2.1. Aging-Related Genes and Signaling Pathways
Aging is the most important risk factor for a broad array of diseases, including neurodegenerative diseases, cardiovascular disease, metabolic syndrome, chronic inflammation, and cancer. Additionally, genetic mutations that delay aging have been also found to delay the onset of age-related diseases
[26][27][36,37]. Genes that regulate aging are relatively conserved among species and are enriched in certain signaling pathways
[6] (
Figure 1). The association between aging and disease makes fighting aging an even more attractive proposition; it is likely to fight the occurrence and progression of other diseases as well. In this section,
rwe
searchers s summarize the most important pathways and genes to the aging process.
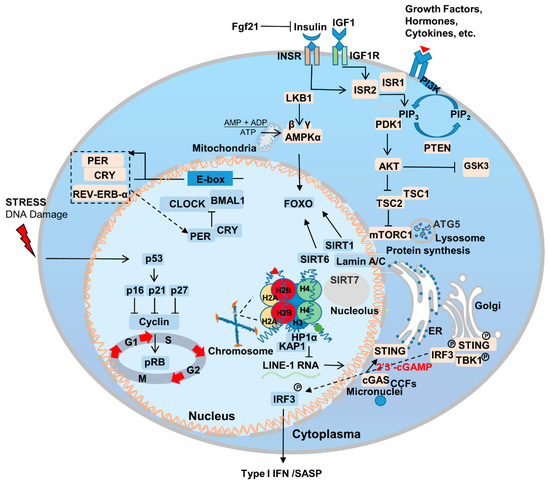
Figure 1.
Genetic and signaling mechanisms underlying aging. Aging involves multiple genetic alterations in a range of pathways, including, but not limited to, nutrient sensing, sirtuins, nuclear skeleton proteins, immunity, inflammation and circadian rhythm. PI3K/AKT, AMPK, and mTORC1 serve as the core members of lipid, glucose, and amino acid sensing. Lamin A/C interacts with SIRT1, 6 and 7 to regulate chromatin and intracellular homeostasis. cGAS-STING responds to internal and external nuclear pressures and regulates senescence-associated secretory phenotype (SASP). The feedback regulation of circadian rhythm-associated genes is also affected by other aging-related genes. p16, cyclin-dependent kinase inhibitor 2A; p21, cyclin-dependent kinase inhibitor 1A; p27, cyclin-dependent kinase inhibitor 1B; Rb, retinoblastoma protein.
2.2. Nutrient Sensing
Cells rely on nutrient sensing for both the detection of stresses and, ultimately, their survival
[28][38]. Nutrient availability and perception are important material basis for maintaining cell growth and normal function, and cellular metabolic homeostasis imbalance and cellular senescence complement each other
[29][39]. For example, in
Caenorhabditis elegans (
C. elegans), mutations in the highly conserved
daf-2 gene, which encodes an insulin-like receptor and regulates the insulin/insulin-like growth factor 1 (IGF-1) pathway, have been found to significantly prolong lifespan
[30][40]. During aging, the mechanistic target of rapamycin (mTOR) signaling pathway is also important for perceiving stress signals and nutrient sensing, and protein translation
[31][32][41,42]. Genetically inhibiting the insulin/IGF and mTOR pathways has also been demonstrated to extend mouse lifespan
[33][43].
In 1939, researchers discovered that calorie restriction (CR) can ameliorate aging
[34][44]. CR induces various metabolic changes in the body, and crosstalk between CR and proteins related to nutrient sensing-related pathways is an important reason for ameliorate aging
[35][45]. To date, CR has been shown to extend the lifespan of
Saccharomyces cerevisiae, C. elegans, normal and progeria mouse models, and non-human primate rhesus monkeys; at present, CR represents the most effective lifespan-extending intervention across species
[36][37][38][39][40][41][42][46,47,48,49,50,51,52]. This is due to the fact that most molecular pathways involved in longevity are associated with increased stress resistance
[43][53]. Compared with ad libitum access to food (AL), every-other-day feeding (EOD) increases the healthy lifespan of mice. Dietary restriction has also been found to limit the growth of various types of tumors
[44][54]. The phosphatidylinositol-3-kinase (PI3K) pathway, which is a key insulin signaling component, is an important regulator of CR
[40][45][50,55]. In addition, restricting the amount of branched-chain amino acids (BCAAs), such as leucine, in the diet has also been demonstrated to prolong the lifespan of
LmnaG609G/G609G and
Lmna–/– mice. In terms of physiological aging, a low-BCAA diet reduces weakness, but does not extend lifespan
[46][56]. Thus, achieving CR via the regulation of metabolism and diet represents a promising anti-aging intervention.
2.3. Sirtuins
Sirtuins are another gene family that can extend the lifespan of
C. elegans [47][57]. They are mainly responsible for regulating cell metabolism, genome stability, gene expression, signal transduction, and important for maintaining the health of the body
[48][58]. There are seven sirtuins in mice and humans, and, under CR, SIRT1 expression is upregulated. This prolongs lifespan and is closely associated with the IGF signaling pathway
[49][59]. Meanwhile, SIRT6 regulates the IGF1 levels and, thus, aging, with SIRT6 overexpression extending lifespan in male mice
[17][27]. Recent studies have also confirmed that SIRT1 is a key protein in the regulation of endothelial cell aging; vascular endothelial cells are essential for maintaining the health and growth of blood vessels. Reducing the expression of SIRT1 in endothelial cells accelerates cellular aging and hinders the normal function of blood vessels
[50][60]. Endothelial cell senescence performs a pivotal role in systemic aging, but the effects can be lessened via the overexpression of SIRT7
[51][52][61,62]. Furthermore, SIRT6 expression in endothelial cells has been shown to be important for maintaining heart function
[53][63]. Taken together, these findings indicate that aging-related genes show tissue-dependent effects, and targeting specific types of senescent cells may represent an effective way to treat systemic aging.
