Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Tawhida Islam and Version 2 by Peter Tang.
Acceptance of nanoparticles (NPs) in therapeutic applications has increased because of their outstanding physicochemical features. By overcoming the drawbacks of conventional therapy, the utilization of metal NPs, metal-oxide, or metal supported nanomaterials have shown to have significant therapeutic applications in medicine. This is proved by a lot of clinical and laboratory investigations that show improved treatment outcomes, site-specific drug delivery, and fewer side effects compared to traditional medicine. The metal NPs interaction with living cells (animal and plant) showed many ways to develop therapeutic models with the NPs.
- metal nanoparticles
- nanotherapy
- therapeutic uses
1. Introduction
The implementation of nanoparticles (NPs) for the treatment and diagnosis of disease is a revolutionary concept that has been developed over the past few decades. The nanotechnological approach can be divided into two branches: one is nanodevices and the other is nanomaterials. The nanodevice can be defined as such tiny devices at the nanoscale range, which includes microarrays and some devices such as respirocytes [1][2][3][1,2,3].
Particles smaller than 100 nanometers (nm) in any one of the dimensions are considered nanomaterials. Biomedical science found successful result by using nanoparticles as therapeutic agents in the treatment of various diseases. As it is selective on the target organ and receptors, it overcomes several limitations of conventional therapy, such as nonspecificity, unwanted side effects, less efficiency, and low bioavailability [4].
Therefore, current research projects are considerably more focused on developing and designing new drug delivery systems, and the most promising area is ensured by NPs for their uniqueness in biological and physicochemical characteristics, as they can deliver molecules to specific locations in the body [5].
The therapeutic molecules which are insoluble in water can be complexed with NPs, resulting in greater bioavailability and significantly fewer physiological barriers; for example, NP carriers assist medication in passing the blood–brain barrier (BBB) [6][7][8][6,7,8]. Nevertheless, because it is targeted, it will require lower doses than conventional therapy, and the therapeutic index will be higher as it will minimize the toxicity in the biological system. The utilization of NPs in various fields of the health sector is possible because of their ability to provide a visual image of the targeted delivery location by using some agents; moreover, their pathway can be tracked.
Several studies have recently focused on the method of producing metal NPs using green synthesis, which has shown positive results against pathogens, cancer cells, helminths, fungi, etc. using the metal NPs Zn, Ag, Au, Pt, Mn, Ni, and Ti [9]. Currently, among other NPs, Ag-NPs are one of the top listed compounds being researched [10]. In 1857, Michael Faraday was the first person to study Au-NPs in a colloidal system and report Au-NP’s optical features [11].
2. Therapeutic Applications of Metal NPs
2.1. Therapeutic Interventions of Gold Nanoparticles (Au-NPs)
When Robert Koch discovered that gold cyanide had a bacteriostatic effect on Mycobacterium TB, the medical use of gold for the treatment of tuberculosis was established for the first time. This led to the introduction of gold as a medicine in the 1920s [12][110]. Au-NPs have a tendency to aggregate at tumor sites [13][95]. Tumor cells can be killed by Au-NPs in a variety of ways, including as drug delivery systems for mechanical damage, anticancer medicines, and photothermal ablation [14][111]. In particular, Au-NPs are used in drug delivery, imaging, photo-thermal therapy, sensing, catalysis, and antimicrobials [15][112]. The list of applications of Au-NPs is much longer because of their unique properties. The biocompatibility of gold nanoparticles has been well documented; however, the typical reduction procedures used to create them can leave behind harmful chemical species [16][113]. Consequently, Au-NPs manufactured in an environmentally friendly manner hold far more promise in a variety of settings. Although Au-NPs are not as widely used as Ag-NPs as antibacterial agents, they nonetheless have considerable impact against a wide range of diseases due to their inherent biocidal qualities [15][17][112,114]. Au-NPs of 60 nm showed a positive result in retinoblastoma treatment [18][115], Au nanopopcorn 28 nm in size is used to diagnose prostate and breast cancer [19][116], and Au nanostars (Au-NS) 30 and 60 nm in size can be used to identify brain tumors, and this same NP showed a satisfying result against bladder cancer [20][117]. Silica-coated Au nanorods showed effective antitumor activity, both in vivo and in vitro, against breast cancer by targeting CD44+ receptors [21][118]. Colloidal Au-NPs are of interest as nontoxic carriers for drug delivery [22][23][24][119,120,121]. In a study, it was found that the internalization of the 50 nm spherical gold nanoparticles (AuNPs) was the best of all the nanoparticles investigated [25][122]. TrxR (thioredoxin reductase) function can be inhibited by gold compounds, which causes tumor cells to accumulate reactive oxygen species (ROS) and experience oxidative stress, which ultimately kills the tumor cells [26][27][123,124] and the proposed anticancer mechanism of Au-NPs is illustrated in Figure 1.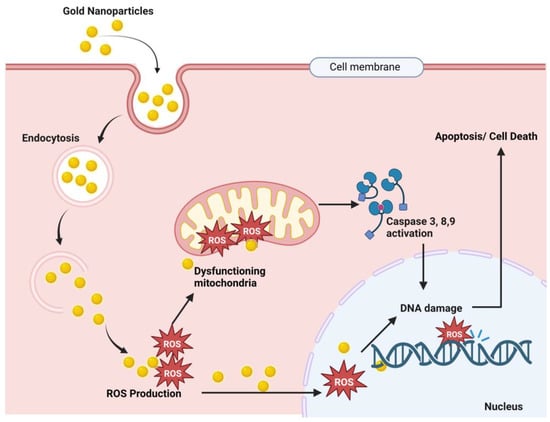
Figure 1. Proposed anticancer mechanism of gold nanoparticles. Here, Au-NPs pass through the cancer cell membrane by endocytosis, and endosomal release causes ROS (reactive oxygen species) production. These ROS cause mitochondrial dysfunction and result in caspase 3, 9, and 8 activations, which results in DNA damage and finally cell death [26][27][28][29][123,124,125,126].
