Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Jorge Sanz Ros.
Similar to other subtypes of EVs (extracellular vesicles), MitoEVs (mitochondrial extracellular vesicles) are altered in several diseases, including cancer, neurodegenerative disorders, and cardiovascular disease. MitoEVs contain a variety of molecular components from releasing cells, including proteins, lipids, and nucleic acids, which may serve as indicators of disease status.
- MitoEVs
- mitochondria
- extracellular vesicles
1. Cancer
The search for new methods to diagnose cancer in its early stages and distinguish between states of the disease has led to the development of liquid biopsies. The analysis of body fluids such as blood or urine to gather information about a person’s cancer status has emerged as a powerful tool for cancer diagnosis, prognosis, and treatment monitoring, as it allows for the detection of cancer-related genetic alterations in a minimally invasive manner [81,82][1][2].
Cancer cells often release various types of molecules into the bloodstream, including DNA, RNA, and proteins [83][3]. These molecules can be used to detect cancer cells and track their progression over time. The results of a liquid biopsy can provide important information about the type and stage of cancer, as well as help monitor the effectiveness of the treatment and detect the early signs of cancer recurrence [82][2].
The analysis of EVs (extracellular vesicles) in liquid biopsies has emerged as a novel method to provide new insights into the role of EVs in several diseases, as the content in EVs varies across disease status [84][4]. Currently, the main application of the analysis of EVs in liquid biopsies is in the detection and characterization of cancer-specific biomarkers [84,85][4][5]. This approach offers several advantages over traditional diagnostic methods, such as tissue biopsy or imaging. Firstly, EVs are readily available in the bloodstream, making liquid biopsy with EVs a minimally invasive and convenient option for cancer diagnosis and monitoring. Secondly, EVs contain a wealth of information about cancer cells, including their genetic and epigenetic alterations, which can provide valuable insights into cancer’s biology, progression, and treatment response [86][6].
The mitochondria, the cellular organelles responsible for energy production, have emerged as crucial players in the development and progression of cancer. Growing evidence links mitochondrial dysfunction to various aspects of cancer biology, including metabolism, apoptosis, and signaling pathways [87][7]. In this context, it has been shown that cancer cells release EVs that contain specific mitochondria-derived molecules, such as proteins or mtDNA [18,88,89][8][9][10].
mtDNA present in EVs has an important role in cancer biology and progression, making it an interesting source in cancer diagnosis. mtDNA transfer between cancer cells acts as an oncogenic signal, promoting the escape of cells from metabolic quiescence [20][11]. Similarly, mtDNA contained in metastatic tumor cells is transferred to low-metastatic tumor cells via MitoEVs (mitochondrial extracellular vesicles), enhancing the metastatic potential during tumor progression [22][12]. In a more recent study, the authors showed that the protein PINK1 mediates the packaging of mtDNA in EVs from cancer cells and that this mtDNA can promote invasiveness through the activation of Toll-like receptor 9 in recipient cells [21][13].
Some studies have proposed that MitoEVs could serve as new biomarkers of cancer. Jang et al. discovered that EVs released by melanoma tissue contain higher levels of mitochondrial membrane proteins when compared with non-cancerous cells. In addition, they found that patients with melanoma or other types of cancer such as ovarian or breast cancer have a higher concentration in the plasma of these MitoEVs [25][14]. Regarding mtDNA, it was recently shown that patients with pancreatic ductal adenocarcinoma have a higher enrichment of mtDNA in circulating EVs, detecting specific mtDNA mutations that could serve as a tool for early cancer detection [90][15]. Moreover, mtDNA contained in MitoEVs obtained from the plasma exhibit different characteristics among patients with hepatocellular carcinoma, hepatitis, or healthy individuals, indicating a potential role as a diagnostic biomarker in these conditions [91][16].
