Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Erkan Topkan.
Radiotherapy (RT) plays a pivotal role in the oncological management of head and neck cancers (HNC). Radiotherapy is the only curative alternative for patients with early-stage squamous cell HNC who are medically inoperable. It is also the only option for inoperable locally advanced salivary gland carcinomas where effective chemotherapy alternatives are not accessible. Locally advanced HNC (LA-HNC) patients may benefit from RT as an adjuvant therapeutic option after surgery and as the backbone of organ-sparing treatment when combined with concurrent chemotherapy. Furthermore, RT can be an effective primary palliative strategy for recurrent or metastatic disease.
- radiotherapy
- osteoradionecrosis
- radiotherapy modality
- radiotherapy technique
- radiotherapy toxicities
- head and neck cancer
1. Predisposing Anatomical and Physiological Characteristics
The mandibular processes gradually grow and fuse in the midline during the fourth and fifth weeks of the embryological period. The left and right Meckel cartilages, which are cartilaginous rods that serve as the cores around which the membranous bone of the lower jaw develops, are made by neural crest cells of the first pharyngeal arch between the fifth and eighth weeks of development. The regions of the lower lip, lower jaw, and lower cheek are formed by the mandibular processes. The mentum designates the location of the midline fusion of the two mandibular processes.
The mandible is a flat bone with two rami and a body that forms a horseshoe shape. Apart from the middle ear bones, the mandible is the only moveable bone in the skull and the largest bone in the viscerocranium, or fascial skeleton. In contrast to the other skull bones, which are joined by sutures, the mandible articulates with the surrounding bones via a synovial joint called the temporomandibular joint. This anatomical c0haracteristic makes it possible to chew and speak while maintaining the mandible’s connection to the skull.
The mandible receives its main blood supply from the inferior alveolar artery, which also nourishes the nerves, gingivae, and teeth. It is a small muscular artery that arises from the first portion of the maxillary artery and runs alongside the inferior alveolar nerve, the main nerve that innervates the mandible and its associated structures. The inferior alveolar artery splits into the mylohyoid and lingual branches before to entering the mandibular foramen. It then gives rise to the incisor arteries as it traverses the mandibular foramen and the mental artery as it leaves the mental foramen. It is worth noting that the inferior alveolar artery can occasionally arise from the external carotid artery rather than the maxillary artery and can be found in duplicated form. Necrosis of the mandible and structures perfused by its branches is possible after an injury or occlusion of the inferior alveolar artery.
In healthy adults, the mandible, like other bones, is a dynamic tissue with a balance of bone formation and resorption—the remodeling process—that is regulated systematically by hormones and locally by growth factors and cytokines [1][2][3]. The mandible has a compact-to-trabecular bone ratio similar to the distal radius and other long bones: 80% compact and 20% trabecular bone [4]. Due to its unique architecture of trabeculae, plates, and rods, the mandible has ten times the surface area of compact bones, resulting in a more pronounced remodeling process owing to an abundance of endosteal surfaces and cells [5][6]. The rate at which trabecular bone in the mandible remodels is six times faster than in the femur and twice as fast as in the maxilla [7]. There is also a difference in remodeling rates between the parts of the mandible. The rate of alveolar bone turnover at the alveolar crest, for example, is twice as fast as that of the mandibular canal and three to five times faster than that of the inferior compact border of the mandible [8]. Regardless of the type of injury, any damage to the mandible may logically result in a faster devitalization of the bone tissue than its comparators, including the maxilla. This difference is partially due to the mandible’s restricted blood supply, which is nearly one-sixth that of the maxilla, making the mandible more susceptible to necrosis than the maxilla due to a delayed or insufficient repair process in the absence of sufficient oxygenation, where the supply cannot meet the increased demand.
2. Radiobiological Considerations
Recent decades have seen significant technological advancements that have improved the quality of localized RT. While delivering ionizing radiation to the tumor is critical, minimizing damage to surrounding healthy tissue remains a significant obstacle despite these advancements. Effective management of RT’s early and late adverse effects requires knowledge of how healthy tissues respond to ionizing radiation. Radiation exposure has been shown to cause adverse changes in the physiologic levels of many factors, including cytokines, which play a crucial role in the onset of radiation-induced normal tissue injury [9]. Ionizing radiation causes the immediate formation of highly reactive free radicals following exposure, culminating in immediate protein changes and damage to cell membranes, ribonucleic acid (RNA), and deoxyribonucleic acid (DNA). A chronic increase of reactive oxygen species (ROS) is created and maintained for long periods as a consequence of secondary reactions after the initial hydrolysis of water and direct ionization following radiation exposure. The production of ROS by various cell types during injury and inflammation can also result in reactive nitrogen species, which can further exacerbate oxidative damage [10]. Damaged endothelial, epithelial, and inflammatory cells, for instance, may intensify localized oxidative stress and long-lasting radiation injury by producing chemicals like superoxide and nitric oxide [11]. Superoxide is a very toxic NADPH metabolite that is produced as a result of radiation-induced injury, which may destroy any sort of cellular component, including DNA, either directly or by producing other hazardous secondary radical species [12][13]. The direct effects of radiation occur when it interacts directly with the atoms of the DNA molecule or another vital biological component. However, because the amount of DNA molecule is too small, the likelihood of radiation interacting with it is less probable. Therefore, it is more likely that ionizing radiation will exert the majority of its effects indirectly (almost two-thirds), by ionizing water molecules and producing free radicals that lead to double-strand DNA breaks. Regardless of the mechanism of action, these early wounds may cause changes in cellular vitality and functioning as well as a local or systemic inflammatory response that may last for months or years, depending on the inherent radiosensitivity of the injured cells [14].
