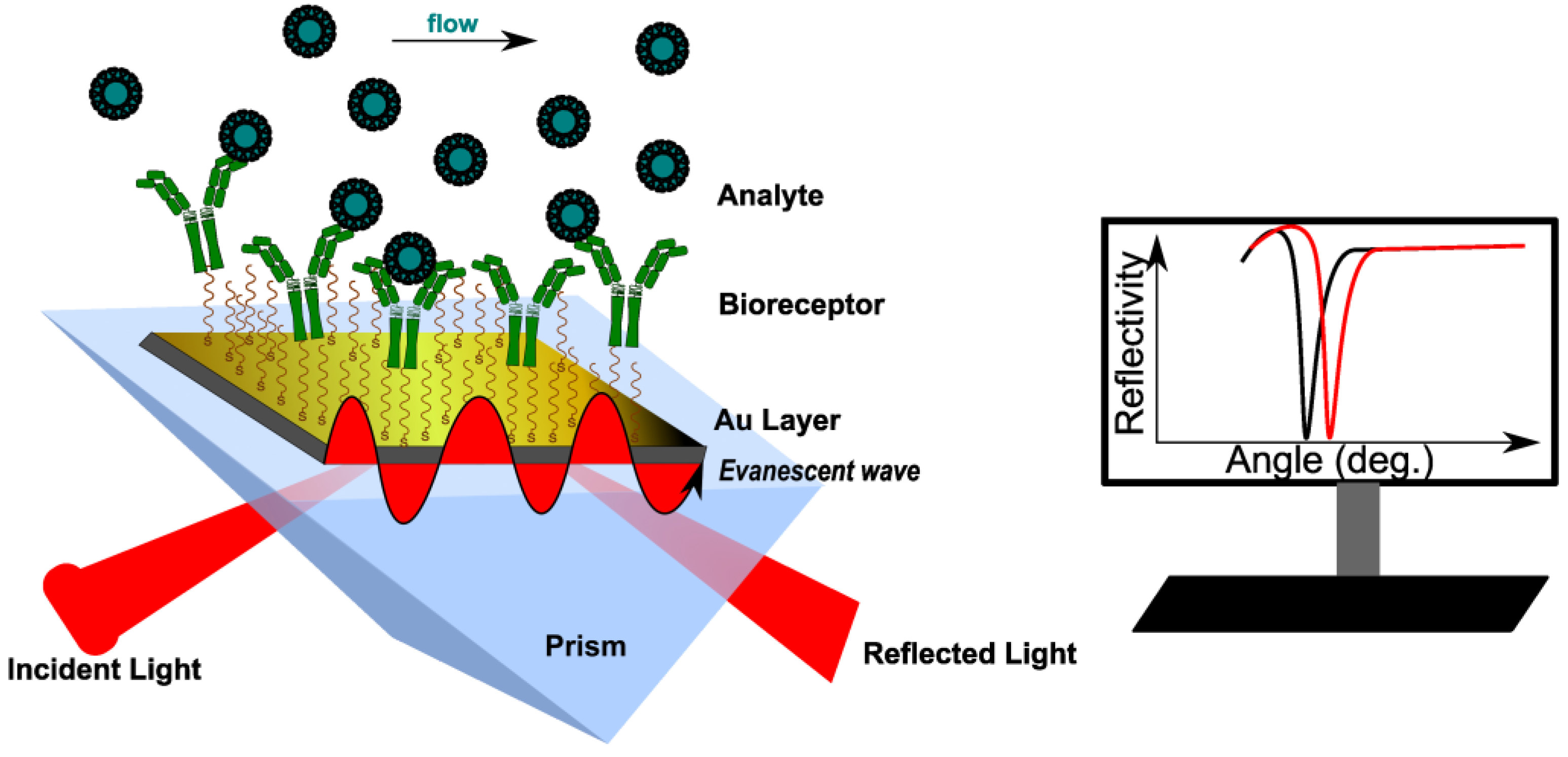
2. SPR Operating Principle
Surface plasmon resonance (SPR) is an optical method that measures changes in the refractive index (RI) of the medium in close proximity to a metallic surface, caused by binding events occurring at the surface
[6]. Most SPR devices use the Kretschmann configuration, where an incident polarized light beam goes through a prism on a sensor chip, which consists of two parts: glass and a metal layer (usually gold)
[8][9]. Surface plasmons (SPs) are generated because of the coupling of the incident oscillating electric field of the electromagnetic (EM) wave with the electrons at the metal/dielectric interface. When an SP couples with a photon, the resulting excitation is called surface plasmon polariton (SPP)
[10]. The incident light, after total internal reflection (TIR) within a glass prism, generates an electric field on the interface’s opposite site. The SPPs also create an electric field that extends into the medium on either side of the metallic film. This field is called an evanescent wave (EW) because the amplitude of the wave drops exponentially with the distance from the interface. The evanescent field wave and the incident light have the same wavelength. At a specific angle (SPR angle, φ), the SPPs can be excited to resonate for a given monochromatic light. In specific conditions, φ depends only on the refractive index (RI) of the medium (or the dielectric constant)
[11]. This causes a sharp dip in the intensity of the reflected light (
Figure 1). The most used sensing configurations are prism, grating, waveguide and fiber-optic, with two ways to excite the surface plasmons: attenuated total reflection (ATR) and diffraction.
