2. DNA-Based Materials
2.1. DNA Tetrahedron
Since the one-step synthesis of a tetrahedron (TDN) was first introduced, it has become one of the most widely used DNA nanostructures in biomedicine. TDNs, like other DNA nanostructures, are formed by mixing all four DNA oligonucleotides during a thermal annealing process
[9][14]. Since TDN can be modified with various agents and has good biocompatibility, the use of TDN can increase the effectiveness of off-target anticancer drugs
[10][15]. According to Fan et al., TDNs may successfully enter the dermis layer of mice and people’s skin and load and distribute the drug doxorubicin (DOX) to subcutaneous tumor locations
[11][16].
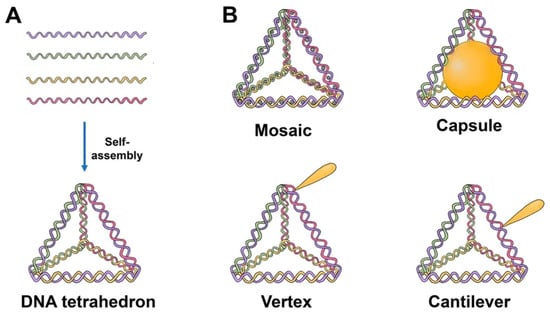
The majority of TDNs used today are duplexes and double bundles, duplexes receiving the most research attention. TDNs can be modified with fluorescent dyes, bioligans, proteins, chemotherapy drugs, and various types of nucleic acids. Based on the various positions of the added functional groups or molecules, there are four major ways to modify TDN: vertex modification, mosaic modification, capsule modification, and cantilever modification (
Figure 1)
[12][17].
Figure 1. Schematics of the TDN structure and possibilities for drug loading. (A) Self-assembly of TDN by annealing. Each single strand consists of three sequence blocks, each complimentary to the sequence of another single strand. Hence, four triangles of DNA helices form a solid tetrahedral structure upon hybridization. (B) Tetrahedron modified with drugs. Mosaic: chemotherapy drug (doxorubicin, paclitaxel, actinomycin D). Capsule: nanoparticle (AuNP, for example), cytochrome c, peptide (melittin). Vertex: CpG, aptamer, mAbs, 5-fluorouracil, peptide. Cantilever: folate, siRNA, KillerRed, camptothecin.
Vertex modification is the modification of functional groups in the top position of the TDN, such as azide groups for the ensuing click reaction or amino or sulfhydryl groups to stabilize the structure. Mosaic modifications occur when functionalized molecules or groups, such as chemotherapy drugs or fluorescent molecules, are used. The anticancer medicine is loaded into a DNA tetrahedron, which may then pass through the negatively charged cell membrane with minimal cytotoxicity, hence enhancing drug delivery. This method can also considerably lessen the drug’s negative side effects on the body.
The molecules of interest are put into the TDN in the case of capsular modification. By encapsulating nanoparticles in DNA, Mao et al. created nanocomplexes of a DNA tetrahedron with a gold nanoparticle. Cantilever modification of the TDN refers to the attachment of functional molecules or groups to the sides of the TDN. An example of such a modification was created by Tian et al. TDN associated with the angiopep-2 peptide
[12][17].
Tetrahedrons have been used in a number of investigations thus far on medication delivery. TDNs created by Xie et al. and loaded with paclitaxel demonstrated stronger cytotoxicity on non-small cell lung cancer A549 cells and a PTX-resistant cell line than PTX alone. Moreover, drug resistance was overcome. TDNs appear to act as a P-glycoprotein inhibitor because the expression of the mdr1 gene and P-glycoprotein was shown to be downregulated in A549/T cells. Additionally, it was demonstrated that TDN-loaded PTX can induce apoptosis in A549/T cells
[13][18].
Kim et al. showed that TDN can be employed as a carrier to deliver a variety of physiologically active molecules.
[14][15][19,20]. They can include DNA-intercalating medicines such as doxorubicin, which can be easily loaded and efficiently delivered even to drug-resistant cells to demonstrate the desired action
[16][21]. Photosensitizers may be loaded into TDN and transmitted into the cell with a high uptake efficiency since it is also known that methylene blue interacts with DNA duplexes
[17][18][22,23].
