new-onset blood pressure (BP) ≥ 140/90 mmHg at or after 20 gestational weeks. The risk of progression to PE is estimated as 17.1–25% [7,8,9,10,11,12,13][5][6][7][8][9][10][11]. About 50% of patients with gestational hypertension continue to have high BP levels after delivery. Conversely, about 10% of the women who are normotensive during pregnancy may develop hypertension up to 6 weeks postpartum [14][12]. In the latter case, the patients have the same risk of short- and long-term cardiovascular complications as women affected by hypertension during gestation [15][13].
While the prevalence of chronic hypertension increases with age, it can affect all patients of childbearing age, both in its idiopathic and secondary forms, and is estimated to be present in 1–2% of pregnancies [16][14]. Apart from the specific susceptibility of women of Afro-American ethnicity, the prevalence of chronic hypertension in pregnancy is rising, as shown by a population-based cross-sectional study of more than 150 million hospital deliveries in the United States, where the increase in chronic hypertension was closely related to increased maternal age and body mass index (BMI) [17][15]. The clinical relevance of chronic hypertension in pregnancy is high, as it is associated with a higher rate of maternal and neonatal complications, including the need for cesarean section (41%), small-for-gestational-age (SGA) newborns (17%), preterm delivery (28%), the need for a neonatal intensive care unit (NICU) (21%), and perinatal death (4%) [18][16]. In addition, hypertension is an independent risk factor for “superimposed” PE (relative risk (RR), 5.1; 95% CI, 4.0–6.5) [16[14][17][18][19],19,20,21], defined by the development of proteinuria or end-organ damage in hypertensive patients after 20 gestational weeks.
Several issues are not yet clear: the blood pressure target is still a matter of discussion and, while reaching lower targets was not associated with an improvement in maternal and fetal health, the presence of known chronic kidney disease (CKD) is acknowledged as a reason for trying to reach lower BP targets. Things are not as simple as they may seem, considering that CKD is probably the most common cause of secondary hypertension in a non-obese, non-diabetic young woman, and that many forms of CKD are not detected unless specifically searched for using both biochemical assessment and renal imaging [22,23][20][21].
Overall, the current guidelines on hypertension in pregnancy recommend treating all cases of severe hypertension (>160/110 mmHg) and suggest starting antihypertensive treatment in milder cases (BP between 140–159/90–109 mmHg) only in the presence of cardiovascular diseases, diabetes, CKD or acknowledged risk factors for cardiovascular diseases. Once more, nephrologists often have a different view of the situation, as is suggested by the best-practice guidelines of the Italian Society of Nephrology, which, while acknowledging the risks of hypotension in pregnancy, recommend the correction of hypertension, as in the case of young patients outside the context of pregnancy (target BP ≤ 130/80 mmHg), under strict clinical surveillance [24][22]. The main risks to be avoided are seen as those related to hypercorrection, since hypotension can cause placental hypoperfusion, with detrimental effects on the fetus. Careful follow-up is needed to allow optimal blood pressure to be reached [24][22]. The issue is similar to those problems that may arise in attempting to control diabetes, where hypercorrection and frequent hypoglycemia can have negative effects on pregnancy outcomes [25][23]. When setting BP goals in pregnancy, wresearchers should keep in mind that outside the context of pregnancy, the results for CKD patients under tight hypertension control in the recent ACCORD BP trial demonstrated that the benefits obtained in reducing cardiovascular events and mortality were offset by a higher risk of adverse kidney outcomes, which are mainly represented by AKI [26][24]. No such data are available for pregnancy.
2.2. Preeclampsia
PE is a multisystem disease, generally defined as a condition in which the onset of arterial hypertension after 20 weeks of gestation is accompanied by at least one sign of renal, hepatic, central nervous system, or hematologic impairment [7,8,9,10,11,12][5][6][7][8][9][10] (Figure 1). Proteinuria levels ≥0.3 g/24 h or a protein-to-creatinine ratio (PCR) ≥ 0.3 g/g is present in about 75% of the cases defined as PE. While, in the past, proteinuria was required for a PE diagnosis, international boards now consider it to be of the same importance as other symptoms and signs [7,8,9,10,11,12][5][6][7][8][9][10]. In this regard, the new guidelines underline the central role of kidney involvement, basing a diagnosis not only on proteinuria but also on a reduction in kidney function from the baseline pre-gestational level [7,8,9,10,11,12][5][6][7][8][9][10].