Bones are highly specialized anatomical structures susceptible to the damaging effects of ionizing radiation, especially at higher dosages. The bone is a common location of radiation-induced damage because of its high calcium concentration and propensity to absorb roughly 40% more radiation than surrounding tissues [15]. Radiation causes an excessive release of cytokines and chemokines during the damage response, leading to the symptoms of acute inflammation such as increased vascular permeability, localized edema, endothelial cell death, and vascular thrombosis [15][16]. In the later stages, RT also promotes fibroatrophic processes, which cause tissue to have poor vascularization and obstruct effective repair. This adverse circumstance renders tissue more fragile and triggers inflammation to flare up or reoccur after local injuries, such as tooth extraction or dental implant placement procedures. Similar to osteoporotic diseases, RT decreases the trabecular bone volume and skeletal stem cell populations while raising bone marrow adiposity and serum CTX/TRAP5 levels, resulting in more sluggish and less effective fracture repair [17][18]. Blood levels of osteocalcin and TRAP5 quickly increase after bone irradiation, indicating an increase in osteoclast activity [19]. At 12 weeks post-irradiation, trabecular bone volume decreases significantly, likely due to drastically reduced osteoblastogenesis, whereas osteoclastogenesis returns to nearly normal levels [19]. Such observations confirm the emergence of a decreased bone formation-to-resorption ratio and deteriorated bone quality. Irradiated bones’ skeletal stem cells appear to favor adipogenesis over osteogenesis, which further contributes to RT-induced bone loss. The rapid increase in osteoclast activity after RT and the gradual decrease in osteoblast activity in the weeks that followed are thought to be responsible for this phenomenon [18][20]. High-dose RT may alter the differentiation characteristics of skeletal stem cells in favor of decreased differentiation potential but increased radiation-induced cellular senescence, as evidenced by a robust galactosidase labeling signal that overlaps with cell death patterns [17][21].
The hallmarks of ORNJ include the development of hypovascular, hypocellular, and hypoxic bone and soft tissues, along with a chronic inflammatory process that worsens with time. These RT-induced changes increase cell death and collagen breakdown above and beyond the standard homeostasis capacity of cell repair and collagen synthesis, resulting in fibroatrophic and necrotic bone formation [15][22]. In a ground-breaking study, radiation-free samples from HNC patients were compared to radiation-treated samples from 40 ORNJ patients who received 50.4–70.4 Gy [15]. According to a histopathology examination of the bone and soft tissue samples, hyperemia and endarteritis were the early effects of irradiation that persisted for up to 6 months after exposure. Irradiated bone specimens had more cellular loss than their soft tissue counterparts, and indications of increased hypocellularity emerged quickly after irradiation in bone. The vascular structures were found to contain dense fibrous material years after being exposed to radiation, providing proof that thrombosis had formed. It was also discovered that radiation-induced damage had end-stage indicators like a decline in vascular content and an increase in tissue fibrosis, both of which got worse over time [15][22].
Modern molecular research has demonstrated that damaged tissue may also have metabolic derangements, which may be attributed to hypoxia, inflammation, and the activation of various pathways, such as hypoxia-inducible factor-1 alpha (HIF-1α) and the mechanistic target of rapamycin [23]. Irradiation-induced HIF-1α enhanced signaling induces the overproduction of transforming growth factor β1 (TGF-β1) and vascular endothelial growth factor (VEGF) [24][25]. TGF-β1 is a multifunctional cytokine secreted latently by cells. However, radiation-induced ROS exposure and the actions of activated proteinases cause the active TGF-β1 protein to be released and bind to one of several TGF-β1 receptors. Immediately following irradiation, a rise in TGF-β1 expression may be observed, and the increase is dose and time-dependent [26][27]. Although the TGF-β1 expression may normalize after this initial spike, it often rises again in chronically injured tissue after radiation exposure [28]. Numerous experimental and clinical studies have proven that TGF-β1 plays a role in clinical radiation damage. TGF-β1 exerts its action through canonical (Smad-dependent) and non-canonical (Smad-independent) mechanisms. The canonical route is crucial to the regulation of fibrosis genes such as collagen I, collagen III, and fibronectin. Non-Smad TGF-β1 activated signaling pathways, including Rho/ROCK, p38, and JNK, seem essential for fibrosis regulation. Even though TGF-β is a growth factor that is known for being anti-inflammatory, the interaction between TGF-β-R1 and TGF-β-R2 can cause upregulation of TRAF4 and TRAF6, which in turn activates inflammatory mediators like the multifunctional transcription factor NFκB. The expression of COX-2, iNOS, and STAT3 can be induced by NFκB, which can exacerbate fibrosis, EMT, and inflammation. TGF-β1 is one of the most prevalent cytokines released after tissue exposure to radiation, and its release correlates directly with radiation dose [29], indicating that TGF-β1 is a crucial marker of radiation toxicity and DNA damage in irradiated cells. If a cell survives DNA damage, the first genetic consequence of ionizing radiation is the increased production of TGF-II [30]. Consequently, it is plausible to hypothesize that radiation-induced release of TGF-β1 may influence fibrotic cell death, the final stage of ORNJ development; however, additional fundamental research is required to confirm this hypothesis. But a few reliable studies, like Delanian’s research on the fibroatrophic theory, have shown that ROS and TGF-β1 play significant roles in the early inflammation, fibrosis, and remodeling that result in terminal tissue necrosis in the development of ORNJ [15]. Similar hypothetical findings were also reported by Lyons et al. [31] and Bras et al. [32], lending support to the role of radiation-induced cytokines, fibrosis, and vascular abnormalities in the pathogenesis of ORNJ.