Moreover, Kim et al. created a TDN streptavidin-mirror hybrid as an enzyme delivery vehicle. The biotinylated enzymes—caspase-3, Cre recombinase, and β-galactosidase—were loaded onto streptavidin. These TDNs were capable of penetrating cells, localizing in tumors, and delivering all of the mentioned enzymes, even intracellularly. As the hybrid could intracellularly carry the enzyme β-galactosidase, which is considerably larger than the antibody, it was concluded that the use of such a hybrid is not limited by the size of the delivery molecules
[19][24].
The novel TDN was created by Tian et al. as a biocompatible nanocarrier of metal complexes. The TDN was linked noncovalently with two aptamers, AS1411 and MUC-1, and loaded with an iridium-based photocatalyst. The combined targeting of MUC-1 and AS1411 aptamers improved the selective cellular uptake and cytotoxicity of the iridium photocatalyst against U251 glioma cells. This compound successfully blocked signaling pathways by causing considerable fragmentation of mitochondria, inducing ROS-dependent apoptosis, and effectively inhibiting the migration of cancer cells
[20][25].
Yang et al. integrated antisense oligonucleotides suppressing the c-raf protooncogene and nuclear targeting peptides into the double-bundle TDN. With the use of this delivery technique, the c-raf gene was successfully knocked down while also increasing the degree of target mRNA suppression at low concentrations in the nucleus and cytoplasm
[21][26].
The use of affibody-TDNs as nanocarriers opens up new possibilities for the transport of nucleoside anticancer drugs. Affibody molecules are small polypeptides that can bind a number of different target proteins. The affibody molecule was conjugated to one of the vertices of the tetrahedron, and the 5-fluorouracil nucleoside analog (FUdR) preparation was attached to the other three vertices. Affibody-TDN complexes have demonstrated high selectivity and inhibition both in vitro and in vivo in HER2-overexpressing breast tumors
[22][27].
2.2. DNA Origami
Paul Rotumend introduced the concept of DNA origami technology in 2006. DNA origami is a self-assembling nanostructure in which hundreds of short “staple” oligonucleotides fold a long single-stranded DNA “scaffold” into multilayer DNA assemblies
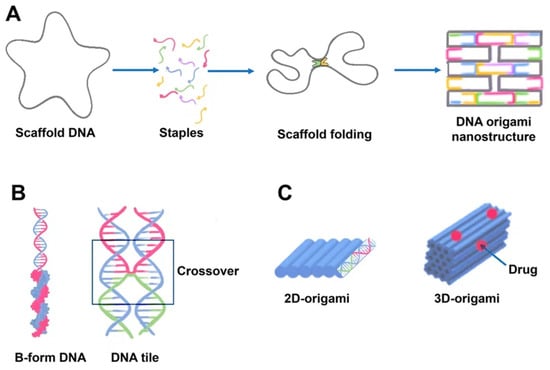
[23][28]. The scaffold is folded into a predefined form using approximately 200 complimentary short strands (“staples”) by forming crossovers at every DNA helical turn. During thermal annealing, each staple specifically links various scaffold components together according to its own sequence, folding the scaffold into the desired form (
Figure 2). The advantage of using DNA origami is concluded in the addressability of specific structural components with subnanometer accuracy and precision by changing individual staples
[24][29].
Figure 2. Schematics of DNA origami assembly and its structural features. (A) DNA origami assembly from the directed folding of the scaffold strand (gray) by using complementary staple strands (multicolored). Scaffold DNA functions as a guide or seed in the folding process, increasing folding efficiency and strand stoichiometry robustness. Each staple, for example, the blue strand, is incorporated specifically based on its sequence. The staples can connect distant sections of the scaffold by base pairing resulting in formation of a predefined form. The helices are formed by a scaffold chain (black) and a number of staple chains (colored). The lattice geometry is the consequence of crossings between the helix and its neighbors occurring seven base pairs apart. (B) The geometry of DNA crossovers in DNA helices for the creation of 2D origami designs. Every staple oligonucleotide binds to various regions of the scaffold DNA, producing double-helical tracts and connecting them. Individual interhelically connected tracts form a dense lattice in space. The connections, or crossovers, resemble an antiparallel Holliday four-way junction. Individual helical domains are connected by interhelix crossovers (in frame). Helices are linked to one another at regular intervals by junctions where scaffold or staples cross from one helix to the next. (C) 2D and 3D origami. DNA origami may be used to generate higher-order structures as a unit tile. A linear arrangement of DNA helices may be used to create 2D DNA origami. Cylinders are double-stranded DNA strands generated by base pairing of the DNA strands. 3D origami can be made by stacking multiple DNA helices on various lattices.