-
Nanotherapeutic Application of Gold
2.2. Therapeutic Interventions of Silver Nanoparticles (Ag-NPs)
Silver has excellent physicochemical features, such as catalytic, optical, electric, and, of course, antibacterial capabilities, and these qualities make silver nanoparticles the most marketable nanoparticles. In the presence of Ag-NPs, the synergistic impact of antibiotics such as cefotaxime, azithromycin, cefuroxime, chloramphenicol, and fosfomycin against E. coli was greatly boosted as compared to antibiotics alone [30][80]. Other metal NPs may exhibit equivalent efficacy against particular germs, but overall, silver is said to be the most effective material against a variety of pathogens. Ag-NPs inhibit the extracellular activity of severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) [31][154]. Ag-NPs are the preferred metal when antibacterial characteristics are required. The antibacterial, antiviral, antioxidant, and anticancer characteristics of silver are well recognized, and it has the potential to be developed into a unique therapeutic agent. Ag also has antiparasitic, antiviral, and anticancer qualities [32][33][155,156], and the mechanisms of action of these effects are illustrated in Figure 2. Ag-NPs, after entering cells by endocytosis, produce ROS that damage the endoplasmic reticulum and mitochondria. The cellular pathways NF-kB, PI3K/AKT/mTOR, Wnt/beta-catenin, MAPK/ERK, and ERK activation result in DNA fragmentation, cell cycle arrest, and cell apoptosis [34][35][36][37][38][157,158,159,160,161].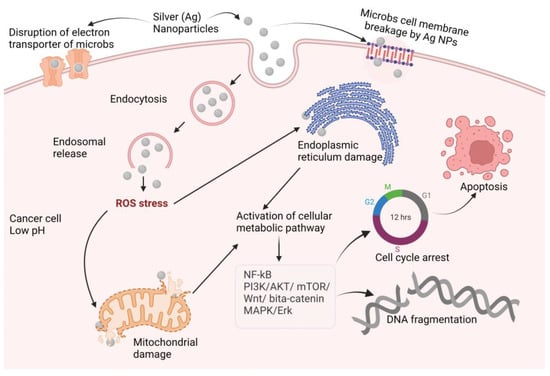
Figure 2. Proposed anticancer mechanisms of silver nanoparticles (NF-kB: nuclear factor kappa-light-chain-enhancer of activated B cells; PI3K: phosphoinositide 3-kinases AKT: protein kinase B; mTOR: mammalian target of rapamycin; Wnt: wingless and Int-1; MAPK: mitogen-activated protein kinase; ERK: extra-cellular receptor kinase).