A precise balance must be maintained between bone formation by osteoblasts (OBs) and bone resorption by osteoclasts (OCs) to maintain a healthy bone microenvironment and a functioning skeletal system throughout the course of a person’s lifetime [33]. Additionally, healthy levels of various hormones and cytokines are necessary for strictly and appropriately regulated bone metabolism; however, any dysregulation in this complex system can lead to osteoporosis or osteoporotic diseases depending on the dominant remodeling process unrelated to the bone type. Tumor necrosis factor-alpha (TNF-α) is a pro-inflammatory cytokine that plays a crucial role in maintaining bone homeostasis by inhibiting osteoblast activity and boosting osteoclastogenesis during bone remodeling. However, TNF-α also contributes to the development of chronic inflammation. In confirmation, TNF-α has been shown to play active roles in the development of inflammatory joint disorders such as rheumatoid arthritis (RA), which causes extensive juxta-articular bone degradation, and ankylosing spondylitis (AS), which causes simultaneous bone breakdown and excessive formation. Existing in vitro research has shown that TNF-α inhibits osteoblastic differentiation and stimulates osteoclastogenesis through differential expression of many transcription factors, including NF-κB. TNF-α together with interleukin-1 (IL-1) has been shown to induce osteocyte apoptosis. Because they attract osteoclasts, apoptotic osteocytes have a significant impact on osteoclastic bone resorption during bone remodeling, which is in part regulated by TNF-α. Furthermore, TNF-α has been shown to significantly reduce F-actin levels, nitric oxide (NO) production, and intracellular calcium. This physiological imbalance may result in a decrease in osteocyte elasticity, providing a potential mechanism to explain how inflammation contributes to bone mass loss. Briefly, TNF-α disturbs bone homeostasis by activating osteoclastic resorption, suppressing osteoblastic proliferation and matrix production, and activating TNF receptor-associated Factor-2 (TRAF-2), which stimulates NF-κB, AP-1, and MAPKs signaling pathways, resulting in reduced bone formation. In this regard, it has been noted that proinflammatory and proresorptive IL-1, IL-6, IL-17, and TNF-α are significantly elevated 24 to 48 h after radiation exposure, which causes early bone loss after irradiation, confirming the crucial roles of proresorptive and inflammatory cytokines in post-irradiation bone loss [34][35]. Considering that TNF-α is an inflammatory cytokine involved in the acute phase reaction and is rapidly and persistently expressed in irradiated and adjacent tissue, it is reasonable to assume that rapid rise of TNF-α after irradiation may also play significant roles in ORNJ formation after head and neck RT. But before any meaningful conclusions about the precise function of TNF and related cytokines in ORNJ development can be drawn, this inference needs to be examined in thoroughly conducted pathophysiologic research.
According to the findings of basic research that provided the basis for Marx and colleagues’ 3H (hypovascular, hypocellular, and hypoxic) theories and Delanian’s radiation-induced fibroatrophic ORNJ theories [21][27], hypoxia, inflammation, and related cytokines appear to play a significant role in ORNJ genesis. Microvascular damage and subsequent vascular occlusion following RT are additional drivers of compromised bone integrity, which leads to hypovascularity, hypocellularity, local hypoxia, and fibroatrophic healing. After irradiation of a well-designed minipig model, the endothelial linings of vascular structures edematized in just 1-day, followed by the obliteration of small luminal vessels, indicating an induced hypoxic condition [36]. Although there was a transitory increase in blood flow two weeks after irradiation, this was followed by a progressive decline, demonstrating that microvascular damage occurred far sooner than bone damage. It further confirms the centrality of vascular occlusion and the associated chronic hypoxia in the evolution of ORNJ, which may set in motion a self-perpetuating cycle of persistent inflammation and heightened fibrosis. Dekker et al. [37] recently provided new evidence that radiation exposure causes microvascular damage in human mandibles. The authors assessed 20 irradiated, edentulous patients who had received mandibular dental implants, with the radiation-free implant patients acting as the control group. Bone biopsies at doses >50 Gy showed reduced vascular density and preferential obliteration of microvascular structures in the irradiated group. Clinical evidence also supports a vascular origin for ORNJ given that it can occur up to six times more frequently in the mandible than in the maxilla, which has better blood flow [38][39][40]. In a recent clinical trial including 263 patients with locally advanced nasopharyngeal carcinoma (LA-NPC) who underwent CCRT, Yilmaz et al. [41] shown the relevance of hypoxia in the development of ORNJ. The connection between pretreatment hemoglobin (Hb) levels and ORNJ rates served as this restudyearch’s primary outcome measure. The authors reported an 8.7% ORNJ prevalence rate. The optimal pre-CCRT Hb cutoff was 10.6 g/dL. When patients were divided into two groups based on this criterion, the Hb ≤ 10.6 group had a considerably higher ORN rate (32.5% vs. 1.5% for Hb > 10.6; p < 0.001). The encouraging findings of this pivotal restudyearch may serve as the foundation for additional basic and clinical research examining the crucial role of systemic and local hypoxia in the genesis of ORNJ, even though Hb is only an indirect indicator of tissue hypoxia.
ORNJ is a multifaceted complication of RT in patients with HNC, involving multiple physiological disruptions and suppressed or activated cytokines. Despite the substantial body of literature associating tissue hypoxia, elevated apoptosis, chronic inflammation, and hyper-fibrosis with the emergence of ORNJ, no clinical study has been reported that evaluates the potential utility of related biomarkers in the precise prediction of HNC patients receiving RT or CCRT (Figure 1). The exception to this is the most recent work by Yilmaz and colleagues [41]. If these findings are replicated, it might pave the way for a better understanding of ORNJ’s complicated biology and the creation of new, more effective approaches for preventing and treating this devastating disease.