2. Other Diseases
Although cancer is currently the most studied disease in terms of liquid biopsies and MitoEVs, recent studies have found that the content in MitoEVs can be altered in other diseases, such as neurological or cardiovascular conditions [69][17].
Multiple lines of evidence suggest that mitochondrial dysfunction plays a key role in the pathogenesis of Parkinson’s disease (PD). Post-mortem studies have shown that there is a reduction in the number and size of mitochondria in the substantia nigra region of PD patients’ brains [92][18]. Additionally, there is evidence of decreased mitochondrial respiratory chain activity and increased ROS generation in PD patients [93][19]. Furthermore, mutations in genes that regulate mitochondrial function, such as PINK1 and Parkin, are associated with some forms of PD [94][20]. Recently, it was shown that these proteins are involved in mitochondrial quality control through the regulation of mitochondria-derived vesicle trafficking [64,95][21][22]. Along with these results, a clinical study with PD patients suggested that circulating EVs were altered in the disease; more specifically, they found that a specific mitochondrial signature was present in these patients [96][23].
Another neurological condition characterized by mitochondrial dysfunction is Down syndrome (DS). Patients have impairments in mitochondrial function, which leads to a decrease in energy production that may contribute to the cognitive impairments seen in individuals with Down syndrome [97][24]. Additionally, studies have shown that people with Down syndrome have an increased susceptibility to oxidative stress [98][25]. A recent study that presents a new approach to isolate and separate EV subpopulations from the brain extracellular matrix, identifies a unique subset of EVs of a mitochondrial origin, which they term mitovesicles. The authors found that the number and composition of brain mitovesicles are altered in individuals with DS, indicating their possible role in the neuropathological process [27][26].
