You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Shlomo Margel.
Proteinoids are random polymers composed of amino acids synthesized by stepwise thermal polymerization. They were discovered and studied in the 1950s by Fox and coworkers, who suggested that they formed spontaneously by high heat at the beginning of life on Earth. Nanocapsules (NCs) form spontaneously by heating proteinoids to about 70 °C in an aqueous solution to completely dissolve the polymers, followed by slow cooling to room temperature.
- proteinoid nanocapsules
- fluorescent nanocarriers
1. Introduction
Proteinoids are random polymers composed of amino acids synthesized by stepwise thermal polymerization. They were discovered and studied in the 1950s by Fox and coworkers, who suggested that they formed spontaneously by high heat at the beginning of life on Earth. Fox et al. demonstrated that proteinoids may self-assemble in spherical micrometer structures, proteinoid microspheres, presented as the protocells of life [1,2,3][1][2][3]. This process occurs at rather high temperature (170–180 °C, for instance) in an inert atmosphere without a catalyst or a solvent. Lysine or aspartic/glutamic acid are critical, as they form cyclic products which act as solvents [4]. Various proteinoids may be prepared by using natural and synthetic amino acids at different ratios. The special features of each proteinoid influence the character of particles that are composed from it [5,6][5][6].
In early work by Rao and coworkers [7[7][8],8], an acidic proteinoid was prepared by thermal condensation of seven natural amino acids, mainly Glu, Asp and Gly [7]. The proteinoid was expected to be non-antigenic due to its low molecular weight (<10 kDa). The acidic gastric irritating drug methotrexate was encapsulated in self-assembled microspheres rather efficiently (~50% with ~10% loading) [8], conferring gastric (pH~1) stability (<10% released in two hours) and thus improving the prospects of oral delivery. The spherical microspheres were of uniform diameter (1–3 μm). Complete solubility in neutral blood pH allowed release of most of the encapsulated drug within one hour.
During the last decade, Margel and coworkers produced proteinoids by bulk stepwise polymerization of 3–4 amino acids without or with a biopolymer such as poly(L-lactic acid) (PLLA) [9,10,11,12,13,14][9][10][11][12][13][14]. Nanocapsules (NCs) form spontaneously by heating proteinoids to about 70 °C in an aqueous solution to completely dissolve the polymers, followed by slow cooling to room temperature. Different drugs and/or imaging reagents were encapsulated by dissolution in the proteinoid solution, and covalent binding to the surface of the NCs was achieved with or without a spacer arm. Proteinoid NCs suit many applications owing to their non-toxicity, biocompatibility and immune safety [10,11][10][11]. Incorporation of the tri-amino acid sequence arginine–glycine–aspartic acid (RGD) in the random proteinoid backbone enables targeting of tumors.
Kwon, Park & Kim [15] demonstrated that proteinoids could also act as carriers of a certain kind of drugs and be programmed to release them depending on the external conditions. This is made possible by introducing a disulfide bond in the proteinoid that lets it assemble in an aqueous phase. In an external environment which is reducing in nature, the disulfide bond is cleaved, and the micelle is likely to be loosened. As a result, the payload (drug) inside the micelle would be released.
Recent work by Adamatzky investigated the potential application of proteinoids in the field of computing systems [16]. Proteinoids have the potential to be utilized in unconventional computing owing to their unique electrical properties. Such proteinoids were also prepared by step-growth polymerization of amino acids in an aqueous environment. These proteinoids swell into hollow microspheres that produce an endogenous burst of electrical potential spikes and exhibit the capacity to alter patterns of their electrical activity in response to illumination. By forming interconnected networks through pores and tubes, proteinoid microspheres can enable programmable growth and have potential for creation of intricate computing systems. This capacity for novel growth patterns and electrical activity allows for the possibility of developing computing architectures that are more versatile and efficient than traditional systems.