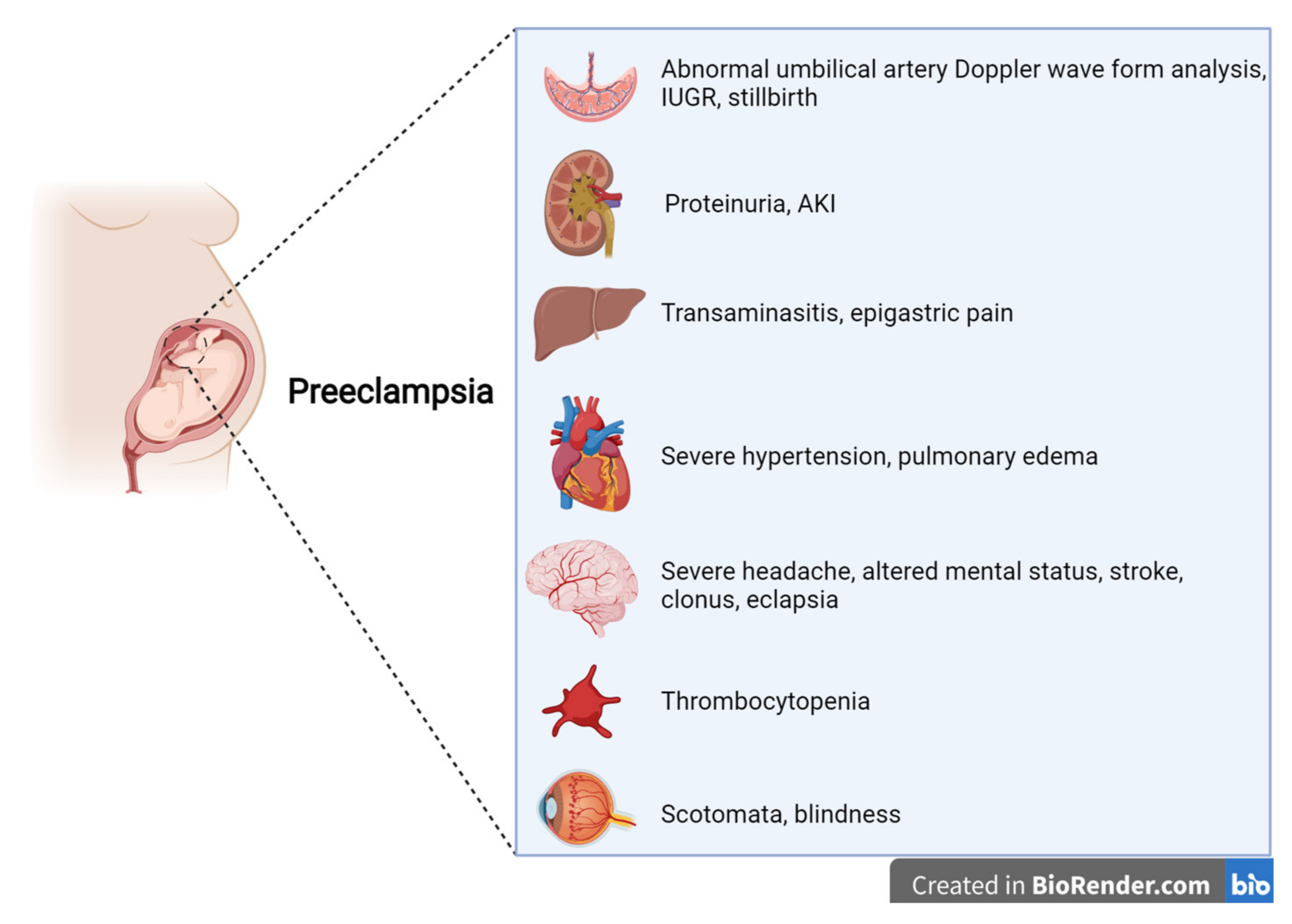
Figure 1.
Features of preeclampsia, according to international guidelines.
Other “new” features of the definition of PE are laboratory findings regarding liver involvement, with an increase in aminotransferases (alanine aminotransferase and/or aspartate aminotransferase at >40 IU/L, whether or not this is accompanied by right-upper quadrant or epigastric abdominal pain), thrombocytopenia (platelets at <109/L), disseminated intravascular coagulation (DIC), and hemolysis. In this regard, the more extensive PE definition merges with the definition of HELLP syndrome, indirectly alluding to a univocal definition of HDPs.
Symptoms reported by patients range from severe headaches, persistent visual scotomata, or blindness to alterations in mental state and complications such as clonus, eclampsia, and stroke. The Society of Obstetricians and Gynaecologists of Canada (SOGC) and the Society of Obstetric Medicine of Australia and New Zealand (SOMANZ) also include cardiac and/or pulmonary involvement in the diagnostic criteria for PE [7,8,9,10,11,12][5][6][7][8][9][10]. Moreover, except for the American College of Obstetrics and Gynecologists (ACOG), all other societies include signs of uteroplacental dysfunction, such as fetal growth restriction, an abnormal umbilical artery Doppler wave, and stillbirth as being diagnostic criteria of PE [7,8,9,10,12][5][6][7][8][10]. Once more, this choice reflects the view that all HDPs are interlinked, even though they do not necessarily occur with the same severity (Figure 2).