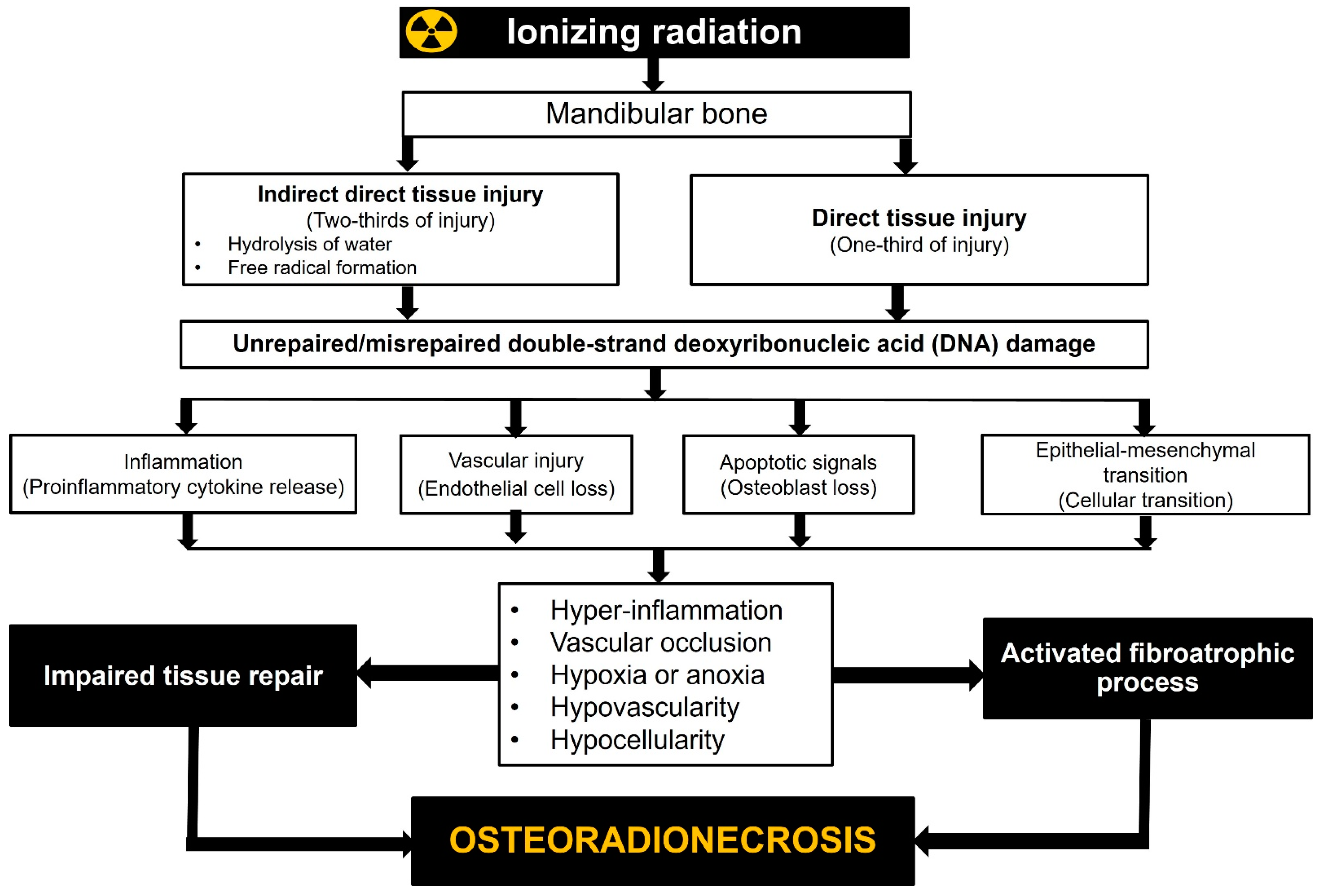
Figure 1.
Radiobiological mechanisms of jaw osteoradionecrosis.
3. Radiotherapy Modality and Technique
For radiation-induced toxicity in general and ORNJ in particular, the RT modality (equipment) and technique are two of the most influential factors. This is mainly because various RT techniques have varying degrees of success when delivering the prescribed dose to the treatment’s focal volume while still ensuring the safety of the “organ at risk” (OAR). Optimizing tumor coverage by focusing high doses on the affected area while limiting exposure to healthy tissue is a top priority in today’s RT techniques. In contrast to the primary tumor and lymphatic regions, which can receive nearly comparable doses with any 2-dimensional RT (2D-RT), 3-dimensional conformal RT (3D-CRT), IMRT, or heavy ion therapy, such as proton therapy and carbon ion therapy, doses to OARs can only be reduced to desired values using computer-aided, sophisticated treatment plans and their delivery by modern treatment machines (Figure 2).
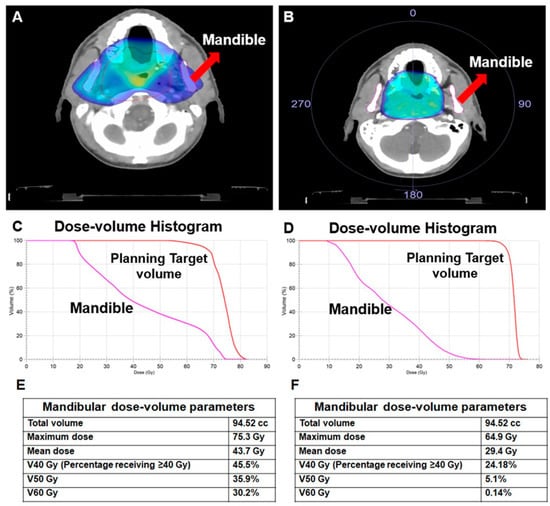
Figure 2. A comparison of two different treatment plans for the same patient with nasopharyngeal carcinoma illustrates the effect of the radiotherapy technique on the doses received by the mandible. (A) 3-dimensional conformal radiotherapy (3D-CRT), (B) Volume-modulated arc therapy (VMAT), (C) Dose-volume histogram for 3D-CRT, (D) Dose-volume histogram for VMAT, (E) Dosimetric measures for 3D-CRT, (F) Dosimetric measures for VMAT.