2. Synthesis and Characterization of Proteinoids and NCs
2.1. Preparation of Proteinoids
Proteinoid chains were prepared by step-growth polymerization of amino acids at high temperature (depending on the amino acids, e.g., 180 °C) in an inert atmosphere with no solvent, initiator or catalyst [11,12][11][12]. A tri-functional amino acid—Glu/Asp/Lys—is an essential component, providing a solvent by cyclization and serving as an initiator (see Figure 1) [11]. Proteinoid preparation was recently reviewed [17]. Different proteinoid polymers can be obtained due to the extensive range of both natural and synthetic amino acids available (in most cases, the ratio between the monomers was 1:1). This entreviewy presents several examples of proteinoid polymers that have been studied for the purpose of cancer diagnostics, therapy and theranostics (see Table 1).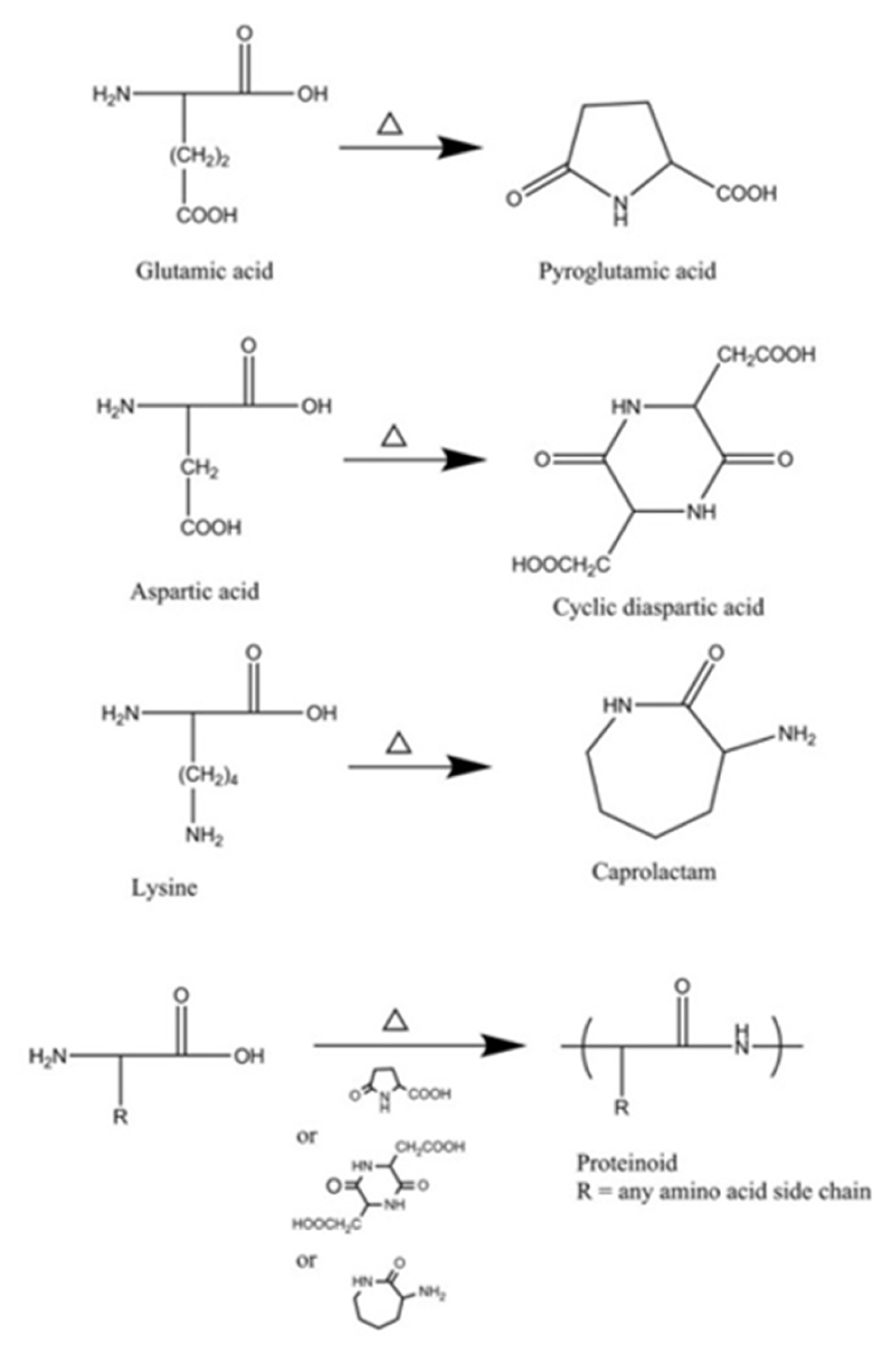
Figure 1.
Table 1.