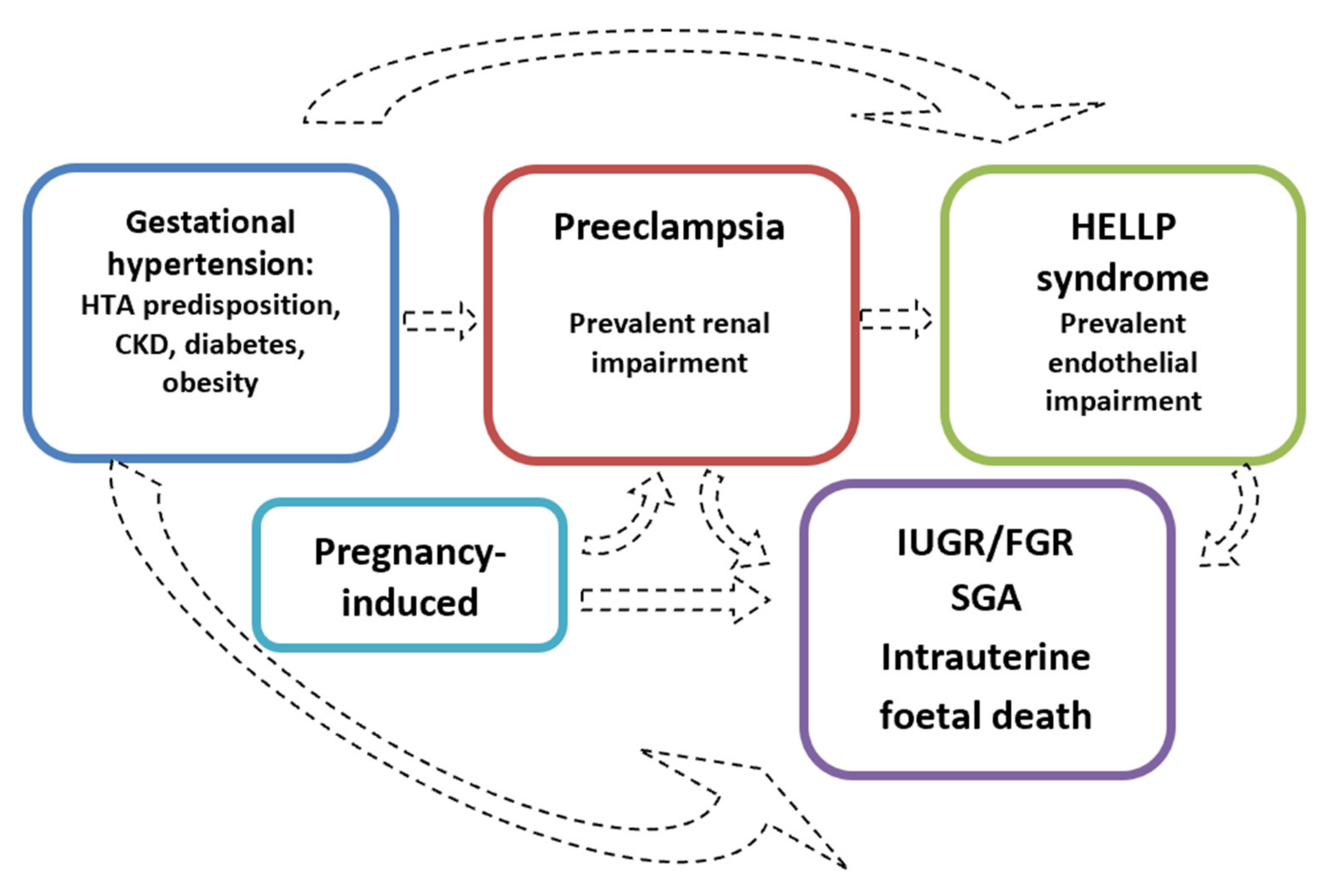
Figure 2.
The hypothesis of distinct disorders that may merge into one another.
A recent retrospective cohort study of 22,094 pregnancies, followed up at Monash Health in Australia, showed that the wider criteria for defining PE, such as those adopted by the International Society for the Study of Hypertension in Pregnancy (ISSHP) in 2018, made it possible to classify a further 14.8% of women, who in the past would have been excluded from consideration by the ISSHP’s 2018 or ACOG’s 2018 guidelines, as having experienced PE [7,11,33][5][9][25]. The ISSPH’s 2018 guidelines highlighted that among the signs included for the diagnosis of PE, FGR, thrombocytopenia, proteinuria, AKI, or liver impairment had the strongest association with the risks of severe adverse maternal-fetal outcomes [33][25].
This acknowledgment of the high heterogeneity of PE, in parallel with the proposal to unify the pathogenesis of PE and other hypertensive disorders of pregnancy, has led to an attempt to stratify PE, based on a variety of elements from severity to the period of onset and pathogenic mechanisms [34][26]. While none of these has proved to be sufficient to clearly distinguish between phenotypes and, above all, to be invariably correlated with maternal-fetal short- and long-term outcomes, these approaches are the basis of a better description of the phenotype of PE, which, albeit with wide overlaps, can enable clinicians to perform a prognostic evaluation of individual cases. In fact, even though late-onset PE may be life-threatening for both mother and fetus, early-onset PE is more often “placental”, severe, and associated with profound angiogenic-antiangiogenic imbalance, and, at least according to some studies, has a higher risk of recurrence in subsequent pregnancies [35,36,37][27][28][29].
2.3. Small for Gestational Age, Intrauterine Growth Restriction, Fetal Growth Restriction (FGR), and Intrauterine Death
The hypertensive disorders of pregnancy have been related to defective placentation, which may be severe enough to impair the physiologic development of the fetus during pregnancy. These include babies that are small for gestational age (SGA), intrauterine growth restriction (IUGR) or its synonymous fetal growth restriction (FGR), and an increased risk of pre-term delivery, which is usually classified as very early pre-term (<28 gestational weeks), early pre-term (28–34 weeks or, presently, more often, 28–32 gestational weeks) and late pre-term (according to the previous definitions: 32–37 or 34–37 gestational weeks), or intrauterine death [7,8,9,10,11,12][5][6][7][8][9][10]. In this scenario, FGR, newborns that are small for gestational age, and intrauterine death, are part of the spectrum of the hypertensive disorders of pregnancy; while they can occur in association with hypertension or full-blown PE, they may also be isolated and without other PE features [7,8,9,10,11,12][5][6][7][8][9][10].