If the mandible is designated as an OAR during treatment planning, the use of IMRT, image-guided radiation therapy (IGRT), or hadron therapy may reduce the doses received by the mandible and, consequently, the risk of ORNJ. Therefore, in addition to the other OARs, the mandible should be distinguished as a separate OAR, and mandibular dosages should be kept as low as possible to reduce the risk of ORNJ in HNC patients. Maesschalck et al. [42] compared the incidence of ORNJ after IMRT in comparison to 3D-CRT techniques. The cohorts included 145 patients in the 3D-CRT group and 89 patients in the IMRT group. Total incidence rate of ORNJ was similar for both groups with rates of 11% versus 10% (p = 1.0). Nevertheless, in contrast to the findings of Maesschalck and colleagues, existing research data often indicate that cutting-edge RT techniques result in reduced ORNJ incidence rates. Nguyen et al. [43] analyzed 83 patients treated with definitive CCRT, post-operative RT/CCRT, or RT alone to determine the efficacy of IMRT and IGRT in reducing the risk of ORNJ. The mean mandibular doses for IMRT and IGRT were 43.6 Gy and 43.8 Gy, respectively, with only 1 (1.2%) ORNJ incidence at a median follow-up of 28 months, which is less than the generally cited range of 2% to 28%. These results lend credence to the efficacy of advanced RT techniques in reducing the risk of ORNJ, even though the median follow-up time was shorter than the commonly mentioned 36 months for ORNJ development. The prevalence and risk factors for ORNJ in patients with OCC and OPC were investigated by Moon et al. [44]. Out of 252 consecutively treated patients, 14 (5.5%) were found to have ORNJ upon review of their medical records. On univariate analysis, factors associated with ORNJ included a primary diagnosis of OCC versus OPC [hazard ratio (HR): 3.0; p = 0.04], continued smoking during RT (HR: 3.1; p = 0.04), mandibular invasion of the primary (HR: 3.7; p = 0.04), tooth extraction before RT (HR: 4.52; p = 0.01), and treatment with 3D-CRT versus IMRT (HR: 5.1; p = 0.003). The presence of pre-RT dental extractions, as well as the RT technique, were both confirmed to be significant by multivariate analysis. Aarup-Kristensen and colleagues examined the prevalence of ORNJ and associated risk variables in 1224 HNC patients treated with 66–68 Gy [45]. IMRT was used to treat the vast majority of patients and controls (94%). Cases of ORNJ were identified through cross-referencing the national Danish Head and Neck Cancer database with clinical observations at follow-up and hospital code diagnostics following oral-maxillofacial surgery. Documentation of dental procedures, including mandibular surgery, performed prior to RT for patients with ORNJ cases and two controls (1:2) was collected in a nested case-control study. ORNJ was observed in 56 cases (4.6%), with a median time to occurrence of 10.9 months (range: 1.8–89.7) following RT; 90% of cases occurred within 37.4 months. This study’s authors hypothesized that IMRT was responsible for the significantly lower ORNJ incidence rate (4.6% vs. 21.0%) they observed compared to the previously published DAHANCA 7 trial, in which 3D-CRT was the primary RT technique. Recently, Yilmaz et al. [41] utilized IMRT in 263 locally advanced nasopharyngeal carcinoma patients and reported 8.7% ORNJ rate. A recent meta-analysis by Balermpas et al. [46] provided additional support for these studies by showing that fewer than 5% of patients who underwent tooth extractions before IMRT experienced ORNJ as a side effect.
Theoretically, compared to IMRT (photon RT), more advanced intensity-modulated proton therapy (IMPT) may further lower ORNJ rates due to its inherent physical properties. Protons stop in the tissue after depositing their maximum energy (Bragg Peak), leading to a reduced integral dose and sparing of normal tissue. A constant relative biological effectiveness of 1.1 relative to photon RT is used for treatment planning. However, it is worth to note that increased relative biological effectiveness values may occur particularly at the field edges, which are usually located in the normal tissue due to clinical safety margins. Zhang et al. [47] compared mandibular doses and ORNJ rates in patients with oropharyngeal cancer (OPC) after IMRT or IMPT. A total of 584 patients who received definitive RT were included. Mandibular doses (minimum: 0.8 vs. 7.3 Gy; mean: 25.6 vs. 41.2 Gy; p < 0.001) and ORNJ (2.0% vs. 7.7%; p = Not specified) rates were lower for patients treated with IMPT than that with IMRT. These findings showed that using IMPT decreased excess irradiation of the mandible and, as a consequence, the risk of ORNJ for OPC. Singh et al. [48] recently published their IMPT experience with 122 OPC patients at Memorial Sloan Kettering Cancer Center. During a median follow-up of 40.6 months, ORNJ was documented in 13 (10.6%) individuals. The most involved ORNJ location in this investigation was the posterior ipsilateral mandible inside the radiation field that received the entire targeted IMPT dosage. This disheartening ORNJ rate indicates that ORNJ continues to be a clinical challenge even in the era of highly conformal IMPT, despite its unquestionable dosimetric advantages.
Along with the dosimetric benefits of IMPT, carbon ion therapy, a different type of hadron therapy, also has radiobiological benefits. However, no significant research findings in this field have been released as of yet. A retrospective evaluation by Musha et al. [49] included 199 HNC patients who received carbon-ion therapy. However, only 11 individuals with OPC and floor of mouth cancers were examined. The treatment consisted of 57.6 Gy or 64 Gy (relative biological effectiveness) administered in 16 fractions. The association between radiation dose and mandibular ORNJ was investigated. ORNJ was diagnosed in 5 (45.5%) of the patients after a median follow-up of 68 months. With cut-off values of 16.5 cc (p = 0.002) and 1.8 cc (p = 0.0059), respectively, doses of 30 Gy (relative biological effectiveness) to the mandible and teeth had the most striking impact on ORNJ development.
Although the dose distribution characteristics of IMPT and carbon ion therapy may offer superior tissue sparing than photon-based RT techniques, data on ORNJ is limited, and the results are contradictory. For instance, compared to the frequently cited <5% ORNJ rates with IMRT, the recently reported 10.6% ORNJ rate with IMPT in the study by Singh et al. seems discouraging [41]. Therefore, it is essential to conduct large-scale, prospectively evaluated hadron therapy studies in order to reach more reliable conclusions regarding this issue of utmost importance.