DNA origami as combinations of single-layered complanate structures can be synthesized in different sizes (approximately 100 nm) and shapes (triangular, simple rectangular, five-point stars, complicated smiling faces)
[8].
There are now a number of approaches for altering DNA origami structures. All of these techniques are based on the concept of backbone functionalization to obtain reactive end groups (e.g., biotinylation) of backbones and the addition of amino groups to backbones or chains associated with backbone extensions. These chains can be conjugated with other chains that carry drug molecules and nanoparticles
[25][30].
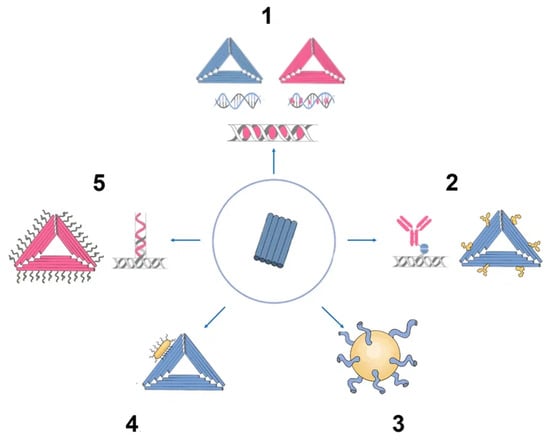
There has been much interest in using DNA origami structures as drug delivery systems. First, DNA is a naturally occurring biomaterial that is both biodegradable and almost noncytotoxic. Second, various interactions (intercalation, base pairing, covalent binding) can easily load a variety of therapeutic compounds and materials onto carriers, including DOX, immunostimulatory nucleic acids, small interfering RNAs, antibodies, and enzymes (
Figure 3). In addition, they can function as containers: DNA origami structures can house docking sites inside of them or in separate cavities that keep the payloads safe from the outside environment
[26][31].
Figure 3. Origami as nanocarrier in cancer therapy. (1) Chemotherapy drugs. (2) DNA origami epitopes for IgGs. (3) DNA origami gold nanoparticle. (4) DNA origami gold nanorod nanocomplex. (5) Aptamer (e.g., MUC-1) + chemotherapy drug (e.g., doxorubicin).
Recently, DNA origami has been used to develop useful cancer therapeutic applications, including sensory nanoplatforms and drug carriers
[27][32]. When combined with anticancer medications, DNA origami-based molecular recognition parts can provide precise location data on tumor cells and treat cancer simultaneously
[28][33].
Currently, research is being conducted to optimize the size and structure of DNA origami for passive targeting to tumor cells. Active targeting has been accomplished primarily by the incorporation of aptamers, the attachment of cell surface receptor ligands, and the use of cell-penetrating peptides. By hybridization, aptamer sequences can be easily integrated into origami backbones or conjugated on the origami surface
[29][34].
Since extracellular and intracellular environments are chemically diverse, smart carriers must be capable of detecting environmental stimuli at different stages of delivery and switching their structures and properties to readjust. Douglas et al., for example, created a logic-gated nanorobot with a DNA origami container sealed by two aptamer motifs. This container opens only when both aptamers bind to the appropriate cell surface receptors, allowing conditional presentation of the drug molecules to certain cell types.
Jiang et al. discovered that when a 2D DNA origami triangle was compared to a 3D DNA origami tube structure, both structures were equally effective in delivering DOX inside cells of a breast cancer cell line. When compared to controls, both variants showed significantly higher cytotoxicity
[30][35]. While drug DNA intercalation is a simple loading method, it does not provide any quantitative or qualitative control over the amount of loaded drug. The kinetics of drug release and the rate of carrier degradation in vivo are not strongly correlated, although the release of DOX from DNA origami has been examined in vitro
[29][34]. Several articles have shown the therapeutic activity of different DOX-loaded DNA origami nanostructures employing in vitro and in vivo models. Using various DNA origami nanostructures, et al. demonstrated different efficiencies in DOX delivery to human breast cancer cells
[31][36].