-
Nanotherapeutic Application of Silver
2.3. Therapeutic Interventions of Copper Nanoparticles (Cu-NPs)
Researchers and health care professionals have been drawn to cupric oxide (CuO) NPs for their physical, chemical, high temperature, and photocatalytic capabilities, but most notably for their antibacterial properties [39][185]. Copper nanoparticles’ synergistic activity with amoxicillin, ampicillin, ciprofloxacin, and gentamicin against both Gram-positive and Gram-negative bacteria was investigated, and ampicillin showed comparatively improved activity compared to alone [40][186]. Cu-NPs inactivate glycosidase to provide an antidiabetic effect, and the study found that Cu-NPs showed an anticancer effect by activating BAX and p53 and by decreasing Bcl-2 expression, which result in apoptosis in cancer [41][187]. Cu-NPs increase ROS production in bacterial cells and cause bacterial DNA and protein destruction; on the other hand, accumulation of Cu-NPs in the bacterial cell wall causes cell wall disruption [42][43][44][45][46][47][48][188,189,190,191,192,193,194]. The mechanisms underlying these effects are depicted in Figure 3.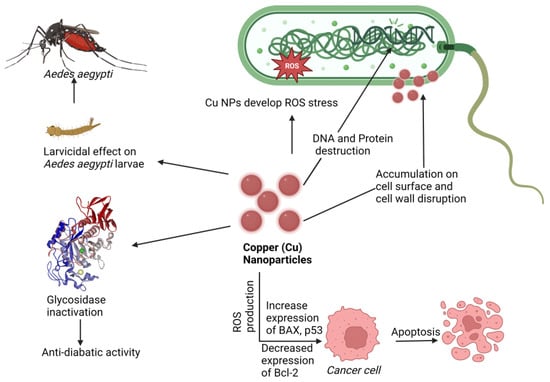
Figure 3. Proposed mechanism of nanotherapeutic applications of copper. Here Cu-NPs showed an anticancer effect by increasing BAX and p53 expression and Bcl-2 downregulating, an antidiabetic effect by glycosidase inactivation, an antimicrobial effect by ROS production cell wall disruption, and a larvicidal effect against Aedes aegypti (Dengue virus carrier).
-
Nanotherapeutic Application of Copper
2.4. Therapeutic Interventions of Zinc Nanoparticles (Zn-NPs)
Zinc is a material that is frequently used in biomedical applications due to its unique features, such as electric conductivity, optical capabilities, and piezoelectric qualities [49][207]. Beyth et al. defined the method of killing bacteria using zinc oxide (ZnO) NPs as having two pathways of action [50][208]. The first involves cell wall penetration, and the second includes the formation of ROS. Zn-NPs follow the Bcl-2/BAX/BAK pathway to cell apoptosis by caspase-3 and -9 and ROS-induced DNA fragmentation leading to cell cycle arrest and apoptosis, and also follow the mitochondrial disruption for an anticancer effect [51][52][53][209,210,211], as shown in Figure 4.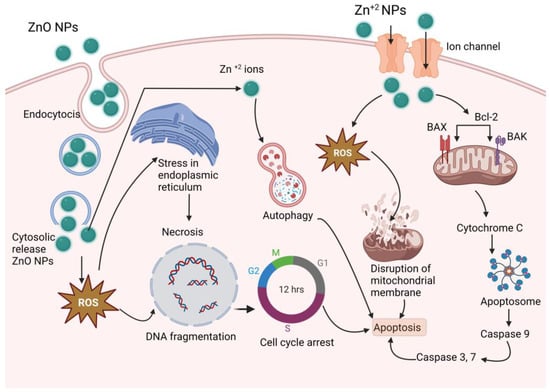
Figure 4. Proposed anticancer mechanism of Zn-NPs (ZnO-NPs create stress in endoplasmic reticulum, and produce ROS, which results DNA fragmentation and cell cycle arrest; on the other hand, produced ROS disrupts mitochondrial membrane and activates caspase 3, 7, and 9, which results in apoptosis).
-
Nanotherapeutic Application of Zinc
2.5. Therapeutic Interventions of Nickel Nanoparticles (Ni-NPs)
Ni-NPs have anticancer action [55][56][260,261]. A complex structure of Qu–PEG–NiGs (48–72 nm), green synthesized by Ocimum sanctum leaf extract, showed mitochondrial-mediated apoptosis against the MCF-7 cell line [57][262], antimicrobial activity, antioxidant action, and activity against human ovarian cancer, liver and spleen injury [55][58][59][60][260,263,264,265], lung inflammation [61][266], human lung cancer [62][267], lymphatic filariasis [63][268], and larvicidal parasitic activity [64][269]. Bacterial protein leakage induced by ROS activation [65][270] and disruption of the cell membrane [66][271] is one way of causing bacterial cell death. The antimicrobial mechanism is shown in Figure 5. It has numerous other therapeutic properties in a single formulation or a complex formulation.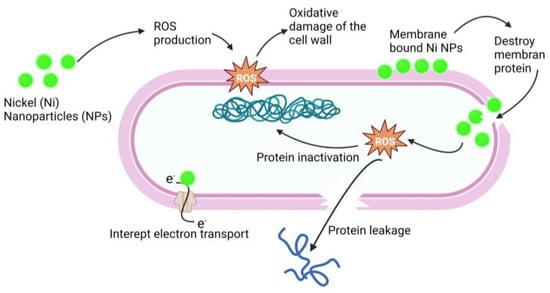
Figure 5. Antimicrobial mechanism of action of Ni-NPs. Ni-NPs cause ROS production that cause oxidative damage of the cell wall and destroy the membrane. ROS cause protein leakage and interrupt electron transport; these processes result in the antimicrobial effect of Ni-NPs.
