Ophthalmic malignancies include various rare neoplasms involving the conjunctiva, the uvea, or the periocular area. These tumors are characterized by their scarcity as well as their histological, and sometimes genetic, diversity. Uveal melanoma (UM) is the most common primary intraocular malignancy. UM raises three main challenges highlighting the specificity of ophthalmic malignancies. First, UM is a very rare malignancy with an estimated incidence of 6 cases per million inhabitants. Second, tissue biopsy is not routinely recommended due to the risk of extraocular dissemination. Third, UM is an aggressive cancer because it is estimated that about 50% of patients will experience metastatic spread without any curative treatment available at this stage. These challenges better explain the two main objectives in the creation of a dedicated UM biobank. First, collecting UM samples is essential due to tissue scarcity. Second, large-scale translational research programs based on stored human samples will help to better determine UM pathogenesis with the aim of identifying new biomarkers, allowing for early diagnosis and new targeted treatment modalities. Other periocular malignancies, such as conjunctival melanomas or orbital malignancies, also raise specific concerns. In this context, the number of biobanks worldwide dedicated to ocular malignancies is very limited.
1. Resources of the NOMA Biobank
1.1. All Tumors
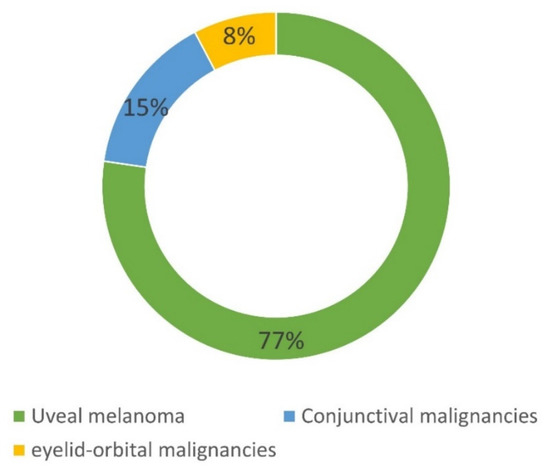
Overall, 207 patients have been included in the NOMA biobank since 2013. As shown in Figure 1, 160 patients had an UM, 31 had a CM, and 16 had an orbital malignancy. Table 1 summarizes the main histological subtypes of the tumors stored in the NOMA biobank. For the 160 patients with an UM, 41% and 77.5% of samples were stored as LBs and solid biopsies, respectively. This preponderance of LB samples is easily explained by the fact that tissue biopsy is not routinely performed in the case of UM and is only obtained when enucleation is performed. The low number of conjunctival and orbital tumors included in the NOMA Biobank is explained by the fact that the tumor size is usually too small to be able to include tissues in a biobank and the samples are only used for clinical diagnostic purposes.
Figure 1. Composition of the NOMA Biobank samples.
Table 1. Detailed composition of the NOMA Biobank samples.
| Tumor Location |
Histological Subtype: Number (%) |
Tissue Biopsy: Number (%) |
Venous Liquid Biopsy: Number (%) |
Intraocular
N = 160 |
Uveal melanoma: 160 (100) |
66 (41) |
124 (77.5) |
Conjunctival
N = 31 |
Conjunctival melanoma: 21 (68)
Conjunctival naevus: 8 (25.5)
Squamous cell carcinoma: 2 (6.5) |
31 (100) |
11 (35.5) |
Orbit
N = 16 |
Lymphoma: 8 (50)
Carcinoma: 4 (25)
Schwanoma: 3 (19)
Solitary fibrous tumor: 1 (6) |
16 (100) |
0 (0) |
1.2. Uveal Melanoma
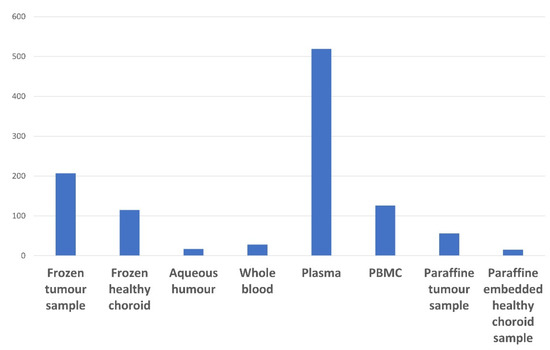
The number of UM samples available in the NOMA Biobank is summarized in Figure 2. Regarding LBs, most samples were prepared and stored as plasma samples. Only a few AH samples (15 patients) were available in the our biobank. This is explained by the fact that resesarcherswe have only systematically collected the AH from enucleated eyeballs since 2020.