FGR is defined by the fetal weight, or an abdominal circumference estimated using ultrasounds, showing growth between the 3rd and 10th percentile for gestational age (moderate fetal growth restriction) or less than the 3rd percentile (the severe form) [38][30]. However, other studies set the cut-off points at the 10th and 5th percentile. The definition of FGR is also dynamic and may need to be raised when there is a flattening of the growth curve [38][30], to distinguish between low-birth-weight babies with harmonic growth, for genetic (or pathological) reasons, and babies whose normal growth was impaired at some point in their intrauterine life. Considering that FGR can also be the result of fetal diseases, a genetic evaluation should be proposed by the obstetric team, in cases of early recognition (<32 gestational weeks) or when in association with polyhydramnios or, more obviously, of fetal malformations [38][30].
Conversely, the definition of small for gestational age (SGA) usually applies if the effective birth weight is less than the 10th percentile of the expected adjusted figure for gestational age, according to local growth curves; once more, the definition is not homogeneous, and the cut-off point of less than the 5th percentile is also used [38][30].
The International Society of Ultrasound in Obstetrics and Gynecology (ISUOG) guidelines distinguish between FGR and SGA, as follows: FGR is defined as the fetus failing to reach its genetically predetermined growth potential, while SGA is diagnosed when the fetus size falls below a predefined threshold for its gestational age (usually the 10th percentile) [39][31]. In addition, these guidelines specify that an SGA fetus may be small but not at increased risk of adverse perinatal outcomes, while a fetus with a size above the 10th percentile may still be considered FGR and at increased risk of adverse perinatal and long-term outcomes [39][31].
Intrauterine death is distinguished from miscarriage, which is defined as fetal loss before 20 gestational weeks in the United States, while the British guidelines still set the cut-off point at 24 weeks, which was previously the agreed point. The anticipation of the definition is due to current improvements in allowing viability in very preterm babies, as the definition of intrauterine death usually applies to gestational ages compatible with survival [40,41,42][32][33][34].
According to the US fetal mortality rate, in 2013, “early” intrauterine death was estimated as occurring in 3.01 per 1000 births before 28 gestational weeks, while “late” cases (after 28 weeks) occurred in 2.97 per 1000 births [43][35]. Over 30% of these fetuses were SGA, often without a causal diagnosis [44][36].
The relevance for nephrologists of the inclusion of FGR in the context of the hypertensive disorders of pregnancy is high. Including these conditions increases the number of cases that, at least according to the Italian Society of Nephrology (to date, the only group to have published a specific statement on follow-up after preeclampsia), should undergo a basic nephrology evaluation to exclude the presence of CKD [45][37].
The probability that PE is associated with pre-existing CKD is high, as has also been reported by the Mayo clinic, and it is probably present in at least 20% of the women who experience a PE episode [22,45,46][20][37][38].
The second element of interest is that being born small for gestational age and/or with a low birth weight is, in turn, associated with the risk of developing CKD, hypertension, diabetes, and metabolic syndrome in adulthood and, for females, with developing preeclampsia once pregnant, thus generating a vicious circle of diseases reappearing over generations, not necessarily with a genetic basis [47,48,49,50][39][40][41][42]. Despite the fact that the relationship between low birth weight and prematurity and subsequent disorders during pregnancy has been described for decades, the message that the risk of preeclampsia in the mother decreases with increased gestational age has not been fully integrated into clinical practice, and no specific surveillance programs have been set up for such high-risk pregnancies (the odds ratio (OR) of developing PE was about 4 for mothers born earlier than 34 gestational weeks, and is even higher for those born under 4.5 lb, about 2 kg) [47][39].
Among the numerous subsequent studies on this important issue, the recent large population study by Gjerde and co-workers deserves mention: considering over two million individuals, after a mean follow-up period of 26 years, low birth weight was associated with an odds ratio of 1.72 for CKD, 1.79 for SGA, and 1.48 for preterm birth (95% CI, 1.33 to 1.66) [51][43].
2.4. HELLP Syndrome
2.4. Low Platelet-Count Syndrome
The position of the low platelet-count (HELLP) syndrome in the context of the hypertensive disorders of pregnancy is still debated, although this severe condition is currently included in most guidelines regarding HDP [7,8,9,10,11,12][5][6][7][8][9][10]. Currently, there are two different approaches: one that considers HELLP syndrome a severe variant of PE (Figure 3), and one that sees it as a distinct disease, while acknowledging that there is a shared pathogenesis and risk factors as well as an interplay between all these disorders [34][26].