4. Radiotherapy Dose
Even though RT modality and technique are established predictors of ORNJ, the dosage to the mandible is the ultimate determinant of the probability of this complication. If the total dosage, dose per fractionation, and dose distribution within the tissues remain the same, the radiobiological consequences will be identical regardless of the photon delivery technique. Consequently, modern RT techniques appear to mitigate this risk by reducing mandibular doses rather than by altering the behavior of ionizing radiation in irradiated tissues. Over the years, numerous studies have evaluated the risk of ORNJ in HNC patients based on different mandibular dosimetric parameters (Table 21). Despite significant methodological differences, the vast majority of studies have consistently suggested a strong correlation between mandibular dosages and the risk of ORNJ: the higher the dose, the greater the risk of ORNJ.
Table 21.
Accessible published dosimetric parameters associated with ORNJ incidence.
| Reference | Study Year | Patients (N) |
Tumor Primary | Recommended Parameter | |
|---|---|---|---|---|---|
| Kubota et al. | [50] | 2021 | 616 | HNC | V60 ≤ 14% |
| MD Anderson Head and Neck Cancer Symptom Working Group | [51] | 2017 | 68 | OPC | V44 < 42% V58 < 25% |
| De Felice et al. | [52] | 2016 | 36 | HNC | Dmean < 57.6 Gy D2% < 65 |
| van Dijk et al. | [53] | 2021 | 1259 | HNC | For <5% ORNJ: D30% < 42 Gy (without tooth extraction) For <5% ORNJ: D30% < 35 Gy (without tooth extraction) |
| Aarup-Kristensen et al. | [45] | 2021 | 1224 | HNC | Dmean < 37 Gy |
| Tsai et al. | [54] | 2013 | 402 | OPC | Dmean < 37.5 Gy V50 (continously) V60 (continously) |
| Caparrotti et al. | [55] | 2017 | 1196 | OPC | V50 (continously) V60 (continously) |
| Lang et al. | [56] | 2022 | 89 | OCC | Dmean ≤ 45 Gy Dmax ≤ 60 Gy PTV proportion intersecting the mandible ≤ 40% |
| DeLuke et al. | [57] | 2022 | 83 | HNC | V50 (continously) V65 (continously) |
| Lee et al. | [58] | 2022 | 174 | OPC | V44 (continously) V58 (continously) |
| Yilmaz et al. | [41] | 2023 | 263 | NPC | V59.8 ≥ 36% Gy |
Abbreviations: HNC: Head and neck cancer; OPC: Oropharyngeal cancer; OCC: Oral cavity cancer; NPC; Nasopharyngeal cancer; ORNJ: Osteoradionecrosis of the jaw; Vx: Volume receiving X Gray or higher dose: Dx: Percentage of the prescription dose received by the X% of the mandible; Dmean: Mean dose: Dmax: Maximum dose; PTV: Planning target volume.
References
- Lerner, U.H. Bone remodeling in post-menopausal osteoporosis. J. Dent. Res. 2006, 85, 584–595.
- Reddy, M.S.; Morgan, S.L. Decreased bone mineral density and periodontal management. Periodontol. 2000. 2013, 61, 195–218.
- Von Wowern, N. Bone mass of mandibles. In vitro and in vivo analyses. Dan. Med. Bull. 1986, 33, 23–44.
- Kanis, J.A.; Melton, L.J., 3rd; Christiansen, C.; Johnston, C.C.; Khaltaev, N. The diagnosis of osteoporosis. J. Bone Miner. Res. 1994, 9, 1137–1141.
- Jonasson, G.; Rythén, M. Alveolar bone loss in osteoporosis: A loaded and cellular affair? Clin. Cosmet. Investig. Dent. 2016, 8, 95–103.
- Huja, S.S.; Fernandez, S.A.; Hill, K.J.; Li, Y. Remodeling dynamics in the alveolar process in skeletally mature dogs. Anat. Rec. Discov. Mol. Cell Evol Biol. 2006, 288, 1243–1249.
- Marx, R.E.; Cillo, J.E., Jr.; Ulloa, J.J. Oral bisphosphonate-induced osteonecrosis: Risk factors, prediction of risk using serum CTX testing, prevention, and treatment. J. Oral Maxillofac. Surg. 2007, 65, 2397–2410.
- Citrin, D.E.; Mitchell, J.B. Mechanisms of normal tissue injury from irradiation. Semin. Radiat. Oncol. 2017, 27, 316–324.
- Khan, M.A.; Van Dyk, J.; Yeung, I.W. Partial volume rat lung irradiation; assessment of early DNA damage in different lung regions and effect of radical scavengers. Radiother. Oncol. 2003, 66, 95–102.
- Choi, S.H.; Kim, M.; Lee, H.J. Effects of NOX1 on fibroblastic changes of endothelial cells in radiation-induced pulmonary fibrosis. Mol. Med. Rep. 2016, 13, 4135–4142.
- Hodgson, E.K.; Fridovich, I. The interaction of bovine erythrocyte superoxide dismutase with hydrogen peroxide: Chemiluminescence and peroxidation. Biochemistry 1975, 14, 5299–5303.
- Hayyan, M.; Hashim, M.A.; Al Nashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085.
- Martin, M.; Lefaix, J.; Delanian, S. TGF-beta1 and radiation fibrosis: A master switch and a specific therapeutic target? Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 277–290.
- Curi, M.M.; Cardoso, C.L.; de Lima, H.G.; Kowalski, L.P.; Martins, M.D. Histopathologic and histomorphometric analysis of irradiation injury in bone and the surrounding soft tissues of the jaws. J. Oral Maxillofac. Surg. 2016, 74, 190–199.
- Delanian, S.; Lefaix, J.L. The radiation-induced fibroatrophic process: Therapeutic perspective via the antioxidant pathway. Radiother. Oncol. 2004, 73, 119–131.
- Chandra, A.; Park, S.S.; Pignolo, R.J. Potential role of senescence in radiation-induced damage of the aged skeleton. Bone 2019, 120, 423–431.
- Herberg, S.; Kondrikova, G.; Hussein, K.A.; Periyasamy-Thandavan, S.; Johnson, M.H.; Elsalanty, M.E.; Shi, X.; Hamrick, M.W.; Isales, C.M.; Hill, W.D. Total body irradiation is permissive for mesenchymal stem cell-mediated new bone formation following local transplantation. Tissue Eng. Part A 2013, 20, 3212–3227.