In cardiovascular disease, mitochondrial dysfunction has been linked to the development of key pathological changes such as heart failure or atherosclerosis [99,100][27][28]. MitoEVs regulate mitochondrial quality control in the cardiovascular system [101,102][29][30] and serve as pro-inflammatory signaling between monocytes and endothelial cells in cardiovascular disease [103][31]. This particular subtype of vesicles has a crucial role in the maintenance of mitochondrial homeostasis in the heart, as cardiomyocytes release dysfunctional mitochondria taken up by resident macrophages [104][32].
Thereby, MitoEVs can be detected in biological fluids and seem to play a role in the regulation of mitochondrial biology and intercellular communication, making them an interesting subtype of EVs that could be used as diagnostic markers for several diseases (Figure 1 2 and Table 1).
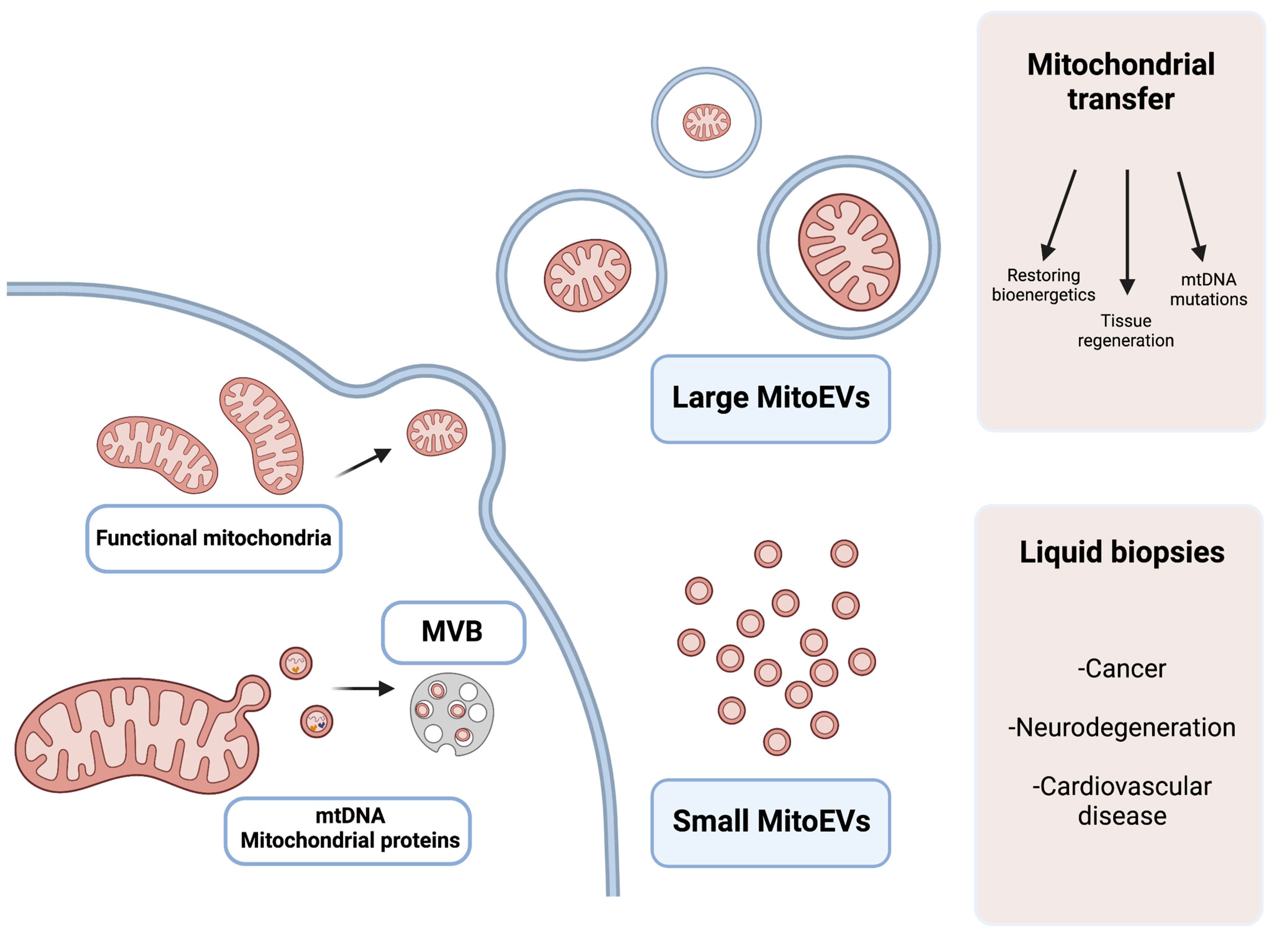
Figure 21. Graphical explanation of the potential therapeutic and diagnostic use of large and small MitoEVs. Larger MitoEVs refer to EVs that are shed from the plasmatic membrane of the cell and can include whole mitochondria; these EVs are particularly interesting in the field of mitochondrial transfer as therapeutic vehicles. Small MitoEVs are included in MVB (multivesicular bodies) previous to their release and contain material from the mitochondrial origin (mtDNA and proteins); its analysis may serve as a diagnostic tool in liquid biopsies.
Table 1. Summary of applications of MitoEVs in human diseases, regarding the sources of the vesicles and the key findings in different studies.