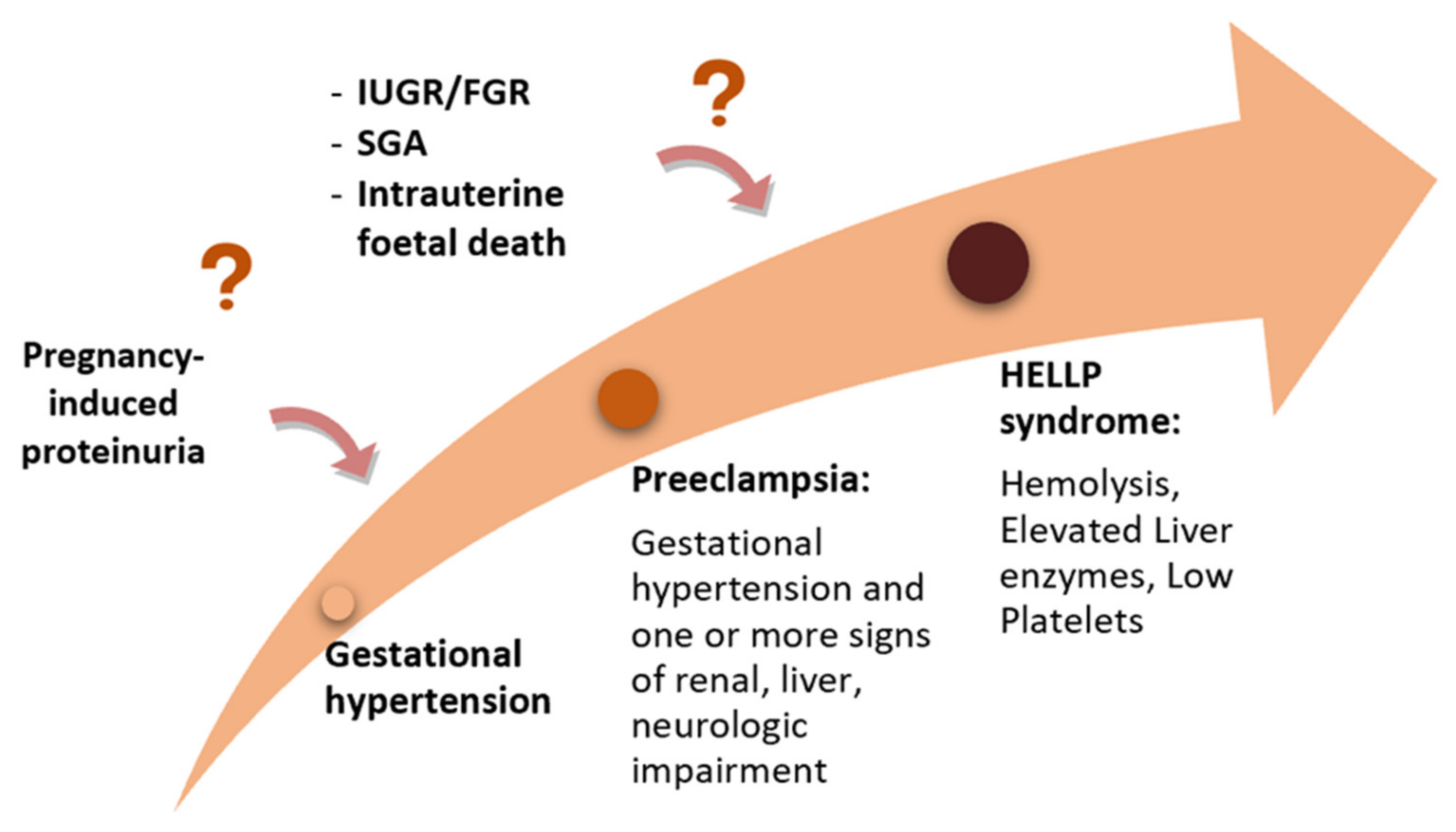
Figure 3.
The “sequential hypothesis” of a continuum of severity of the hypertensive disorders of pregnancy.
Along with hemolysis, thrombocytopenia, and liver impairment, other typical features are hypertension, present in 82–88% of pregnant women, and proteinuria, which is found in 86–100% of pregnant women, according to the cohorts evaluated [52][44].
Conversely, an interesting “unifying” approach includes HELLP syndrome, together with pregnancy-induced thrombotic microangiopathies (TMAs), mainly because they share patterns of endothelial cell injury (primarily affecting the kidneys and the cardiovascular and central nervous systems, with a hemolytic uremic syndrome, thrombotic thrombocytopenic purpura, and catastrophic antiphospholipid syndrome) [53][45]. Laboratory findings, including peripheral thrombocytopenia, mechanical hemolytic anemia (defined by hemoglobin levels of ≤10 g/dL, lactate dehydrogenase (LDH) at the upper limit of normal, undetectable haptoglobin, and the presence of schistocytes on the blood smear), as well as signs of impaired organ function, such as a rise in serum creatinine or transaminases, can be present in PE and HELLP as well as in TMAs [53][45]. Although this interpretation is not universally accepted, it offers a change in perspective, making it possible for us to see the hypertensive disorders of pregnancy not only as “transient” alterations due to gestation but also as endothelial disorders that may recur in subsequent pregnancies or evolve into long term endothelial dysfunction and, in turn, CKD.