Various proteinoid polymers and their amino acid content
a
.
| Proteinoid Polymer | Amino Acid Content | Main Component of Amino Acids a | Segment |
|---|---|---|---|
| P(EF-PLLA) | L-glutamic acid L-phenylalanine |
L-glutamic acid | Poly-L-lactic acid (PLLA) |
| P(KRHF) | L-lysine L-arginine L-histidine L-phenylalanine |
L-lysine | PLLA |
| P(RGD) | D-arginine L-glycine L-aspartic acid |
L-aspartic acid | ------ |
a A tri-functional amino acid—Glu/Asp/Lys—is a main component, providing a solvent by cyclization and serving as an initiator.
2.2. Preparation of Nanocapsules (NCs)
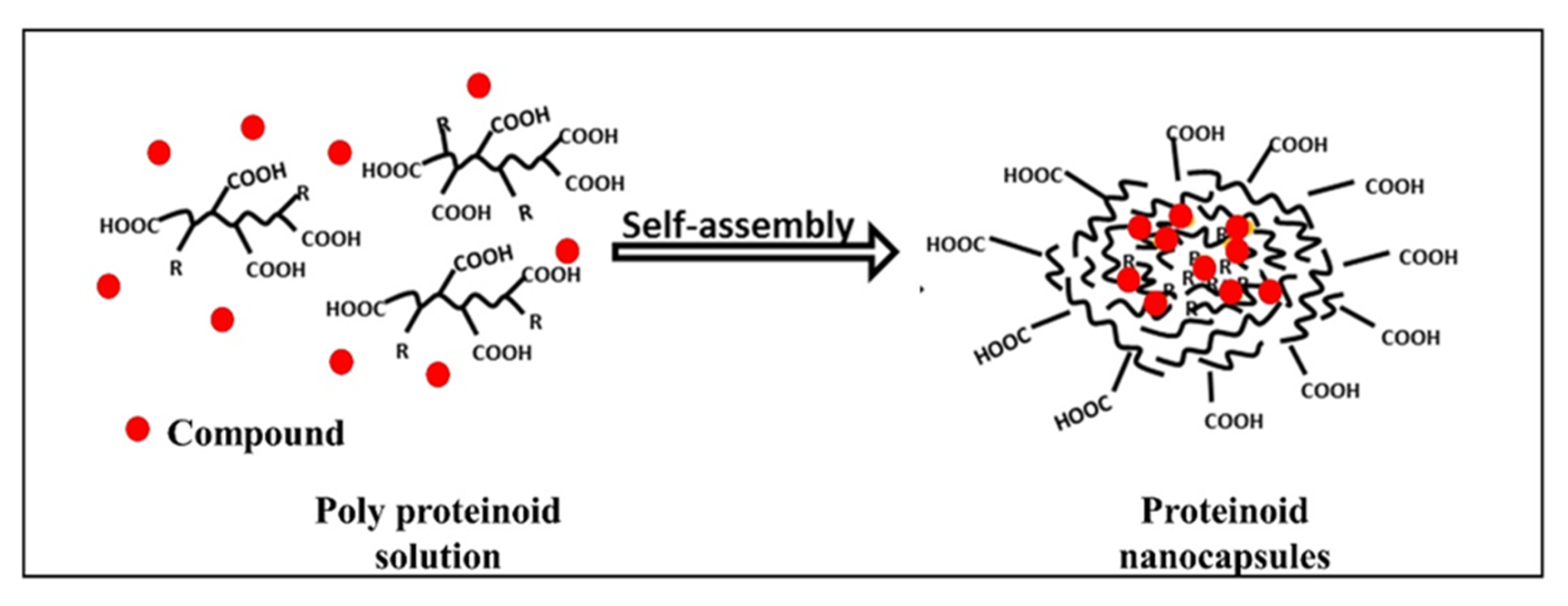
Hollow proteinoid NCs were produced by a self-assembly process in an aqueous continuous phase [17]. In this process, the first step was to heat the aqueous phase containing the proteinoid to about 70–80 °C until a full dissolution of the polymer was observed, followed by slow cooling to room temperature for precipitation and formation of proteinoid NCs. The NCs form biocompatible carriers with a hydrophobic core and hydrophilic groups on the surface. Various compounds were encapsulated during self-assembly, as presented in Figure 2. Near infrared (NIR) fluorescent dyes were used for cancer diagnosis [12,17,18][12][17][18]. Recently, a synergistic combination of anti-cancer drugs was encapsulated [19] for personalized therapy.