| Application | Disease | Source | Findings | References |
|---|---|---|---|---|
| Diagnosis | Melanoma, ovarian and breast cancer | Plasma | Mitochondrial protein enriched EVs from cancerous cells are present at higher concentrations in patients’ plasma | [25][14] |
| Pancreatic ductal adenocarcinoma | Plasma | EVs from mitochondria carrying specific mtDNA mutations from cancer cells are present can be detected in patients’ plasma | [90][15] | |
| Hepatocellular carcinoma and hepatitis | Plasma | mtDNA profile in plasma MitoEVs differs between patients with hepatocellular carcinoma, hepatitis, and healthy individuals | [91][16] | |
| Parkinson’s disease | Plasma | Circulating EVs from PD patients have a specific mitochondrial signature | [96][23] | |
| Therapy | Myocardial ischemia-reperfusion injury | Healthy cells | Mitochondrial transfer improved tissue regenerative capacity, enhanced ATP production, improved cell viability, and reduced pro-inflammatory markers | [105][33] |
| Brain ischemia | Xenogenic and muscle mitochondria, MSCs | Mitochondrial transfer improved neurogenesis, and reduced pro-inflammatory markers, oxidative stress and apoptosis | [106,107,108][34][35][36] | |
| Limb ischemia | Healthy cells | Mitochondrial transplantation improved tissue regenerative capacity, enhanced ATP production, improved cell viability, and reduced pro-inflammatory markers | [109][37] | |
| Lung ischemia-reperfusion injury | Healthy cells | Mitochondrial transplantation improved tissue regenerative capacity | [110][38] | |
| Acute kidney injury | Healthy cells | Intra-arterial mitochondrial transplantation improved tissue regenerative capacity | [111][39] | |
| Doxorubicin injury to cardiomyocytes | MSCs | Mitochondria-rich EVs improve cell viability in induced cardiomyocytes from patients with doxorubicin injury | [112][40] | |
| Alzheimer’s disease | Healthy cells | Mitochondrial transfer improves cognition and lower neuronal loss and gliosis in mice | [113][41] | |
| Parkinson’s disease | Allogenic and xenogenic mitochondria | Mitochondrial transplantation restored mitochondrial function and attenuated 6-hydroxydopamine-induced neurotoxicity in mice | [114][42] | |
| Mitochondrial diseases | Healthy cells | Mitochondrial transfer can improve mitochondrial bioenergetics in cells from patients with mutations in mtDNA | [46,115,116,117][43][44][45][46] |
References
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid biopsy: A step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 2022, 21, 79.
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid biopsy enters the clinic—Implementation issues and future challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312.
- Corcoran, R.B.; Chabner, B.A. Application of Cell-free DNA Analysis to Cancer Treatment. N. Engl. J. Med. 2018, 379, 1754–1765.
- Zhou, B.; Xu, K.; Zheng, X.; Chen, T.; Wang, J.; Song, Y.; Shao, Y.; Zheng, S. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct. Target. Ther. 2020, 5, 144.
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359.
- Yu, D.; Li, Y.; Wang, M.; Gu, J.; Xu, W.; Cai, H.; Fang, X.; Zhang, X. Exosomes as a new frontier of cancer liquid biopsy. Mol. Cancer 2022, 21, 56.
- Zong, W.X.; Rabinowitz, J.D.; White, E. Mitochondria and Cancer. Mol. Cell 2016, 61, 667–676.
- Guescini, M.; Genedani, S.; Stocchi, V.; Agnati, L.F. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. J. Neural Transm. 2010, 117, 1–4.
- Mears, R.; Craven, R.A.; Hanrahan, S.; Totty, N.; Upton, C.; Young, S.L.; Patel, P.; Selby, P.J.; Banks, R.E. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics 2004, 4, 4019–4031.
- Staubach, S.; Razawi, H.; Hanisch, F.G. Proteomics of MUC1-containing lipid rafts from plasma membranes and exosomes of human breast carcinoma cells MCF-7. Proteomics 2009, 9, 2820–2835.
- Sansone, P.; Savini, C.; Kurelac, I.; Chang, Q.; Amato, L.B.; Strillacci, A.; Stepanova, A.; Iommarini, L.; Mastroleo, C.; Daly, L.; et al. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9066–E9075.
- Takenaga, K.; Koshikawa, N.; Nagase, H. Intercellular transfer of mitochondrial DNA carrying metastasis-enhancing pathogenic mutations from high- to low-metastatic tumor cells and stromal cells via extracellular vesicles. BMC Mol. Cell Biol. 2021, 22, 52.