Figure 2. Summary of the UM tissue and liquid biopsy samples available in the NOMA Biobank.
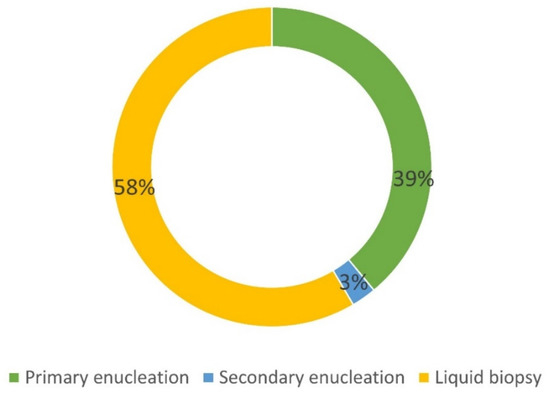
Among the 160 UM patients included in the NOMA Biobank, 66 (41%) were enucleated, thus allowing for tissue collection (Figure 3).
Figure 3. Details of the origin of UM samples included in the NOMA Biobank. Primary enucleation: no treatment before surgery. Secondary enucleation: treatment before surgery (proton therapy).
The histological features of the enucleated eyeballs are provided in Table 2. Among the patients who underwent secondary enucleation, only those referred for tumor recurrence were included. Patients who underwent secondary enucleation for painful and blind eye due to extensive necrosis of the tumor with proton therapy were not included in the biobank. The cold ischemia time was defined as the time between enucleation and sample freezing for the biobank. The BAP 1 status was assessed by immunohistochemistry. Most of the UM tumors showed histological signs of aggressiveness (67% of cases with epithelioid cells, 20% of cases with extraocular extent, 33% of cases with ciliary body involvement).
Table 2. Histological features of enucleated UM patients. Cold ischemia time (time between resected specimen collection in the operative room and the freezing procedure); * n = 40 patients.
| Number of Enucleated Patients (%) |
66 (100) |
| Primary Enucleation |
62 (94) |
| Secondary Enucleation |
4 (6) |
| Tumor thickness in mm: mean (range) |
12.1 (0.2–20) |
| Epithelioid subtype: number (%) |
44 (67) |
| Extraocular extent: number (%) |
13 (19.7) |
| Ciliary body infiltration: number (%) |
22 (33) |
| Mitoses/mm2: mean number (range) |
1.9 (0–21) |
| % of tumor cells in frozen sample: mean (range) |
75.2 (0–95) |
| Cold ischemia time in min: mean (range) |
34.8 (15–180) |
| BAP 1 loss: number (%) * |
30 (75) |
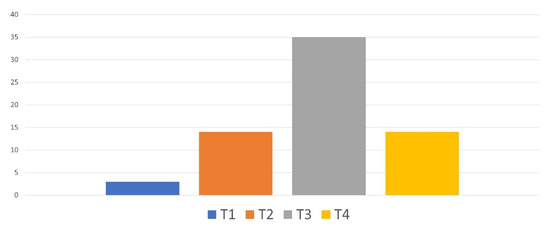
The pathological Tumor–Node–Metastasis (pTNM) classification of the tumors of enucleated patients is provided in Figure 4. About half of the patients had a pT3 UM.
Figure 4. Pathological TNM classification of the tumors of enucleated patients.
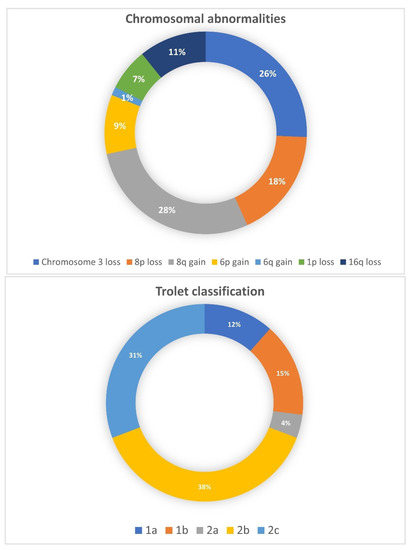
Among the 66 enucleated patients, genetic analyses were performed in 28 (42%) patients. The chromosomal abnormalities as well as the subsequent Trolet classification are shown in Figure 5. Overall, most patients were classified as “poor prognosis” with the loss of a chromosome 3 and a chromosome 8q gain and 64% were classified as “Trolet 2b and 2c”. These findings were consistent with the histological features described above.