Jiang et al. noncovalently linked daunorubicin molecules to rod-like origami DNA nanostructures to overcome drug resistance in a leukemia cell line
[30][35]. This rod-like origami DNA may be controllably loaded with daunorubicin, and this sort of origami has been demonstrated to dramatically boost medication effectiveness due to quick self-assembly and strong stability in cell culture, demonstrating a robust DNA nanostructure design. The scientists also demonstrated that at therapeutically relevant medication doses, origami DNA nanostructures might overcome drug resistance in leukemic cells
[32][37].
BMEPC, a photosensitizer carbazole derivative created by Zhuang et al., was loaded into DNA origami. During irradiation, the DNA origami efficiently protected BMEPC molecules from photobleaching, and BMEPC fluoresced for a longer period of time; therefore, free radicals triggered cellular death in MCF-7 cells
[33][38].
For drug delivery and medicinal applications, origami DNA nanostructures coupled to metal nanoparticles are becoming more widespread. A novel approach to cancer theranostics uses gold nanorods and DNA origami constructs. Compared to gold nanorods alone, DNA origami and gold nanorods together demonstrated improved cell uptake and greater antitumor effectiveness. This complex has the ability to image cells and photothermal ablation of malignant cells. In another example, the complex of nanoparticles with DNA origami provided a high ability to be loaded with ligands for binding to many molecules or drugs
[34][39].
In addition, DNA origami has been created that can selectively target nucleolin in tumor blood vessels. This allowed the encapsulated thrombin to be exposed locally and promoted intravascular thrombosis, inhibiting tumor growth in mice and resulting in tumor necrosis
[26][31].
2.3. DNA Nanotube
DNA nanotubes are crystalline self-assemblies made of 10 nm-diameter DNA tiles that can grow to tens of micrometers in length. The rigidity of DNA nanotubes is hundreds of times greater than that of double-stranded DNA in general
[35][40]. DNA nanotubes are special 3D structures that have great promise for biomedical applications, such as filament supporting tracks and cargo transporting carriers
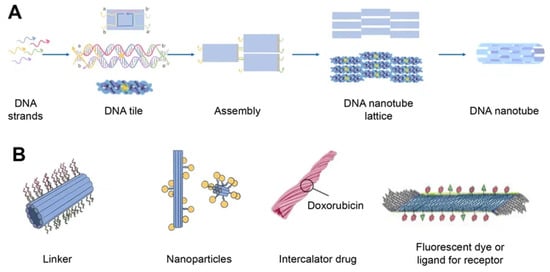
[36][41]. Regardless of how they are assembled, DNA nanotubes can have well-defined or indefinite lengths. Single-stranded DNA tiles, multi-crossover tiles, DNA origami, and multi-rung design methodologies for DNA nanotubes are the key assembly techniques. Tile-based motifs have been employed as DNA nanostructure building blocks (
Figure 4A) since the 1990s. Recently, single-stranded tile-based 2D and 3D DNA bricks were also developed. Self-assembly of DNA nanotubes is often achieved by heating and cooling mixtures of DNA strands with a given sequence, even though the complexity of DNA nanotubes varies. Importantly, the invention of 3D DNA nanostructure assembly helped the development and use of DNA nanotubes
[37][42]. Typically, DNA nanotubes are created by vertically aligning DNA duplexes into a curved motif, then closing it, or by rolling and cyclizing a two-dimensional DNA origami array
[38][43].
Figure 4. Schematics of DNA nanotube folding, modifications, and drug loading. (A) DNA nanotube self-assembly (schematically) using the single-stranded tile method. Five DNA strands form DNA double-crossover tiles. A DNA tile is formed with four short single-stranded sections called sticky ends (marked a, b, a′, and b′) that act as binding domains. Individual helical domains are connected by interhelix crossovers. Each domain is complementary to one domain of neighboring tiles and several domains hybridized with each other to form nanotubes (the single-stranded tile method of assembly of DNA nanotube). This interaction is provided due to complementary interactions of their sticky ends. Tiles can be schematically represented as molecular bricks with complementary connectors. The sticky end arrangement directs the hybridization of DNA tiles to form tubular DNA structures with a range of diameters. Their distribution is determined by the thermodynamics and kinetics of the DNA nanotube assembly process. (B) Modifications of DNA nanotubes: linker (CpG, cholesterol, aptamers, etc.); nanoparticles (Au); chemotherapy or photodynamic therapy drug; other ligands.