- Zou, Q.; Hong, W.; Zhou, Y.; Ding, Q.; Wang, J.; Jin, W.; Gao, J.; Hua, G.; Xu, X. Bone marrow stem cell dysfunction in radiation-induced abscopal bone loss. J. Orthop. Surg. Res. 2016, 1, 3.
- Green, D.E.; Adler, B.J.; Chan, M.E.; Rubin, C.T. Devastation of adult stem cell pools by irradiation precedes collapse of trabecular bone quality and quantity. J. Bone Miner. Res. 2012, 27, 749–759.
- Preciado, S.; Muntión, S.; Rico, A.; Pérez-Romasanta, L.A.; Ramos, T.L.; Ortega, R.; Borrajo, J.; Corchete, L.A.; Rodríguez, C.; Díez-Campelo, M.; et al. Mesenchymal stromal cell irradiation interferes with the adipogenic/osteogenic differentiation balance and improves their hematopoietic-supporting ability. Biol. Blood Marrow Transplant. 2018, 24, 443–451.
- Hoffmann, L.; Marschner, S.N.; Kakoschke, T.K.; Hickel, R.; Sabbagh, H.; Wölfle, U.C. Dental management before radiotherapy of the head and neck region: 4-year single-center experience. Clin. Exp. Dent. Res. 2022, 8, 1478–1486.
- Marx, R.E.; Johnson, R.P. Studies in the radiobiology of osteoradionecrosis and their clinical significance. Oral Surg. Oral Med. Oral Pathol. 1987, 64, 379–390.
- Sun, K.; Tordjman, J.; Clement, K. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477.
- Rabbani, Z.N.; Mi, J.; Zhang, Y. Hypoxia inducible factor 1alpha signaling in fractionated radiation-induced lung injury: Role of oxidative stress and tissue hypoxia. Radiat. Res. 2010, 73, 165–174.
- Vujaskovic, Z.; Anscher, M.S.; Feng, Q.F. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int. J. Radiat. Oncol Biol. Phys. 2001, 50, 851–855.
- Barcellos-Hoff, M.H.; Dix, T.A. Redox-mediated activation of latent transforming growth factor-beta 1. Mol. Endocrinol. 1996, 10, 1077–1083.
- Yu, Q.; Stamenkovic, I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000, 14, 163–176.
- Rube, C.E.; Uthe, D.; Schmid, K.W. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1033–1042.
- Dieriks, B.; De Vos, W.H.; Derradji, H.; Baatout, S.; Van Oostveldt, P. Medium-mediated DNA repair response after ionizing radiation is correlated with the increase of specific cytokines in human fibroblasts. Mutat. Res. 2010, 687, 40–48.
- Barcellos-Hoff, M.H.; Cucinotta, F.A. New tricks for an old fox: Impact of TGFβ on the DNA damage response and genomic stability. Signal Sci. 2014, 7, re5.
- Lyons, A.; Ghazali, N. Osteoradionecrosis of the jaws: Current understanding of its pathophysiology and treatment. Br. J. Oral Maxillofac. Surg. 2008, 46, 653–660.
- Bras, J.; de Jonge, H.K.; van Merkesteyn, J.P. Osteoradionecrosis of the mandible: Pathogenesis. Am. J. Otolaryngol. 1990, 11, 244–250.
- Koudougou, C.; Bertin, H.; Lecaplain, B.; Badran, Z.; Longis, J.; Corre, P.; Hoornaert, A. Post implantation radiation therapy in head and neck cancer patients: Literature review. Head Neck. 2020, 42, 794–802.
- Lorimore, S.A.; Coates, P.J.; Scobie, G.E.; Miline, G.; Wright, E.G. Inflammation-type responses after exposure to ionizing radiation in vivo: A mechanism for radiation-induced bystander effects? Oncogene 2001, 20, 7085–7095.
- Willey, J.S.; Lloyd, S.A.; Robbins, M.E.; Bourland, J.D.; Smith-Sielicki, H.; Bowman, L.C.; Norrdin, R.W.; Bateman, T.A. Early increase in osteoclast number in mice after whole-body irradiation with 2 Gy X rays. Radiat. Res. 2008, 170, 388–392.
- Xu, J.; Zheng, Z.; Fang, D.; Gao, R.; Liu, Y.; Fan, Z.P.; Zhang, C.M.; Wang, S.L. Early-stage pathogenic sequence of jaw osteoradionecrosis in vivo. J. Dent. Res. 2012, 91, 702–708.
- Dekker, H.; Bravenboer, N.; van Dijk, D.; Bloemena, E.; Rietveld, D.H.F.; Ten Bruggenkate, C.M.; Schulten, E.A.J.M. The irradiated human mandible: A quantitative study on bone vascularity. Oral Oncol. 2018, 87, 126–130.
- Toneatti, D.J.; Graf, R.R.; Burkhard, J.P.; Schaller, B. Survival of dental implants and occurrence of osteoradionecrosis in irradiated head and neck cancer patients: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 5579–5593.
- Schiegnitz, E.; Reinicke, K.; Sagheb, K.; König, J.; Al-Nawas, B.; Grötz, K.A. Dental implants in patients with head and neck cancer-A systematic review and meta-analysis of the influence of radiotherapy on implant survival. Clin. Oral Implant Res. 2022, 33, 967–999.
- Kudo, M.; Matsui, Y.; Ohno, K.; Michi, K. A histomorphometric study of the tissue reaction around hydroxyapatite implants irradiated after placement. J. Oral Maxillofac. Surg. 2001, 59, 293–300.
- Yilmaz, B.; Somay, E.; Topkan, E.; Pehlivan, B.; Selek, U. Pre-chemoradiotherapy low hemoglobin levels indicate increased osteoradionecrosis risk in locally advanced nasopharyngeal cancer patients. Eur. Arch. Otorhinolaryngol. 2023, 280, 2575–2584.