Figure 2. Self-assembly of proteinoid polymers; scribbled lines—polymer chains, dots—encapsulated compound (dyes/drugs) [11].
2.3. Characterization of Proteinoids and NCs
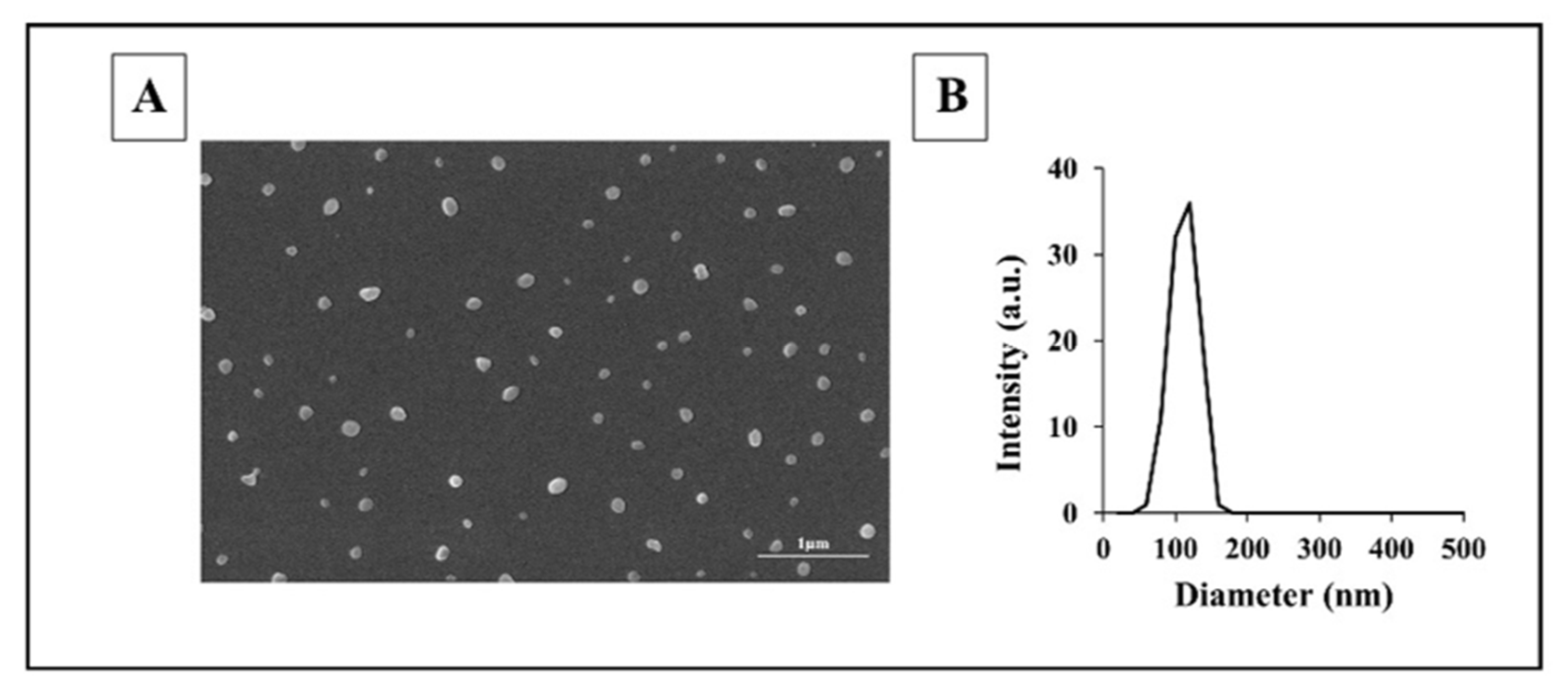
Unexpectedly, the molecular weight of all proteinoids made in the Margel lab was high (25–195 kDa) with a low polydispersity index (PDI of 1.01–1.27) [5]. These very unusual results for stepwise polymerization may be explained by the high temperature, which provides uniform long chains that resemble natural biopolymers. Self-assembled NCs were spherical with a uniform distribution, as shown by dynamic light scattering (DLS) and scanning electron microscopy (SEM) in Figure 3.