The transmembrane channels, bioreactors, and pharmaceuticals can be released over time in a precise nanoscale cavity that is provided by DNA nanotube channels. The exterior surface has a rigid scaffold and several organized connection locations that can be utilized as carriers and templates for cargo delivery. Biomimetic DNA nanotubes have shown considerable potential in bioimaging and therapies because of their strong biological compatibility and addressability
[37][42].
DNA nanotubes can be modified with a range of ligands for various biomedical applications (
Figure 4B). Among them, there may be DNA ligands, among which CpG and aptamers can be distinguished. Aptamers are usually used in this case for binding to a cellular target or as DNAzymes. In addition, intercalating drugs can be loaded into DNA nanotubes or various fluorescent dyes can be conjugated. DNA nanotubes can also be connected to liposomes or nanoparticles using single strand linkers
[39][44].
DNA nanotubes are mostly able to absorb high concentrations of anticancer drugs. Unfortunately, the chemical mechanism of interaction between DNA nanotubes and medicines remains unclear. The impact of the structure of DNA nanotubes on drug dispersion and delivery, in particular, has not been investigated, limiting the utility of DNA nanotubes as drug carriers. Lijun Liang et al. used molecular dynamics simulations to investigate the potential of DNA nanotubes as drug delivery carriers. Due to electrostatic and van der Waals forces, certain hydrophobic anticancer medicines (doxorubicin, daunorubicin, Taxol, and vinblastine) might be stably absorbed at the ends of DNA nanotubes. Moreover, DNA nanotubes inhibited the aggregation of anticancer drugs in aqueous solutions. DNA nanotubes remain more stable after absorbing anticancer drugs
[40][45].
DNA nanotubes were changed with simple ligands such as folic acid in the initial investigations. These DNA nanotubes have excellent cell membrane adherence and can quickly penetrate cancer cells. An increase in the quantity of folic acid fragments led to a 10% increase in DNA nanotube internalization. Several subsequent studies have implemented this change with variable degrees of success
[41][46].
Another well-known modification of DNA nanotubes is the use of Cy3 fluorescent dye, which was delivered to KB epidermal nasopharyngeal carcinoma cells. Cy3 is known to have red fluorescence, which makes it easy to visualize. The combination of folic acid as a ligand for cancer cell receptors and Cy3 as a fluorescent imaging agent has been used to produce multifunctional nanotubes. The resulting DNA nanotubes could be efficiently absorbed by cancer cells without exhibiting cytotoxicity
[42][47].
In addition, cholesterol-modified DNA nanotubes conjugated to cytochrome C have been used by Kokabey et al. for cancer cell apoptosis. The quantity of conjugated cholesterol molecules affects the efficiency with which DNA nanotubes bind to the plasma membrane. The death of cancer cells was linked to an increase in cell membrane permeability and only partially reliant on caspase activity, which in this circumstance suggests that cancer cells underwent both apoptosis and necrosis
[43][48].
Li et al. created telomerase-responsive and nucleolin-targeted DNA nanotubes for drug delivery. Following the Förster resonance energy transfer (FRET) signal shift and RTA-induced cell death, the aptamer-functionalized DNA nanotubes loaded with RTA (ricin A chain) demonstrated improved tumor access and precise drug release in response to tumor cell telomerase. The DNA nanotubes were also effectively used in vivo, when after systemic injection, tumor growth in mice harboring xenografts was clearly inhibited
[44][49].
2.4. Aptamers
Aptamers are typically peptides or single-stranded DNA or RNA that are short in sequence and can bind to cellular targets with high selectivity. Secondary or tertiary structures of aptamers facilitate target binding and determine specificity and affinity
[45][50]. Aptamers can be employed for targeting nanocarriers toward tissue overexpressing the target antigens
[46][51]. Similar to DNA, aptamers can combine to generate complementary base pairs, which can then be used to build a variety of complex structures. They can be secondary structures such as the kissing hairpin, stem, loop, bugle, and pseudoknot. These secondary structures can then be combined to create certain three-dimensional structures, which the cell’s target molecule can subsequently recognize. The major forces that result in the creation of a three-dimensional structure and the attachment of an aptamer to a target include hydrogen bonds, van der Waals forces, and hydrophobic and electrostatic interactions. Similar to how antibodies bind to antigens, the creation of aptamer-target complexes is driven by a unique 3D interaction
[47][52].