2.5. Acute Kidney Injury in Pregnancy
The definition of acute kidney injury (AKI) in pregnancy, in the absence of agreed formulae for calculating the kidney function, is usually based on serum creatinine (the definition of AKI usually corresponds to an increase in serum creatinine of at least 0.3 mg/dL over baseline) [54][46]. However, the physiological reduction in serum creatinine during physiological pregnancies should be considered. In this context, two recent systematic revisewarchs suggest that a normal serum creatinine value in pregnancy should correspond to less than 80% of the pre-pregnancy normal one [55,56][47][48]. However, since serum creatinine assessment is not part of the work-up of physiological pregnancies, the interpretation of a serum creatinine level that is still in the normal range in pregnancy may be difficult, if not impossible. This limitation, and the importance accorded to kidney function in pregnancy, are a further indication of the need for including at least serum creatinine in the routine control tests in pregnancy [57,58][49][50].
Moreover, wresearchers should consider that renal damage may be induced by causes other than the hypertensive disorders of pregnancy, such as hypovolemia, ischemia (e.g., severe postpartum hemorrhage), or sepsis, which can cause acute tubular necrosis or cortical necrosis with adverse outcomes for maternal renal function, both immediately and in the long term [34,59][26][51].
References
- Wang, W.; Xie, X.; Yuan, T.; Wang, Y.; Zhao, F.; Zhou, Z.; Zhang, H. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: A population-based study. BMC Pregnancy Childbirth 2021, 21, 364.
- Macedo, T.C.C.; Montagna, E.; Trevisan, C.M.; Zaia, V.; de Oliveira, R.; Barbosa, C.P.; Lagana, A.S.; Bianco, B. Prevalence of preeclampsia and eclampsia in adolescent pregnancy: A systematic review and meta-analysis of 291,247 adolescents worldwide since 1969. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 248, 177–186.
- Ganchimeg, T.; Mori, R.; Ota, E.; Koyanagi, A.; Gilmour, S.; Shibuya, K.; Torloni, M.R.; Betran, A.P.; Seuc, A.; Vogel, J.; et al. Maternal and perinatal outcomes among nulliparous adolescents in low- and middle-income countries: A multi-country study. J. Obstet. Gynaecol. 2013, 120, 1622–1630, discussion 1630.
- Cox, L.S.; Redman, C. The role of cellular senescence in ageing of the placenta. Placenta 2017, 52, 139–145.
- Brown, M.A.; Magee, L.A.; Kenny, L.C.; Karumanchi, S.A.; McCarthy, F.P.; Saito, S.; Hall, D.R.; Warren, C.E.; Adoyi, G.; Ishaku, S.; et al. The hypertensive disorders of pregnancy: ISSHP classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens 2018, 13, 291–310.
- Magee, L.A.; Pels, A.; Helewa, M.; Rey, E.; von Dadelszen, P.; Canadian Hypertensive Disorders of Pregnancy Working Group. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. Pregnancy Hypertens 2014, 4, 105–145.
- Lowe, S.A.; Bowyer, L.; Lust, K.; McMahon, L.P.; Morton, M.; North, R.A.; Paech, M.; Said, J.M. SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. Aust. N. Z. J. Obstet. Gynaecol. 2015, 55, e1–e29.
- National Institute for Health and Care Excellence. Hypertension in Pregnancy: Diagnosis and Management. NICE Guideline . Available online: https://www.nice.org.uk/guidance/ng133 (accessed on 22 April 2022).
- Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol 2020, 135, e237–e260.
- Poon, L.C.; Magee, L.A.; Verlohren, S.; Shennan, A.; von Dadelszen, P.; Sheiner, E.; Hadar, E.; Visser, G.; Da Silva Costa, F.; Kapur, A.; et al. A literature review and best practice advice for second and third trimester risk stratification, monitoring, and management of pre-eclampsia: Compiled by the Pregnancy and Non-Communicable Diseases Committee of FIGO (the International Federation of Gynecology and Obstetrics). Int. J. Gynaecol. Obstet. 2021, 154 (Suppl. S1), 3–31.
- Yemane, A.; Teka, H.; Ahmed, S.; Temesgen, H.; Langen, E. Gestational hypertension and progression towards preeclampsia in Northern Ethiopia: Prospective cohort study. BMC Pregnancy Childbirth 2021, 21, 261.
- Goel, A.; Maski, M.R.; Bajracharya, S.; Wenger, J.B.; Zhang, D.; Salahuddin, S.; Shahul, S.S.; Thadhani, R.; Seely, E.W.; Karumanchi, S.A.; et al. Epidemiology and Mechanisms of De Novo and Persistent Hypertension in the Postpartum Period. Circulation 2015, 132, 1726–1733.
- Katsi, V.; Skalis, G.; Vamvakou, G.; Tousoulis, D.; Makris, T. Postpartum Hypertension. Curr. Hypertens. Rep. 2020, 22, 58.
- Magee, L.A.; Khalil, A.; Kametas, N.; von Dadelszen, P. Toward personalized management of chronic hypertension in pregnancy. Am. J. Obstet. Gynecol. 2022, 226, S1196–S1210.
- Ananth, C.V.; Duzyj, C.M.; Yadava, S.; Schwebel, M.; Tita, A.T.N.; Joseph, K.S. Changes in the Prevalence of Chronic Hypertension in Pregnancy, United States, 1970 to 2010. Hypertension 2019, 74, 1089–1095.