-
Nanotherapeutic Application of Nickel
2.6. Therapeutic Interventions of Iron Nanoparticles (Fe-NPs)
Among the Fe-NPs, prominently used NPs include magnetite (Fe3O4), hematite, or iron (III) oxide (Fe2O3), and the less abundant iron (II) oxide (FeO) [67][281]. Magnetite (Fe3O4) NPs are used in biomedical applications due to their magnetic characteristics, biocompatibility, and, in particular, their superparamagnetic capabilities [68][282]. Magnetic NPs, also known as superparamagnetic iron oxide, are used in drug delivery [69][70][283,284] and hyperthermia therapy [71][72][73][285,286,287]. Magnetite NPs can produce receptive oxygen species (ROS), which kill microbes, making them a promising contender for an antimicrobial agent. Lung cancer cells terminated by ferroptosis as a result of Zerovalent Fe-NPs (ZVI-NPs) induce mitochondrial malfunction, intracellular oxidative stress, and lipid peroxidation; here, AMPK/mTOR activated by ZVI-NPs cause upregulation of GSK3/β-TrCP, which results in NRF2 degradation and ultimately results ferroptosis, which causes cancer cell damage [74][75][76][77][78][288,289,290,291,292], as shown in Figure 6.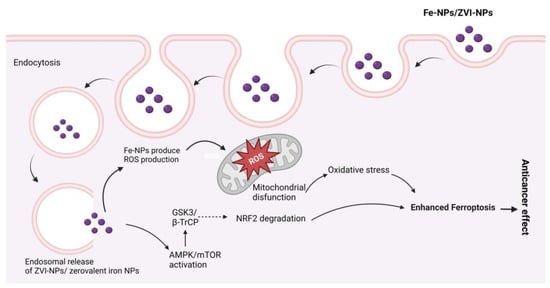
Figure 6. Possible anticancer mechanisms of iron (Fe) nanoparticles (zerovalent Fe-NPs cause ROS production, AMPK/mTOR activation, NRF2 degradation by GSK3/β-TrCP, and mitochondrial disfunction, which results in ferroptosis.
-
Nanotherapeutic Application of Iron
3. Metal Nanoparticles Elimination from Body
The elimination of NPs depends on their particle size, intrinsic biodegradability, core density, surface charge, and surface chemistry [83][307]. The liver is the major clearance organ in the oral administration of NPs. Intravenously administered NPs are cleared from the bloodstream by two main mechanisms: (i) renal elimination and (ii) hepatobiliary elimination. Choi et al. [84][308] reported that smaller-sized (<5.5 nm diameter) quantum dots undergo efficient urinary excretion due to the pore size limit of glomerular filtration in the kidneys. According to estimates of Si-NPs in rats, 7–8% of NPs were eliminated in urine and 75–80% were expelled in feces [85][309]. Nonbiodegradable and larger-sized (>5.5 nm) NPs are supposed to be eliminated through the hepatobiliary route. The hepatobiliary elimination involved the following pathways: (1) the liver sinusoid; (2) the space of Disse, a tiny perisinusoidal space containing blood plasma, nutrients, oxygen, and body waste that has become crucial in the treatment of liver disease, which is located between endothelial cells and hepatocytes; (3) hepatocytes; (4) bile ducts; (5) intestines; and finally (6) out of the body, as shown in Figure 7. In hepatobiliary elimination, the liver nonparenchymal cells (e.g., Kupffer cells and liver sinusoidal endothelial cells) influence and determine the elimination fate. The removal of Kupffer cells increased the fecal elimination of NPs by more than 10-fold [86][310].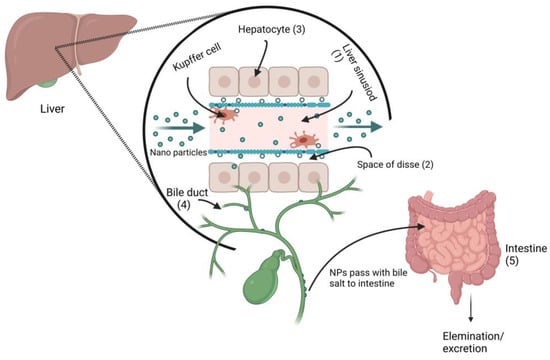
Figure 7. Proposed metal nanoparticles hepatobiliary clearance pathway (when metal NPs pass through the liver sinusoid, they enter the space of Disse via Kupffer cells, and then enter the bile duct, followed by fecal elimination.