Systematic evolution of ligands by exponential enrichment (SELEX) selects nucleic acid aptamers from a library of random sequences, which then bind to the selected compounds with great specificity and affinity. Exponentially enriching a population of random sequence nucleic acid libraries enables the SELEX technique to evolve and select molecules with the highest affinities. SELEX is used for nucleic acids because of the possibility of conveniently amplifying affinity-selected molecules by RT–PCR or PCR. Aptamers can circumvent some of the disadvantages associated with the use of antibodies. For instance, aptamers are produced in vitro and can be chosen to target almost any protein, including toxins or nonimmunogenic proteins, in a relatively short amount of time, whereas the use of living animals is a drawback for antibody production
[48][53].
Aptamers have received much attention recently in the field of biomedicine. Aptamers have characteristics similar to those of antibodies as well as particular benefits such as thermal stability, simplicity in synthesis, reversibility of target binding, and minimal immunogenicity.
Chemical techniques make it simple to change aptamers by adding functional groups and/or lengthening them (
Figure 5)
[45][50]. Changes can confer nuclease resistance and prolong the circulation half-life. For instance, aptamers’ circulation half-life can be extended to many hours by adding carrier molecules to their ends, such as polyethylene glycol or cholesterol
[49][54]. Moreover, aptamers might be conjugated to therapeutic compounds such as medications, carriers for drugs, poisons, or photosensitizers
[48][53].
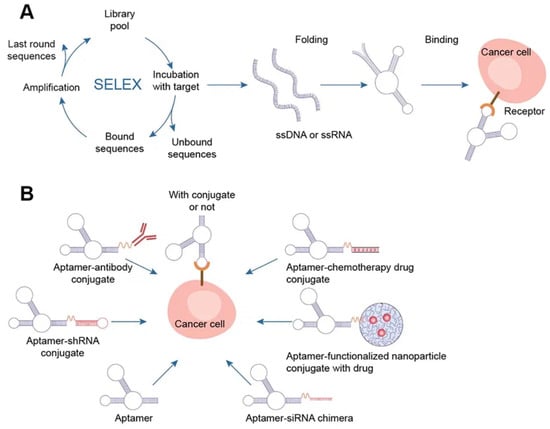
Figure 5. Aptamers. (A) Schematics of SELEX, aptamer folding, and binding to a target protein. A target-aptamer complex is created when the aptamer folds into a certain three-dimensional structure and interacts with a target molecule (such as a protein). (B) Aptamers for drug delivery in cancer.
There are several methods for the direct conjugation of aptamers to various secondary DNA structures, such as chemotherapy drugs or therapeutic oligonucleotides (siRNAs, miRNAs, and anti-miRNAs), that are easy to deliver and affordable. These methods take advantage of the chemical characteristics of aptamers. Drug compounds can simply be conjugated to aptamers via covalent or noncovalent bonding for targeted therapy. Most researchers have discussed the coupling of chemotherapeutic DNA structures to aptamers in their articles, and DOX is one of the most widely used drugs in this context
[50][55]. Different DNA nanocarriers have been combined with therapeutic molecules that have unique properties. The most appealing nanostructures contain aptamers with chemotherapy drugs (DOX, PTX, 5-FU, etc.). They effectively damaged tumors, overcame multidrug resistance, and promoted photodynamic abilities. The idea of multifunctional complexes was brought up to improve their targeting capabilities. Moreover, complexes with aptamers play a significant role in immunostimulation, biosensing, and bioimaging when combined with fluorescent dyes (such as FAM) and bioactive DNA molecules (such as CpG)
[8].
Some clinical investigations have been carried out to investigate the possibility of using aptamers in medicine. Pegaptanib, an RNA aptamer that targets VEGF in age-related macular degeneration, is the only aptamer that has been commercialized thus far. Nonetheless, more aptamers, such as Zimura, Fovista, NOX-H94, and BT200, are still being clinically tested
[50][55].
Approximately 50 national clinical trials of aptamers are currently underway. Clinical trials using aptamers for the detection of cancer are also ongoing. In a clinical trial that was launched in 2015 (NCT02957370), novel bladder cancer biomarkers were found in urine samples. Ptamers may hold promise for in vivo tumor imaging or clinical diagnosis. Protein tyrosine kinase-7 (PTK7), which is expressed in a variety of human malignancies, has a particular ligand that has been discovered as a single-stranded DNA aptamer (Sgc8). The 68Ga-tagged aptamer was studied as a novel radiotracer for PTK7 positron emission tomography to distinguish between benign and malignant colorectal cancer in the most recent trial of this aptamer (NCT03385148).