- Bramham, K.; Parnell, B.; Nelson-Piercy, C.; Seed, P.T.; Poston, L.; Chappell, L.C. Chronic hypertension and pregnancy outcomes: Systematic review and meta-analysis. BMJ 2014, 348, g2301.
- Bartsch, E.; Medcalf, K.E.; Park, A.L.; Ray, J.G. Clinical risk factors for pre-eclampsia determined in early pregnancy: Systematic review and meta-analysis of large cohort studies. BMJ 2016, 353, i1753.
- Townsend, R.; Khalil, A.; Premakumar, Y.; Allotey, J.; Snell, K.I.E.; Chan, C.; Chappell, L.C.; Hooper, R.; Green, M.; Mol, B.W.; et al. Prediction of pre-eclampsia: Review of reviews. Ultrasound Obstet. Gynecol. 2019, 54, 16–27.
- Giannakou, K.; Evangelou, E.; Papatheodorou, S.I. Genetic and non-genetic risk factors for pre-eclampsia: Umbrella review of systematic reviews and meta-analyses of observational studies. Ultrasound Obstet. Gynecol. 2018, 51, 720–730.
- Cabiddu, G.; Mannucci, C.; Fois, A.; Maxia, S.; Chatrenet, A.; Osadolor, S.; Kimani, E.; Torreggiani, M.; Attini, R.; Masturzo, B.; et al. Preeclampsia is a valuable opportunity to diagnose chronic kidney disease: A multicentre study. Nephrol. Dial. Transplant. 2021, gfab225.
- Filali Khattabi, Z.; Biolcati, M.; Fois, A.; Chatrenet, A.; Laroche, D.; Attini, R.; Cheve, M.T.; Piccoli, G.B. Chronic kidney disease in preeclamptic patients: Not found unless searched for-Is a nephrology evaluation useful after an episode of preeclampsia? J. Nephrol. 2019, 32, 977–987.
- Cabiddu, G.; Castellino, S.; Gernone, G.; Santoro, D.; Moroni, G.; Giannattasio, M.; Gregorini, G.; Giacchino, F.; Attini, R.; Loi, V.; et al. A best practice position statement on pregnancy in chronic kidney disease: The Italian Study Group on Kidney and Pregnancy. J. Nephrol. 2016, 29, 277–303.
- Alexopoulos, A.S.; Blair, R.; Peters, A.L. Management of Preexisting Diabetes in Pregnancy: A Review. JAMA 2019, 321, 1811–1819.
- Roehm, B.; Weiner, D.E. Blood pressure targets and kidney and cardiovascular disease: Same data but discordant guidelines. Curr. Opin. Nephrol. Hypertens 2019, 28, 245–250.
- Reddy, M.; Fenn, S.; Rolnik, D.L.; Mol, B.W.; da Silva Costa, F.; Wallace, E.M.; Palmer, K.R. The impact of the definition of preeclampsia on disease diagnosis and outcomes: A retrospective cohort study. Am. J. Obstet. Gynecol. 2021, 224, 217.e1–217.e11.
- Piccoli, G.B.; Zakharova, E.; Attini, R.; Ibarra Hernandez, M.; Covella, B.; Alrukhaimi, M.; Liu, Z.H.; Ashuntantang, G.; Orozco Guillen, A.; Cabiddu, G.; et al. Acute Kidney Injury in Pregnancy: The Need for Higher Awareness. A Pragmatic Review Focused on What Could Be Improved in the Prevention and Care of Pregnancy-Related AKI, in the Year Dedicated to Women and Kidney Diseases. J. Clin. Med. 2018, 7, 318.
- Ma’ayeh, M.; Costantine, M.M. Prevention of preeclampsia. Semin Fetal Neonatal. Med. 2020, 25, 101123.
- Dong, X.; Han, M.; Zeb, S.; Tong, M.; Li, X.; Chen, Q. Active Management Reduces the Incidence of Recurrent Pre-eclampsia and Improves Maternal and Fetal Outcomes in Women with Recurrent Pre-eclampsia. Front. Med. 2021, 8, 658022.
- Barton, J.R.; Sibai, B.M. Prediction and prevention of recurrent preeclampsia. Obstet. Gynecol. 2008, 112, 359–372.
- Society for Maternal-Fetal Medicine; Martins, J.G.; Biggio, J.R.; Abuhamad, A. Society for Maternal-Fetal Medicine Consult Series #52: Diagnosis and management of fetal growth restriction: (Replaces Clinical Guideline Number 3, April 2012). Am. J. Obstet. Gynecol. 2020, 223, B2–B17.
- Lees, C.C.; Stampalija, T.; Baschat, A.; da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Kingdom, J.; Poon, L.C.; Salomon, L.J.; et al. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312.
- Dupont-Thibodeau, A.; Barrington, K.J.; Farlow, B.; Janvier, A. End-of-life decisions for extremely low-gestational-age infants: Why simple rules for complicated decisions should be avoided. Semin Perinatol. 2014, 38, 31–37.
- Gkiougki, E.; Chatziioannidis, I.; Pouliakis, A.; Iacovidou, N. Periviable birth: A review of ethical considerations. Hippokratia 2021, 25, 1–7.