Figure 5. Chromosomal abnormalities and Trolet classification of 28 enucleated UM patients.
2. Objectives of Setting Up a Dedicated Ophthalmic Malignancy Biobank
2.1. Diagnosis
The diagnosis of a conjunctival or periocular malignancy is routinely made by pathologists. However, in certain circumstances such as UM, a tissue biopsy is not recommended, and treatment is only based on clinical and radiological findings, incurring a risk of diagnostic inaccuracy, such as the enucleation of a non-tumor intraocular mass. AH collection is not routinely performed for the diagnosis of UM. However, it is a minimally invasive procedure performed on an outpatient basis. Several studies have recently shown that the presence of driver mutations in the
GNAQ and
GNA11 genes could be used as a reliable diagnostic biomarker in UM, thus highlighting the need for a dedicated venous blood and AH biobank in UM
[1][32].
2.2. Translational Research and Precision Medicine
Healthy and tumor tissues as well as LBs from ocular tumors are stored in
theour biobank, allowing translational research and tumorigenesis exploration. As highlighted above, very rare samples, such as AH samples, are systematically collected prospectively. AH samples should permit the identification of biomarkers for the diagnosis of UM and retinoblastoma follow-up
[2][31]. To date, no targeted therapy has been found to be effective in the treatment of metastatic UM nor the prevention of metastatic spread
[3][12]. In 2018, the tyrosine kinase inhibitor sunitinib administrated in adjuvant therapy in high risk (loss of chromosome 3, gain 8q, Class 2, T3–T4 by American Joint Committee on Cancer classification) primary UM was considered promising due to improvements in overall survival
[4][44]. However, the study was not randomized (since the control group was an historical cohort) and results were not subsequently validated in an independent cohort. However, this study highlights the urgent need to discover new targeted therapies that can be administered, and make room for adjuvant therapeutic decision-making during the primary treatment of ocular malignant tumors. In this context, the systematic storage of UM samples exhibits a strong value for translational research by identifying future therapeutic targets.
2.3. Impact of Radiotherapy on UM Genetics
The storing of secondary enucleation samples following radiotherapy is of high interest and has been minimally studied until now. The impact of radiotherapy on genetic abnormalities and subsequent prognosis is still debated since controversial studies on this topic have been reported. Genetic abnormalities have been found to be more prevalent and more complex in previously irradiated tumors
[5][45]. In contrast, another study found that chromosome 3 status and subsequent prognosis were not modified by radiotherapy
[6][46]. There is also a debate regarding the difficulty of assessing genetic status (especially chromosome 3) in previously irradiated UM. Furthermore, karyotyping and FISH detection were found to be significantly altered by radiotherapy
[5][45], whereas in another study, the detection of chromosome 3 microsatellite analysis was successfully determined
[7][47]. In
theour experience, genetic testing depends on the etiology of secondary enucleation. Secondary enucleations performed for blind and/or painful eyes are associated with a high rate of post-radiotherapy necrosis, leading genetic testing to be inconclusive. Conversely, secondary enucleation performed for tumor recurrence can be more easily processed. However, it is not always clear whether genetic testing in this case is carried out on a recurrent tumor (i.e., tumor resistance to radiotherapy) or on a primary tumor located outside the field of irradiation.
2.4. Pretreatment Screening
A biobank may also be useful to investigate whether a patient might be eligible for a given treatment. Until recently, there was not standard of care for metastatic UM. Tebentafusp, a specific anti-GP100 immunotherapy, has recently been approved by the Food and Drug Administration but only in HLA-A*02:01 UM patients
[8][48]. The HLA status of patients with cryopreserved blood samples can be easily determined and might help clinicians determine whether or not a patient is a good candidate for tebentafusp treatment.
2.5. Scientific Output
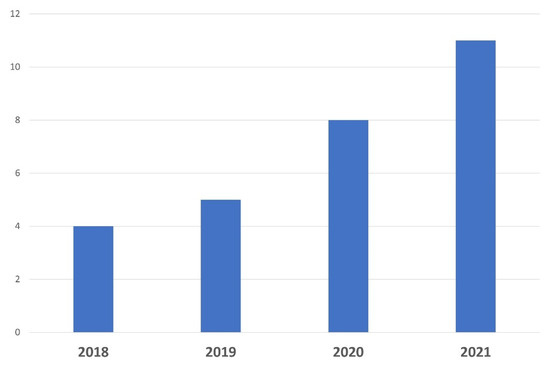
A biobank dedicated to a specific pathology should be managed by an expert team involved in the setting up and development of research projects in tight collaboration with physicians, surgical and molecular pathologists, and researchers. Thus, a specific biobank should be acknowledged and cited in scientific publications. As shown in
Figure 6, the number of publications related to ocular oncology published by
theour department has markedly increased over the last few years, both for fundamental and clinical research programs, mainly through the use of ocular resources from the biobank. Several articles based on the NOMA biobank resources have recently been published in peer-reviewed journals with high impact factors
[9][10][11][12][13][1,6,49,50,51].