Aptamers have been utilized to target cancer stem cells, produce laboratory tumor models, and create a novel breast cancer diagnostic system in another diagnostic clinical study (NCT01830244). A few fundamental studies are currently undergoing clinical trials, and several aptamers are offered commercially
[51][56].
3. Delivery of DNA-Based Nanomaterials
3.1. Biodistribution and Biosafety of DNA-Based Nanomaterials
As of right now, the majority of oligonucleotide treatments (and nearly all licensed nucleic acid preparations) concentrate on either local delivery (for example, to the eyes or spinal cord) or hepatic delivery. As the liver is a strongly perfused organ, absorption of bigger nanoparticles and free oligonucleotides can happen quickly before renal clearance. Moreover, the liver has very high concentrations of receptors that might facilitate fast absorption and/or recycling. The development of effective methods for extrahepatic systemic distribution remains a key objective in the field of oligonucleotide treatment, despite the fact that other organs, such as the kidneys and spleen, are also locations of oligonucleotide accumulation
[52][53][228,229].
Oligonucleotides are hydrophilic, negatively charged polymers that do not pass the plasma membrane by themselves
[54][230]. Cellular DNAses are thought to break oligonucleotides within cells. On the other hand, the degradation process is linked to the uptake pathway of DNA nanostructures
[55][13]. In addition, there are off-target interactions, toxicity depending on the sequence and chemical composition of oligonucleotides, and saturation of endogenous RNA processing pathways
[56][231].
Data on the biodistribution of unmodified therapeutic DNA nanostructures, including aptamers, are limited. All unmodified oligonucleotides (including aptamers) have serious pharmacokinetic problems, including metabolic instability and rapid renal filtration without nonspecific protein binding
[57][232]. For example, the half-lives of unmodified nucleotide aptamers in the blood are on the order of 2 min. Thus, short aptamer stability is a serious issue.
Nucleic acid preparations must resist extracellular degradation
[58][233], prevent the release of the drug bound to specific plasma proteins from circulation
[59][234], and avoid removal by the reticuloendothelial system. Nucleic acid drug platforms must cross the capillary endothelium to the desired target, cross the plasma membrane, avoid lysosomal degradation
[60][235], and be delivered to a specific target in the cell
[54][230]. Therefore, the most commonly used strategies to improve drug delivery and safety from nucleic acids include chemical modification, covalent conjugation with cell-targeting or cell-penetrating molecules, and the use of nanoparticles to lessen adverse effects and boost the treatment efficiency of drug delivery technologies
[54][230]. Most of the aptamers are within 50 nucleotides in length and fall within the renal filtration range. In the case of polymer conjugation, aptamers largely reduce renal filtration
[61][236]. Additionally, chemical modifications are proposed to be incorporated into nucleotide sugars or internucleotide phosphodiester linkages to overcome this problem for aptamers and increase their serum half-life. In addition, mirror L-DNA can solve the problem of low stability of natural DNA in blood serum, which affects the pharmacokinetics and biological distribution
[62][237].
To date, many DNA-based carriers have been shown to have little cytotoxicity and are mainly used as a hub for integrating drug loading, targeting, and release modules together
[63][238]. There is currently very limited information on the safety and efficacy of treatments using DNA nanostructures. Some experiments have been carried out on cell culture, as well as several animal studies
[64][239].
One of the biggest challenges for TDNs in further clinical use is whether TDNs can cause unpredictable gene recombination or deleterious effects in the liver or kidneys. Nevertheless, it was shown that TDN has better biological safety, lower biotoxicity, and higher transport efficiency than other DNA carriers in cell culture
[65][240]. For example, the biosafety of TDN for DOX delivery has been shown
[10][15].
Zhang et al. demonstrated that triangular origami DNA loaded with doxorubicin could be an effective and safe innovation platform for the treatment of breast cancer in nude mice
[66][241]. Recently, the safety of DNA origami at physiological pH for noncancerous cells and its cytotoxicity for cancer cells at the pH of solid tumors have been demonstrated
[67][242].