- Maesschalck, T.; Dulguerov, N.; Caparrotti, F.; Scolozzi, P.; Picardi, C.; Mach, N.; Koutsouvelis, N.; Dulguerov, P. Comparison of the incidence of osteoradionecrosis with conventional radiotherapy and intensity-modulated radiotherapy. Head Neck 2016, 38, 1695–1702.
- Nguyen, N.P.; Vock, J.; Chi, A.; Ewell, L.; Vos, P.; Mills, M.; Khan, R.; Almeida, F.; Davis, R.; Betz, M.; et al. Effectiveness of intensity-modulated and image-guided radiotherapy to spare the mandible from excessive radiation. Oral Oncol. 2012, 48, 653–657.
- Moon, D.H.; Moon, S.H.; Wang, K.; Weissler, M.C.; Hackman, T.G.; Zanation, A.M.; Thorp, B.D.; Patel, S.N.; Zevallos, J.P.; Marks, L.B.; et al. Incidence of and risk factors for mandibular osteoradionecrosis in patients with oral cavity and oropharynx cancers. Oral Oncol. 2017, 72, 98–103.
- Aarup-Kristensen, S.; Hansen, C.R.; Forner, L.; Brink, C.; Eriksen, J.G.; Johansen, J. Osteoradionecrosis of the mandible after radiotherapy for head and neck cancer: Risk factors and dose-volume correlations. Acta Oncol. 2019, 58, 1373–1377.
- Balermpas, P.; van Timmeren, J.E.; Knierim, D.J.; Guckenberger, M.; Ciernik, I.F. Dental extraction, intensity-modulated radiotherapy of head and neck cancer, and osteoradionecrosis: A systematic review and meta-analysis. Strahlenther. Onkol. 2022, 98, 219–228.
- Zhang, W.; Zhang, X.; Yang, P.; Blanchard, P.; Garden, A.S.; Gunn, B.; Fuller, C.D.; Chambers, M.; Hutcheson, K.A.; Ye, R.; et al. Intensity-modulated proton therapy and osteoradionecrosis in oropharyngeal cancer. Radiother. Oncol. 2017, 123, 401–405.
- Singh, A.; Kitpanit, S.; Neal, B.; Yorke, E.; White, C.; Yom, S.K.; Randazzo, J.D.; Wong, R.J.; Huryn, J.M.; Tsai, C.J.; et al. Osteoradionecrosis of the jaw following proton radiation therapy for patients with head and neck cancer. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 151–159.
- Musha, A.; Shimada, H.; Kubo, N.; Kawamura, H.; Okano, N.; Sato, H.; Kaminuma, T.; Okada, K.; Anakura, M.; Adachi, A.; et al. Clinical features and dosimetric evaluation of carbon ion radiation-induced osteoradionecrosis of mandible in head and neck tumors. Radiother. Oncol. 2021, 61, 205–210.
- Kubota, H.; Miyawaki, D.; Mukumoto, N.; Ishihara, T.; Matsumura, M.; Hasegawa, T.; Akashi, M.; Kiyota, N.; Shinomiya, H.; Teshima, M.; et al. Risk factors for osteoradionecrosis of the jaw in patients with head and neck squamous cell carcinoma. Radiat. Oncol. 2021, 16, 1.
- MD Anderson Head and Neck Cancer Symptom Working Group. Dose-volume correlates of mandibular osteoradionecrosis in oropharynx cancer patients receiving intensity-modulated radiotherapy: Results from a case-matched comparison. Radiother. Oncol. 2017, 124, 232–239.
- De Felice, F.; Thomas, C.; Patel, V.; Connor, S.; Michaelidou, A.; Sproat, C.; Kwok, J.; Burke, M.; Reilly, D.; McGurk, M.; et al. Osteoradionecrosis following treatment for head and neck cancer and the effect of radiotherapy dosimetry: The Guy’s and St Thomas’ Head and Neck Cancer Unit experience. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, 28–34.
- van Dijk, L.V.; Abusaif, A.A.; Rigert, J.; Naser, M.A.; Hutcheson, K.A.; Lai, S.Y.; Fuller, C.D.; Mohamed, A.S.R.; On behalf on the MD Anderson Symptom Working Group. Normal Tissue Complication Probability (NTCP) prediction model for osteoradionecrosis of the mandible in patients with head and neck cancer after radiation therapy: Large-scale observational cohort. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 549–558.
- Tsai, C.J.; Hofstede, T.M.; Sturgis, E.M.; Garden, A.S.; Lindberg, M.E.; Wei, Q.; Tucker, S.L.; Dong, L. Osteoradionecrosis and radiation dose to the mandible in patients with oropharyngeal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 415–420.
- Caparrotti, F.; Huang, S.H.; Lu, L.; Bratman, S.V.; Ringash, J.; Bayley, A.; Cho, J.; Giuliani, M.; Kim, J.; Waldron, J.; et al. Osteoradionecrosis of the mandible in patients with oropharyngeal carcinoma treated with intensity-modulated radiotherapy. Cancer 2017, 123, 3691–3700.
- Lang, K.; Held, T.; Meixner, E.; Tonndorf-Martini, E.; Ristow, O.; Moratin, J.; Bougatf, N.; Freudlsperger, C.; Debus, J.; Adeberg, S. Frequency of osteoradionecrosis of the lower jaw after radiotherapy of oral cancer patients correlated with dosimetric parameters and other risk factors. Head Face Med. 2022, 18, 7.
- DeLuke, D.; Carrico, C.; Ray, C.; Stilianoudakis, S.; Holler, S.; Padilla, L.; Song, S. Is dose volume a better predictor of osteoradionecrosis risk than total dose for patients who have received head and neck radiation? J. Oral Maxillofac. Surg. 2022, 80, 1557–1563.
- Rogers, S.N.; D’Souza, J.J.; Lowe, D.; Kanatas, A. Longitudinal evaluation of health-related quality of life after osteoradionecrosis of the mandible. Br. J. Oral Maxillofac Surg. 2015, 53, 854–857.
More