Figure 6. Number of scientific publications related to ocular oncology and translational research published over the last 4 years by Nice University Hospital thanks to the use of samples.
2.6. National and International Collaborations
Data sharing enabled Nice University Hospital to collaborate with several internationally recognized ocular oncology departments, as summarized in Table 3. Of the 369 FFPE samples stored in the NOMA biobank, 44 (11.9%) have been used for collaborative scientific projects. A specific scientific and ethics committee is mandated to assess each public or private request to use samples according to the current regulations.
Table 3. Current national and international collaborations.
| Institution |
Type of Collaboration (Recent Related Articles) |
| Oncology Department, Antoine Lacassagne Cancer Centre, Nice, France |
Fundamental and clinical [9][14][15][16][17][1,26,52,53,54] |
| Team 1, Molecular Mediterranean Medicine Centre (C3M), Nice, France |
Fundamental [12][13][18][19][50,51,55,56] |
| Ophthalmology Department, Lyon University Hospital, France |
Clinical [20][57] |
| Oculoplastic Department, Jules Gonin Eye Hospital, Lausanne, Switzerland |
Clinical [15][21][22][52,58,59] |
| Anatomic Pathology Service, Pathology Department, Centro Hospitalar e Universitário do Porto, Portugal |
Fundamental [10][6] |
| Liverpool Ocular Oncology Research Department, United Kingdom |
Fundamental |
2.7. Information for Patients and Other Health Professionals
Because clear and accurate information is mandatory before obtaining any written informed consent,
theour Ocular Oncology Department has recently developed a dedicated website for patients referred to
theour tertiary care center (
https://www.cancerdesyeux.fr, accessed on 1 January 2023). This website provides valuable information on patients’ care and follow-up, as well as research projects. A dedicated website has also been created by the Clinical and Experimental Pathology Laboratory Department of Nice University Hospital to provide useful information to patients and health professionals (
http://biobank-cotedazur.fr/, accessed on 1 January 2023). Scientific congresses and relevant learned societies such as the ISOO (International Society of Ocular Oncology), OOG (Ocular Oncology Group), ESOPRS (European Society of Ophthalmic Plastic and Reconstructive Surgery), and SFO (Société Francaise d’Ophtalmologie) allow for the diffusion of knowledge about ophthalmic malignancies, including biobanks.
2.8. Education and Training
A Master in Science (MSc) degree entitled “Biobanks and complex data management” has been set up by the Côte d’Azur University (Nice, France). Developed by recognized experts in the field, this program is the first European MSc degree in the field offered by a public university (
https://univ-cotedazur.eu/msc/biobanks-complex-data-management, accessed on 1 January 2023). Dedicated to Biological Sciences, this unique program is intended for students aiming to acquire professional skills in order to provide evolving biobank services, meet regulatory and user requirements, and manage biobanks.
The “Biobanks and Complex Data Management” MSc allows international exchanges through a wide network of leading academic and industry partners.
3. Strengths of the NOMA Biobank
The strengths of the our biobank are as follows:
- (i)
-
The NOMA biobank is certified (ISO 9001, NF-96S-900) and accredited (ISO 20387) and belongs to an already well-established biobank (Cote d’Azur Biobank) (
www.biobank-cotedazur.fr, accessed on 1 January 2023),
-
- (ii)
-
It is stored in a university pathology laboratory (LPCE, Nice) accredited for clinical and molecular pathology according to the ISO 15189 standard,
-
- (iii)
-
Clinical, imaging, histological, biological, and genetic data are collected for each patient,
-
- (iv)
-
Its business model is supported by public funding programs,
-
- (v)
-
It has allowed for an increase in the amount of translational research conducted,
-
- (vi)
-
It allows for an increase in the number of scientific publications and national and international collaborations,
-
- (vii)
-
A master’s degree entitled “Biobanks and Complex Data Management” has been set up by the Côte d’Azur University (Nice, France) to train students to become biobankers.
-