- Rabas, N.; Palmer, S.; Mitchell, L.; Ismail, S.; Gohlke, A.; Riley, J.S.; Tait, S.W.G.; Gammage, P.; Soares, L.L.; Macpherson, I.R.; et al. PINK1 drives production of mtDNA-containing extracellular vesicles to promote invasiveness. J. Cell Biol. 2021, 220, e202006049.
- Jang, S.C.; Crescitelli, R.; Cvjetkovic, A.; Belgrano, V.; Olofsson Bagge, R.; Sundfeldt, K.; Ochiya, T.; Kalluri, R.; Lotvall, J. Mitochondrial protein enriched extracellular vesicles discovered in human melanoma tissues can be detected in patient plasma. J. Extracell. Vesicles 2019, 8, 1635420.
- Vikramdeo, K.S.; Anand, S.; Khan, M.A.; Khushman, M.; Heslin, M.J.; Singh, S.; Singh, A.P.; Dasgupta, S. Detection of mitochondrial DNA mutations in circulating mitochondria-originated extracellular vesicles for potential diagnostic applications in pancreatic adenocarcinoma. Sci. Rep. 2022, 12, 18455.
- Li, Y.; Guo, X.; Guo, S.; Wang, Y.; Chen, L.; Liu, Y.; Jia, M.; An, J.; Tao, K.; Xing, J. Next generation sequencing-based analysis of mitochondrial DNA characteristics in plasma extracellular vesicles of patients with hepatocellular carcinoma. Oncol. Lett. 2020, 20, 2820–2828.
- Popov, L.D. Mitochondrial-derived vesicles: Recent insights. J. Cell. Mol. Med. 2022, 26, 3323–3328.
- Perier, C.; Vila, M. Mitochondrial biology and Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009332.
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013, 3, 461–491.
- Ge, P.; Dawson, V.L.; Dawson, T.M. PINK1 and Parkin mitochondrial quality control: A source of regional vulnerability in Parkinson’s disease. Mol. Neurodegener. 2020, 15, 20.
- McLelland, G.L.; Soubannier, V.; Chen, C.X.; McBride, H.M.; Fon, E.A. Parkin and PINK1 function in a vesicular trafficking pathway regulating mitochondrial quality control. EMBO J. 2014, 33, 282–295.
- Ryan, T.A.; Phillips, E.O.; Collier, C.L.; Robinson, A.J.; Routledge, D.; Wood, R.E.; Assar, E.A.; Tumbarello, D.A. Tollip coordinates Parkin-dependent trafficking of mitochondrial-derived vesicles. EMBO J. 2020, 39, e102539.
- Picca, A.; Guerra, F.; Calvani, R.; Marini, F.; Biancolillo, A.; Landi, G.; Beli, R.; Landi, F.; Bernabei, R.; Bentivoglio, A.R.; et al. Mitochondrial Signatures in Circulating Extracellular Vesicles of Older Adults with Parkinson’s Disease: Results from the EXosomes in PArkiNson’s Disease (EXPAND) Study. J. Clin. Med. 2020, 9, 504.
- Izzo, A.; Mollo, N.; Nitti, M.; Paladino, S.; Calì, G.; Genesio, R.; Bonfiglio, F.; Cicatiello, R.; Barbato, M.; Sarnataro, V.; et al. Mitochondrial dysfunction in down syndrome: Molecular mechanisms and therapeutic targets. Mol. Med. 2018, 24, 2.
- Coskun, P.E.; Busciglio, J. Oxidative Stress and Mitochondrial Dysfunction in Down’s Syndrome: Relevance to Aging and Dementia. Curr. Gerontol. Geriatr. Res. 2012, 2012, 383170.
- D’Acunzo, P.; Perez-Gonzalez, R.; Kim, Y.; Hargash, T.; Miller, C.; Alldred, M.J.; Erdjument-Bromage, H.; Penikalapati, S.C.; Pawlik, M.; Saito, M.; et al. Mitovesicles are a novel population of extracellular vesicles of mitochondrial origin altered in Down syndrome. Sci. Adv. 2021, 7, eabe5085.