Figure 3. SEM image (A) and diameter histogram (B) of proteinoid nanocapsules (NCs) [19]. The diameters of more than 200 NCs were measured with Analysis Auto image analysis software version 3.2 (Soft Imaging System GmbH, Münster, Germany).
References
- Fox, S.W.; Jungck, J.R.; Nakashima, T. From proteinoid microsphere to contemporary cell: Formation of internucleotide and peptide bonds by proteinoid particles. Orig. Life 1974, 5, 227–237.
- Fox, S.W.; Mccauley, R.J.; Fukushim, T.; Windsor, C.R.; Montgome, P.O. Selective action in boundaries of particles of thermal proteinoid. Fed. Proc. 1967, 26, 749.
- Fox, S.W. The proteinoid theory of the origin of life and competing ideas. Am. Biol. Teach 1974, 36, 161–172.
- Harada, K.; Fox, S.W. The thermal condensation of glutamic acid and glycine to linear peptides. J. Am. Chem. Soc. 1958, 80, 2694–2697.
- Matsuno, K. Electrical excitability of proteinoid microspheres composed of basic and acidic proteinoids. Biosystems 1984, 17, 11–14.
- Przybylski, A.T. Excitable cell made of thermal proteinoids. Biosystems 1985, 17, 281–288.
- Kumar, A.B.M.; Jayakumar, R.; Rao, P.K. Synthesis and aggregational behavior of acidic proteinoid. Sci. Polym. Chem. 1996, 34, 2915–2924.
- Kumar, A.B.M.; Rao, P.K. Preparation and characterization of pH-sensitive proteinoid microspheres for the oral delivery of methotrexate. Biomaterials 1998, 19, 725–732.
- Lugasi, L.; Grinberg, I.; Margel, S. Targeted delivery of CBD-loaded poly(RGD) proteinoid nanoparticles for antitumor therapy. J. Nanomed. Nanotech. 2020, 18, 552.
- Kiel, S.; Kolitz-Domb, M.; Corem-Salkmon, E.; Margel, S. Engineered doxorubicin delivery system using proteinoid-poly(L-lactic acid) polymeric nanoparticles of narrow size distribution and high molecular weight for cancer treatment. Int. J. Nanotechnol. Nanomed. 2017, 2, 1–11.
- Kolitz-Domb, M.; Margel, S. Recent advances of novel proteinoids and proteinoid nanoparticles and their applications in biomedicine and industrial uses. Isr. J. Chem. 2018, 58, 1277–1285.
- Hadad, E.; Rudnick-Glick, S.; Ithaki, E.; Avivi, M.Y.; Grinberg, I.; Elias, Y.; Margel, S. Engineering of doxorubicin-encapsulating and TRAIL-conjugated poly(RGD) proteinoid nanocapsules for drug delivery applications. Polymers 2020, 12, 2996.
- Hadad, E.; Rudnick-Glick, S.; Grinberg, I.; Kolitz-Domb, M.; Chill, J.H.; Margel, S. Synthesis and characterization of Poly(RGD) proteinoid polymers and NIR fluorescent nanoparticles of optimal D, L-configuration for drug-delivery applications— in vitro study. ACS Omega 2020, 5, 23568–23577.
- Kolitz-Domb, M.; Grinberg, I.; Corem-Salkmon, E.; Margel, S. Engineering of near infrared fluorescent proteinoid-poly(L-lactic acid) particles for in vivo colon cancer detection. J. Nanobiotechnol. 2014, 12, 30.
- Kwon, K.; Park, D.; Kim, J.C. Disulfide proteinoid micelles responsive to reduction. J. Dispers. Sci. Technol. 2018, 40, 1413–1422.
- Adamatzky, A. Towards proteinoid computers. hypothesis paper. Biosystems 2021, 208, 104480.
- Sharma, S.; Mougoyannis, P.; Tarabella, G.; Adamatzky, A. A review on the protocols for the synthesis of proteinoids. arXiv 2022, arXiv:2212.02261.
- Fox, S.W.; Harada, K. Thermal copolymerization of amino acids in the presence of phosphoric acid. Arch. Biochem. Biophys. 1960, 86, 281–285.
- Itzhaki, E.; Hadad, E.; Moskovits, N.; Stemmer, S.M.; Margel, S. Tumor-targeted fluorescent proteinoid nanocapsules encapsulating synergistic drugs for personalized cancer therapy. Pharmaceuticals 2021, 6, 648.
More