- Nayak, B.; Moon, J.Y.; Kim, M.; Fischhoff, B.; Haward, M.F. Optimism bias in understanding neonatal prognoses. J. Perinatol. 2021, 41, 445–452.
- MacDorman, M.F.; Gregory, E.C.W. Fetal and perinatal mortality: United States, 2013. In National Vital Statistics Reports; National Center for Health Statistics: Hyattsville, MD, USA, 2015; Volume 64.
- Royal College of Obstetricians and Gynaecologists. Late Intrauterine Fetal Death and Stillbirth. RCOG Green-top Guideline No. 55. Available online: https://www.oaa-anaes.ac.uk/assets/_managed/editor/File/Guidelines/Intra-Uterine%20Foetal%20Demise/GTG%2055%20Late%20Intrauterine%20fetal%20death%20and%20stillbirth%2010%2011%2010.pdf (accessed on 27 April 2022).
- Piccoli, G.B.; Cabiddu, G.; Castellino, S.; Gernone, G.; Santoro, D.; Moroni, G.; Spotti, D.; Giacchino, F.; Attini, R.; Limardo, M.; et al. A best practice position statement on the role of the nephrologist in the prevention and follow-up of preeclampsia: The Italian study group on kidney and pregnancy. J. Nephrol. 2017, 30, 307–317.
- Kattah, A.G.; Scantlebury, D.C.; Agarwal, S.; Mielke, M.M.; Rocca, W.A.; Weaver, A.L.; Vaughan, L.E.; Miller, V.M.; Weissgerber, T.L.; White, W.; et al. Preeclampsia and ESRD: The Role of Shared Risk Factors. Am. J. Kidney Dis. 2017, 69, 498–505.
- Innes, K.E.; Marshall, J.A.; Byers, T.E.; Calonge, N. A woman’s own birth weight and gestational age predict her later risk of developing preeclampsia, a precursor of chronic disease. Epidemiology 1999, 10, 153–160.
- Piccoli, G.B.; Torreggiani, M.; Crochette, R.; Cabiddu, G.; Masturzo, B.; Attini, R.; Versino, E.; Pregnancy Study Group of the Italian Society of Nephrology. What a paediatric nephrologist should know about preeclampsia and why it matters. Pediatric Nephrol. 2021.
- Luyckx, V.A.; Brenner, B.M. Low birth weight, nephron number, and kidney disease. Kidney Int. Suppl. 2005, 68, S68–S77.
- Low Birth Weight Nephron Number Working Group. The Impact of Kidney Development on the Life Course: A Consensus Document for Action. Nephron 2017, 136, 3–49.
- Gjerde, A.; Reisaeter, A.V.; Skrunes, R.; Marti, H.P.; Vikse, B.E. Intrauterine Growth Restriction and Risk of Diverse Forms of Kidney Disease during the First 50 Years of Life. Clin. J. Am. Soc. Nephrol. 2020, 15, 1413–1423.
- Sibai, B.M. Diagnosis, controversies, and management of the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet. Gynecol. 2004, 103, 981–991.
- Fakhouri, F.; Scully, M.; Provot, F.; Blasco, M.; Coppo, P.; Noris, M.; Paizis, K.; Kavanagh, D.; Pene, F.; Quezada, S.; et al. Management of thrombotic microangiopathy in pregnancy and postpartum: Report from an international working group. Blood 2020, 136, 2103–2117.
- Lopes van Balen, V.A.; van Gansewinkel, T.A.G.; de Haas, S.; Spaan, J.J.; Ghossein-Doha, C.; van Kuijk, S.M.J.; van Drongelen, J.; Cornelis, T.; Spaanderman, M.E.A. Maternal kidney function during pregnancy: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2019, 54, 297–307.
- Wiles, K.; Bramham, K.; Seed, P.T.; Nelson-Piercy, C.; Lightstone, L.; Chappell, L.C. Serum Creatinine in Pregnancy: A Systematic Review. Kidney Int. Rep. 2019, 4, 408–419.
- Harel, Z.; McArthur, E.; Hladunewich, M.; Dirk, J.S.; Wald, R.; Garg, A.X.; Ray, J.G. Serum Creatinine Levels Before, During, and After Pregnancy. JAMA 2019, 321, 205–207.
- Piccoli, G.B.; Alrukhaimi, M.; Liu, Z.H.; Zakharova, E.; Levin, A.; World Kidney Day Steering Committee. Women and kidney disease: Reflections on World Kidney Day 2018. Kidney Int. 2018, 93, 278–283.
- Piccoli, G.B.; Chatrenet, A.; Cataldo, M.; Torreggiani, M.; Attini, R.; Masturzo, B.; Cabiddu, G.; Versino, E. Pregnancy Study Group of the Italian Society of Nephrology. Adding creatinine to routine pregnancy tests: A decision tree for calculating the cost of identifying patients with CKD in pregnancy. Nephrol. Dial. Transplant. 2022, gfac051.
- Gaber, T.Z.; Shemies, R.S.; Baiomy, A.A.; Aladle, D.A.; Mosbah, A.; Abdel-Hady, E.S.; Sayed-Ahmed, N.; Sobh, M. Acute kidney injury during pregnancy and puerperium: An Egyptian hospital-based study. J. Nephrol. 2021, 34, 1611–1619.
More