- Chistiakov, D.A.; Shkurat, T.P.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. The role of mitochondrial dysfunction in cardiovascular disease: A brief review. Ann. Med. 2018, 50, 121–127.
- Ballinger, S.W. Mitochondrial dysfunction in cardiovascular disease. Free Radic. Biol. Med. 2005, 38, 1278–1295.
- Heyn, J.; Heuschkel, M.A.; Goettsch, C. Mitochondrial-Derived Vesicles-Link to Extracellular Vesicles and Implications in Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 2637.
- Cadete, V.J.; Deschênes, S.; Cuillerier, A.; Brisebois, F.; Sugiura, A.; Vincent, A.; Turnbull, D.; Picard, M.; McBride, H.M.; Burelle, Y. Formation of mitochondrial-derived vesicles is an active and physiologically relevant mitochondrial quality control process in the cardiac system. J. Physiol. 2016, 594, 5343–5362.
- Puhm, F.; Afonyushkin, T.; Resch, U.; Obermayer, G.; Rohde, M.; Penz, T.; Schuster, M.; Wagner, G.; Rendeiro, A.F.; Melki, I.; et al. Mitochondria Are a Subset of Extracellular Vesicles Released by Activated Monocytes and Induce Type I IFN and TNF Responses in Endothelial Cells. Circ. Res. 2019, 125, 43–52.
- Nicolás-Ávila, J.A.; Lechuga-Vieco, A.V.; Esteban-Martínez, L.; Sánchez-Díaz, M.; Díaz-García, E.; Santiago, D.J.; Rubio-Ponce, A.; Li, J.L.; Balachander, A.; Quintana, J.A.; et al. A Network of Macrophages Supports Mitochondrial Homeostasis in the Heart. Cell 2020, 183, 94–109.e23.
- Shin, B.; Cowan, D.B.; Emani, S.M.; Del Nido, P.J.; McCully, J.D. Mitochondrial Transplantation in Myocardial Ischemia and Reperfusion Injury. Adv. Exp. Med. Biol. 2017, 982, 595–619.
- Huang, P.J.; Kuo, C.C.; Lee, H.C.; Shen, C.I.; Cheng, F.C.; Wu, S.F.; Chang, J.C.; Pan, H.C.; Lin, S.Z.; Liu, C.S.; et al. Transferring Xenogenic Mitochondria Provides Neural Protection Against Ischemic Stress in Ischemic Rat Brains. Cell Transplant. 2016, 25, 913–927.
- Zhang, Z.; Ma, Z.; Yan, C.; Pu, K.; Wu, M.; Bai, J.; Li, Y.; Wang, Q. Muscle-derived autologous mitochondrial transplantation: A novel strategy for treating cerebral ischemic injury. Behav. Brain Res. 2019, 356, 322–331.
- Pourmohammadi-Bejarpasi, Z.; Roushandeh, A.M.; Saberi, A.; Rostami, M.K.; Toosi, S.M.R.; Jahanian-Najafabadi, A.; Tomita, K.; Kuwahara, Y.; Sato, T.; Roudkenar, M.H. Mesenchymal stem cells-derived mitochondria transplantation mitigates I/R-induced injury, abolishes I/R-induced apoptosis, and restores motor function in acute ischemia stroke rat model. Brain Res. Bull. 2020, 165, 70–80.
- Orfany, A.; Arriola, C.G.; Doulamis, I.P.; Guariento, A.; Ramirez-Barbieri, G.; Moskowitzova, K.; Shin, B.; Blitzer, D.; Rogers, C.; Del Nido, P.J.; et al. Mitochondrial transplantation ameliorates acute limb ischemia. J. Vasc. Surg. 2020, 71, 1014–1026.