In general, aptamers have also been shown to have little or no side effects and are safe. Aptamers did not lead to the activation of the immune system
[68][243]. Many analyses in preclinical and early clinical trials have not shown complement activation. Additionally, no off-target side effects were identified
[69][244]. In 2022, the first phase I human clinical trial of the ApTOLL DNA aptamer was conducted and the results in healthy male volunteers demonstrated great safety and a suitable pharmacokinetic profile of aptamer. The infusion’s termination resulted in the highest concentration, and the mean half-life was 9.3 h. At any dose or with any researched form of administration, serious adverse effects or biochemical abnormalities were not seen. However, the study showed no accumulation of ApTOLL
[50][55].
However, the shelf life of DNA nanostructures can raise safety concerns. For instance, because DNA sequences in nanostructures are complicated and unpredictable, foreign DNA may at random interact with cellular RNA and result in other possible chronic toxicities. Moreover, the immunological response to DNA nanostructures in vivo has not been well studied. Immunostimulatory activity through a TLR9-independent mechanism should not be disregarded, despite the fact that numerous research claim that CpG-free DNA nanostructures have little immunogenicity
[70][245].
3.2. Cellular Uptake of DNA Nanostructures
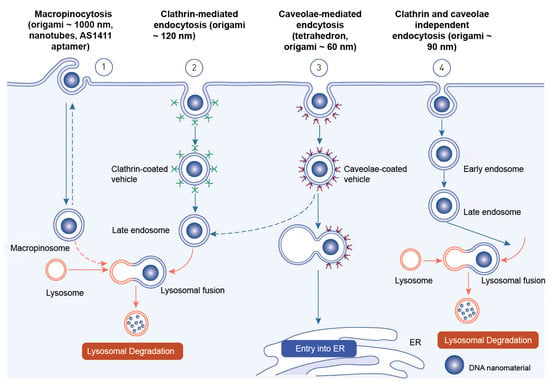
Endocytosis is the process through which macromolecules, their complexes, and large particles enter the cell. Endocytosis is classified into two types: phagocytosis, which consumes particles larger than 250 nm, and pinocytosis, which consumes fluid and particles that phagocytosis does not consume. Pinocytosis occurs in all cell types via four different mechanisms: macropinocytosis, clathrin-mediated endocytosis, caveolar-mediated endocytosis, and clathrin- and caveolin-independent endocytosis
[71][246]. Other pathways, with the exception of macropinocytosis, are directly controlled by cargo molecule activity. Cargo molecules bind to receptors and form receptor–ligand complexes attracting certain effectors to specific parts of the plasma membrane
[72][247].
Depending on their size and the proteins involved, oligonucleotides enter cells through a variety of endocytosis processes (
Figure 6). Despite the fact that all endocytosis routes lead to the creation of endosomes, molecules entering via distinct channels may end up at different downstream locations. The majority of absorbed oligonucleotides end up in late endosomes and lysosomes. There is, nevertheless, some partial translocation to other membrane-bound compartments. Oligonucleotides inside the endomembrane compartment are pharmacologically inactive, and only a tiny percentage of internalized oligonucleotides may reach the cytosol and nucleus on their own. The method of injection may influence oligonucleotide cellular uptake
[73][248].
Figure 6.
Endocytotic pathways involved in the internalization of DNA-based nanomaterials.
Receptor-mediated endocytosis and macropinocytosis are the two primary methods of internalization of DNA-based nanomaterials. The activity of cargo molecules and other proteins controls clathrin-mediated and caveola-mediated endocytosis
[74][249]. Free oligonucleotides, oligonucleotide conjugates, or oligonucleotide-carrying nanocarriers are internalized by endocytosis once they reach the cell surface
[73][248].
The mechanism of endocytosis of DNA-based nanomaterials is not fully understood. The endocytosis process has only been examined for a relatively small variety of DNA nanostructures. The structure, chemical makeup, and cell type of the target cells must all be taken into account in studies of endocytosis and cell fate of each unique design. DNA nanostructures that have been absorbed typically end up being trapped and degraded in lysosomes. As a result, methods that enhance endolysosomal escape and encourage targeted drug delivery to the cytosol and nucleus are needed. These issues will also be resolved by researching a nonendocytic route for DNA nanostructures
[63][238].
Nanocarriers enter cells mostly through endocytosis and are sent to different organelles inside the cell
[75][250]. The loaded drug is typically released from the nanocarriers either extracellularly, in the microenvironment, or intracellularly into the tumor, primarily through cellular uptake via endocytosis
[76][251].