Inorganic polyphosphate (polyP), a simple anionic polymer consisting of even hundreds of orthophosphate units, is a universal molecule present in both simple and complex organisms. PolyP controls homeostatic processes in animals, such as blood coagulation, tissue regeneration, and energy metabolism. Furthermore, this polymer is a potent regulator of inflammation and influences host immune response in bacterial and viral infections. Disturbed polyP systems have been related to several pathological conditions, including neurodegeneration, cardiovascular disorders, and cancer, but researchers lack a full understanding of polyP biogenesis and mechanistic insights into the pathways through which polyP may act.
Inorganic polyphosphate (polyP), a simple anionic polymer consisting of even hundreds of orthophosphate units, is a universal molecule present in both simple and complex organisms. PolyP controls homeostatic processes in animals, such as blood coagulation, tissue regeneration, and energy metabolism. Furthermore, this polymer is a potent regulator of inflammation and influences host immune response in bacterial and viral infections. Disturbed polyP systems have been related to several pathological conditions, including neurodegeneration, cardiovascular disorders, and cancer, but we lack a full understanding of polyP biogenesis and mechanistic insights into the pathways through which polyP may act.
- inorganic polyphosphate
- inflammation
- neurodegenerative diseases
1. Introduction
2. Polyphosphate as a Regulator of Homeostasis in Eukaryotic Cells
PolyP in higher eukaryotes, specifically in mammals, is present in a broad range of tissues. In rodents, it has been found in the brain, heart, kidneys, liver, and lungs [19]. PolyP has been also found in the lysosomes of human fibroblasts, the nucleoli of human myeloma cells, mitochondria, plasma membranes, microsomes, and cytoplasm compartments of various cell types, as well as in the extracellular space, where it can be released by activated platelets and astrocytes [19][20][21][22][23][19,20,21,22,23]. PolyP amounts in mammalian cells oscillate in a micromolar range and are considerably lower than those observed in bacteria [24]. The highest concentration of mammalian polyP was described for platelets, where it reaches around 1 mM [25]. High levels were also observed in bone tissue (several hundred μM polyP in osteoblasts) [26]. In the past century, most research has focused on identifying polyP in various mammalian cells, but the role it may serve was only discussed speculatively [20][27][28][20,27,28]. These speculations covered its function as a regulator of lysosomal transmembrane potential, phosphate storage, or as an energy source. The first indications of the substantial regulatory role of polyP in eukaryotic homeostasis have been described by Ruiz and colleagues [25]. They found that granules of human platelets, which are similar to bacterial acidocalcisomes, are rich in polyP that is released upon thrombin stimulation. Smith et al. described how the polyP of platelets exerts an procoagulant effect and triggers a clotting cascade in the presence of factor XII (FXII), and presented polyP as an activator of the contact pathway of blood clotting [29]. The contact pathway (reviewed by Yi Wu [30]) consists of several plasma proteins activated by negatively charged surfaces or anions (like polyP). PolyP binds and activates FXII, triggering the contact pathway, which leads to blood coagulation and proinflammatory response through the production of the bioactive peptide bradykinin. Furthermore, polyP may be incorporated into fibrin and stabilize fibrin clot structure, making it more resistant to fibrinolysis [31]. Procoagulant effects of polyP are also pronounced; it has the ability to inhibit anticoagulant factors such as tissue factor pathway inhibitor (TFPI) released by endothelial cells [32]. In addition to its procoagulant and proinflammatory functions, polyP released from platelets and platelet-rich plasma have been linked with cell proliferation and tissue regeneration. Müller and colleagues showed that polyP promotes the growth and viability of bone marrow-derived mesenchymal stem cells and upregulates the expression of transcription factors responsible for osteogenesis and chondrogenesis, showing the involvement of polyP in bone and cartilage formation/homeostasis [33]. They also showed that calcium–polyP microparticles are taken up by cells via clathrin-dependent endocytosis; thus, polyP in the form of such microparticles can be manufactured and utilized in treatments of osteoarticular pathologies. Interestingly, polyP can act also as a mediator of signal transmission in the mammalian brain. Astrocytes activated via polyP, similarly to Ca2+ activation, release endogenous polyP which is further