2.4. Nuclear Skeleton-Associated Proteins
Intranuclear proteins, such as Lamins play an important role in regulating and maintaining the balance of aging and tumors. Mutations in the
LMNA gene affect aging through a number of mechanisms. For example, Lamin A/C interacts with SIRT1, 6, and 7 and affects their intracellular activity and stability, thereby regulating aging
[52][54][55][62,64,65]. Interactions between Lamin A/C and SIRT7 also inhibit the transcriptional activation of long interspersed elements-1 (LINE-1, L1)
[56][66], which stabilizes heterochromatin structure. This inhibits the development of a SASP, such as a type I interferon response that triggers natural immune pathways, and can therefore delay the aging of human stem cells by reducing inflammation
[57][67]. IGF-1/AKT signaling pathway protects cells from apoptosis
[58][68]. Furthermore, recent studies have found that the abnormally processed progerin, which is classically located within the nucleus, is also localized outside it. Here, it interacts with IGF-1R and downregulates its expression, thereby impairing IGF-1/AKT signaling, inhibits cellular energy metabolism and accelerates cell aging
[59][69]. Inhibiting isoprenylcysteine carboxylmethyltransferase (ICMT)-associated activation of AKT-mTOR signaling has been found to improve progeria symptoms
[60][70]. Notably, an
mTOR hypomorphic allele (
MtorΔ/+) has also been found to improve aging characteristics and lifespan in
LMNAG608G mice
[61][71]. Taken together, these findings indicate that Lamin A and nutrient sensing share an intricate, important connection to the aging process.
Furthermore, another protein from the nuclear matrix, Lamin B1, is also closely related to aging. Cells respond to carcinogenic pressure by degrading Lamin B1 through autophagy, thus accelerating cell senescence
[62][72]. Recent studies have found that intranuclear SIRT1 protein is the second major nuclear substrate for LC3-mediated selective autophagy, thus influencing cellular senescence through this degradation mechanism
[63][73].
2.5. Immunity and Inflammation
Inflammaging is an important component of aging, which is a pathological phenomenon that brings together our knowledge of age-related chronic diseases, functional decline, and weakness
[64][74]. In the process of aging, the innate and acquired immune system is remodeled, and the reliability and efficiency of the immune system decrease with age, which leads to the upregulation of inflammatory response and the occurrence of related degenerative diseases
[65][75]. The drivers of the inflammatory response mainly include two parts: the degradation of immune receptors/immune sensors and the increase in stimuli that trigger inflammation
[26][66][36,76]. Inflammation is also the result of lifelong exposure of the immune system to antigenic stimuli and complex genetic, environmental, and age-related mechanisms. Inflammation underlies aging and many age-related chronic diseases, which in turn increases the rate of aging
[26][36]. Excess nutrients are an important factor in inflammation, diet performs an important role in the development and treatment of inflammation and related problems, and CR can slow inflammation and improve aging
[67][77]. The activation of innate immune Toll-Like receptors perform an important role in the aging process, and when Toll-like receptors are knocked out, it can significantly ameliorate the aging of heart-related cells
[68][78]. The Janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway plays an important role in regulating inflammatory response, and the inhibition of JAK/STAT signaling pathway can reduce age-related inflammatory response to a certain extent
[69][79].
Innate immunity plays an important role in the aging process. The cytosolic cyclic GMP–AMP synthase (cGAS)-STING pathway is an important signaling pathway in cells whereby cytoplasmic sensory DNA activates immunity (
Figure 1)
[70][80]. During aging, cytoplasmic chromatin fragments (CCFs) leaked from the nucleus, and along with micronuclei or DNA that has escaped from the mitochondria, activate the cGAS-STING pathway, and thus facilitate SASP
[71][81]. SASP promotes the senescence of adjacent or circulatory cells via paracrine signaling
[72][82]. Recent studies have found that yes-associated protein 1 (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ)-mediated control of cGAS-STING signaling is an important molecular mechanism in the regulation of aging in stromal cells and contractile cells. YAP/TAZ is also important for maintaining nuclear envelope stability via the modulation of Lamin B1 expression
[73][83].
2.6. Circadian Rhythm
The production and maintenance of circadian rhythm is the result of positive and negative feedback loops regulated by a series of genes associated with the biological clock, including
BMAL,
CLOCK,
PER,
CRY,
REV-ERB-α, ROR-β, etc.
[74][84] (
Figure 1). The circadian rhythm/clock genes are closely related to aging and two-way adjustment, with aging leading to the transcriptomic reprogramming of circadian genes. For example, the absence of the core clock transcription factor Bmal1 leads to multiple aging-like pathologies in mice
[75][85]. Disturbances in the circadian rhythm accompany the occurrence of aging, and can contribute to the onset and progression of aging-related neurodegenerative diseases
[76][86]. Notably, Salvador Aznar Benitah group and Sassone-Corsi group by comparing mice of different ages, revealed that a low-calorie diet can improve the circadian rhythm of somatic and stem cells, inhibiting the aging process
[77][78][87,88].