- Moskowitzova, K.; Orfany, A.; Liu, K.; Ramirez-Barbieri, G.; Thedsanamoorthy, J.K.; Yao, R.; Guariento, A.; Doulamis, I.P.; Blitzer, D.; Shin, B.; et al. Mitochondrial transplantation enhances murine lung viability and recovery after ischemia-reperfusion injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L78–L88.
- Doulamis, I.P.; Guariento, A.; Duignan, T.; Kido, T.; Orfany, A.; Saeed, M.Y.; Weixler, V.H.; Blitzer, D.; Shin, B.; Snay, E.R.; et al. Mitochondrial transplantation by intra-arterial injection for acute kidney injury. Am. J. Physiol. Renal. Physiol. 2020, 319, F403–F413.
- O’Brien, C.G.; Ozen, M.O.; Ikeda, G.; Vaskova, E.; Jung, J.H.; Bayardo, N.; Santoso, M.R.; Shi, L.; Wahlquist, C.; Jiang, Z.; et al. Mitochondria-Rich Extracellular Vesicles Rescue Patient-Specific Cardiomyocytes From Doxorubicin Injury: Insights Into the SENECA Trial. JACC CardioOncol 2021, 3, 428–440.
- Nitzan, K.; Benhamron, S.; Valitsky, M.; Kesner, E.E.; Lichtenstein, M.; Ben-Zvi, A.; Ella, E.; Segalstein, Y.; Saada, A.; Lorberboum-Galski, H.; et al. Mitochondrial Transfer Ameliorates Cognitive Deficits, Neuronal Loss, and Gliosis in Alzheimer’s Disease Mice. J. Alzheimers Dis. 2019, 72, 587–604.
- Chang, J.C.; Wu, S.L.; Liu, K.H.; Chen, Y.H.; Chuang, C.S.; Cheng, F.C.; Su, H.L.; Wei, Y.H.; Kuo, S.J.; Liu, C.S. Allogeneic/xenogeneic transplantation of peptide-labeled mitochondria in Parkinson’s disease: Restoration of mitochondria functions and attenuation of 6-hydroxydopamine-induced neurotoxicity. Transl. Res. 2016, 170, 40–56.e3.
- Chuang, Y.C.; Liou, C.W.; Chen, S.D.; Wang, P.W.; Chuang, J.H.; Tiao, M.M.; Hsu, T.Y.; Lin, H.Y.; Lin, T.K. Mitochondrial Transfer from Wharton’s Jelly Mesenchymal Stem Cell to MERRF Cybrid Reduces Oxidative Stress and Improves Mitochondrial Bioenergetics. Oxidative Med. Cell. Longev. 2017, 2017, 5691215.
- Lin, T.K.; Chen, S.D.; Chuang, Y.C.; Lan, M.Y.; Chuang, J.H.; Wang, P.W.; Hsu, T.Y.; Wang, F.S.; Tsai, M.H.; Huang, S.T.; et al. Mitochondrial Transfer of Wharton’s Jelly Mesenchymal Stem Cells Eliminates Mutation Burden and Rescues Mitochondrial Bioenergetics in Rotenone-Stressed MELAS Fibroblasts. Oxid. Med. Cell Longev. 2019, 2019, 9537504.
- Lin, H.Y.; Liou, C.W.; Chen, S.D.; Hsu, T.Y.; Chuang, J.H.; Wang, P.W.; Huang, S.T.; Tiao, M.M.; Chen, J.B.; Lin, T.K.; et al. Mitochondrial transfer from Wharton’s jelly-derived mesenchymal stem cells to mitochondria-defective cells recaptures impaired mitochondrial function. Mitochondrion 2015, 22, 31–44.
- Chang, J.C.; Liu, K.H.; Li, Y.C.; Kou, S.J.; Wei, Y.H.; Chuang, C.S.; Hsieh, M.; Liu, C.S. Functional recovery of human cells harbouring the mitochondrial DNA mutation MERRF A8344G via peptide-mediated mitochondrial delivery. Neurosignals 2013, 21, 160–173.
More