cleared from the extracellular space by neuronal uptake, suggesting that polyP acts as a glio- and neurotransmitter [23][34][23,34]. PolyP mediates communication between astrocytes by binding to purinergic receptors P2Y1 in the brainstem [23]. P2Y receptors are G protein-coupled receptors, widely distributed within the cells of the human body [35]. They are activated by extracellular nucleotides and mediate a myriad of signaling cascades involved in cell development, proliferation, and also immune regulation and inflammation [36][37][36,37]. PolyP binding to astroglial P2Y1 results in an increase in central sympathetic activity, stimulates breathing, and raises arterial blood pressure in vivo in rats [23]. Furthermore, several studies have demonstrated polyP as a mediator of proteostasis (reviewed by Xie and Jakob [38]), suggesting a substantial role of polyP in neurodegenerative disorders. Protein aggregation and production of insoluble fibers called amyloid fibrils are the foundation of neuro-diseases such as Alzheimer’s or Parkinson’s diseases. The intermediates preceding mature fibril formation, such as oligomers and protofibrils, accumulate in the extracellular space (and synapses) and alter cell communication, mitochondrial function, eventually triggering apoptosis [39]. PolyP has been shown to act neuroprotectively and abrogate the neurotoxic activity of improperly aggregated amyloid β-peptides/proteins and Tau protein which are responsible for the onset of Alzheimer’s disease [40]. Furthermore, polyP levels have been shown to shrink significantly in the brain with aging [41], when neurodegenerative disorders are most to likely to occur. Figure 1 collects and presents the activity of polyP in different tissues of the human body.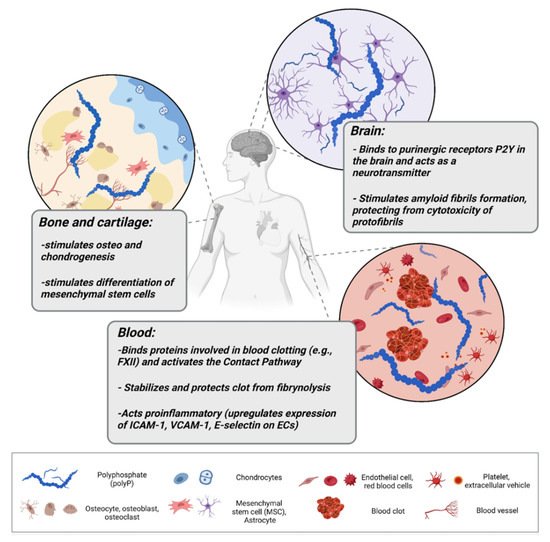
PolyP in Mitochondrial Homeostasis and Cell Energetics
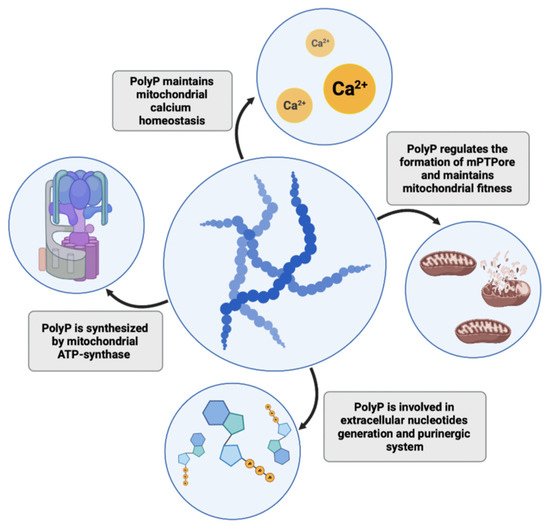
It is well known that mitochondrial dysfunction might be another critical factor and common feature in neurodegeneration [42]. Recently, Angelova and colleagues showed that approximately 40% of cellular polyP in astrocytes resides in mitochondria [43], where it regulates mitochondrial activity and calcium handling [44]. PolyP in mitochondria acts as a buffering system and prevents the formation of calcium phosphate insoluble precipitates, thus maintaining mitochondrial calcium homeostasis and sustaining high levels of calcium in the bioavailable form [45][46][45,46]. Disrupted calcium homeostasis and a decline in mitochondrial function are hallmarks of aging and, in addition to neurodegeneration, have also been associated with coronary heart disease and diabetes [47]. On the other hand, Abramov et al. showed that a depletion of mitochondrial polyP by expression of yeast PPX in several cell lines (including hepatic carcinoma cells, human embryonic kidney cells, and mouse myoblasts) reduces calcium-dependent mitochondrial permeability transition, a key mechanism underlying necrotic and apoptotic cell death [48][49][48,49]. Mitochondrial pores are formed upon stressing stimuli and calcium mishandling and also contribute to the process of neurodegeneration in Parkinson’s, Alzheimer’s, and Huntington’s diseases [50]. Similar results were shown in cardiomyocytes, where polyP depletion also leads to the inhibition of mPTPore (mitochondrial permeability pore) formation. Reduction of polyP in cardiac cells may be cardioprotective, as the formation of mPTPore and dysfunction of mitochondria lead to pathologies in cardiac tissue and irreversible cardiac cell injuries [51]. However, research on cardiac myocytes demonstrated a dual role of polyP, which is directly linked to its chain length. While polyP of 14 phosphates activated mPTPore formation, longer polyP molecules (130 phosphates) suppressed mPTP activity [52]. Seidlmayer et al. hypothesized that such competing actions of polyP may stem from polyP’s chaperone activity and ability to bind proteins involved in mPTPore opening. The authors concluded that mitochondrial polyP chain lengths depend on the metabolic state of these organelles, hence the polyP role in mitochondria should be considered in relation to the function of polyP in cell bioenergetics. Mitochondria are the key energy producers in cells. Interestingly, Pavlov and colleagues described that mitochondrial polyP play an important role in mammalian energetics [53][54][53,54]. They observed dynamic changes in polyP levels in astrocytes that were directly triggered by inhibition or activation of mitochondrial respiration. Inhibition of glycolysis by the addition of iodoacetic acid, which blocked the supply of substrates for mitochondrial respiratory complexes, reduced polyP abundance in mitochondria, suggesting that polyP levels may depend on the activity of the respiratory chain. Confirming this observation, in another study, Nakamura and colleagues observed that degradation of polyP enhances lactic acid fermentation in mice expressing the polyP-degrading PPX enzyme [55]. Their model proposes that elongation of polyP and a subsequent reduction in free intracellular Pi concentration sustains mitochondrial respiration and suppresses anaerobic lactic acid production. In a recently published study, Abramov and his group showed that ATP synthase, the mitochondrial inner membrane enzyme responsible for the formation of ATP, is involved in polyP synthesis similarly to the synthesis of ATP [56]. Using isolated rat brain mitochondria, they showed that polyP production is blocked in the presence of oligomycin, an ATP synthase inhibitor. Moreover, application of ATP before or after oligomycin did not affect polyP concentration, which excludes the possibility of ATP being an intermediate product of polyP synthesis. ATP synthase can also function in the opposite direction, as a proton pump hydrolyzing ATP. The authors observed that in the absence of ATP polyP can by hydrolyzed by ATP synthase, proving that polyP can be utilized by eukaryotic cells as a direct source of energy. However, polyP is not only synthesized in mitochondria. Significant amounts of this polymer can be found in other structures, including the secretory granules of platelets or lysosomes of other cell types (e.g., fibroblasts and glial cells) [20][25][43][20,25,43]. These observations suggest that other enzymes, not only the mitochondrial ones, should also be involved in polyP biogenesis. Reusch et al. proposed that a plasma membrane calcium pump (Ca2+-ATPase) from erythrocytes functions as a polyphosphate kinase due to its ATP/ADP-polyphosphate transferase activities [57]. Some authors have also suggested that multiple enzyme complexes may be involved in the process of polyP formation and that polyP may be a byproduct of several enzymatic reactions [54]. Clarifying the issue of polyP synthesis in eukaryotic cells or finding enzymes responsible for polyP production in other intracellular locations is important to allow for further advances in polyP studies. PolyP may not only act as a direct energy source but also as a phosphate store. For instance, in bacteria, both ATP and polyP are important phosphoryl donors for NAD kinase, which utilizes this polymer to yield NADP+ from NAD+; however, eukaryotic NAD kinases use only ATP, suggesting another purpose of polyP in mammalian phosphate-metabolism [58][59][58,59]. Indeed, recently, an interesting concept has emerged, in which polyP is proposed as both an energy and phosphate source in the extracellular space. Purines and their derivatives, ATP, ADP, and adenosine are important signaling molecules that act through purinergic receptors. Nucleotides can be released from cells by microvesicles, membrane channels, and transporters, or dying cells, and the extracellular adenosine is generated via adenine nucleotide hydrolysis by plasma membrane nucleotidases [60]. Müller et al. hypothesized that polyP may also participate in extracellular nucleotide generation. They found increases in extracellular ATP and ADP levels after polyP treatment of human sarcoma osteogenic (Saos-2) cells [61]. Moreover, they underlined that incubation of Saos-2 cells with polyP leads to translocation of alkaline phosphatase (ALP) and adenylate kinase (AK) to the cell membrane and further release of these enzymes outside of the cell in matrix vehicles. Both of these enzymes are involved in the interconversion and dephosphorylation of extracellular nucleotides. The increase in the ATP pool after polyP stimulation can both be utilized in purinergic signaling or as an energy reservoir, especially in tissues that consist of a large extracellular matrix in which only a few cells are embedded (e.g., bone and cartilage) [53]. It would be interesting to further investigate how polyP influences the extracellular purinergic system or whether it acts through purinergic receptors in other tissues. It is well known that disruption to purinergic signaling contributes to the pathophysiologies of multiple disorders in the immune system, vasculature, heart, kidneys, lungs, and the brain [62]. Nevertheless, polyP influence has not been investigated in the context of Huntington’s disease, a multi-system disorder which comprises both malfunction of purinergic signaling and mitochondrial dysfunction [63]— crucial polyP-associated metabolic events. The collected findings highlight polyP as a multifunctional molecule that plays a key role in maintaining proper cellular homeostasis; thus, deteriorations in its intra- or extracellular levels may lead to the development of multiple pathologies. PolyP functions in mitochondrial homeostasis and energetics are presented in Figure 2.