Figure 1. Principle of conventional SPR biosensor with Kretschmann configuration. The analyte binding to the immobilized bioreceptor causes a change in RI near the surface, recorded as the shift of the resonance angle (here, measured at a fixed wavelength). RI reaches a constant value at equilibrium.
The sensitivity of an SPR sensor is defined as the ratio between the change in sensor output and the change in the refractive index. Thus, the response of the SPR sensor medium is sensitive to changes in the refractive index of the medium near the surface, changes that may be caused by affinity pairing, chemical reaction, adsorption/desorption, temperature, and pH
[12]. The SPR signal can be measured according to three main methodologies: angular, wavelength, and intensity (amplitude)
[10]. In a typical angular scanning SPR experiment, monochromatic light is directed to the metal surface at several incidence angles. The excitation of SPs causes light absorption, recorded as a dip in the angular spectrum of the reflected light
[13]. Data are further processed either by angular scanning of the incident light or by sensorgrams (angle shift versus time). In an intensity-modulated SPR experiment, RI variations are monitored as changes in the resonance intensity, at a fixed incidence angle and wavelength. This methodology is suitable for obtaining images with SPR sensors, and it is commonly called SPR imaging (SPRi)
[14]. In wavelength-modulated SPR measurements, the angle of the incident light is settled at a certain value, whereas the wavelength of the reflected light is adjusted
[9]. The reflected intensity dip is measured against the change in the refractive index over a range of incident wavelengths, and the wavelength with the strongest coupling is used as a sensor output.
Multi-parametric SPR (MP-SPR) was reported recently to enhance the SPR signal. In this case, two or more wavelengths were used; the thickness and refractive of different modified layers were determined by modelling the SPR signal at each wavelength
[15][16].
In a common SPR experiment, the SPR cell works either in batch or flow configuration; either way, the angle shift due to the surface modification is then plotted versus time. Batch configuration is less used because it requires longer time frames for signal stabilization. However, miniaturized instruments such as fiber optic surface plasmon resonance sensors (FO-SPR, where the prism is replaced by the core of an optical fiber) are better suited for monitoring interactions between immobilized ligands and different bacterial strains, similar to in vivo conditions
[17]. On the other hand, flow conditions are mostly used for real-time monitoring and the determination of kinetic parameters.
3. Surface Modification in SPR Assays: Coating Strategies
In SPR assays, the key stage is to modify the metal surface with different coatings, preparing the SPR chip for ligand immobilization. Before any use, the metallic surface should be cleaned and activated. The common substrate for SPR sensors is gold, due to its chemical stability and resistance to corrosion. Besides that, compounds containing thiol groups can be spontaneously deposited onto the surface, via gold–thiol chemistry. Some studies reported the use of silver as an SPR metal layer, but the main drawbacks were the chemical instability and fast oxidation following air exposure
[18]. For these reasons, gold is the preferred substrate in SPR assays.
3.1. Surface Activation
Generally, before coating with an organic layer, the metal must be chemically activated by removing all the contaminants (inorganic or organic) and allowing further modification. The most common surface pre-treatments are (a) immersion in piranha mixture (H
2SO
4/H
2O
2); (b) immersion in concentrated NaOH solution (2.5 M); (c) immersion ammonia-peroxide water mixture (NH
4OH/H
2O
2/H
2O); and (d) O
2-plasma etching
[19]. The roughest surfaces were obtained after piranha and ammonia-peroxide water treatments. Moreover, it was observed that the piranha solution provided the highest adsorption degree of organophosphorus acids. The disadvantages of using piranha solution for gold chip activation are changes in the morphological structure of the gold, an increase in surface hydrophilicity through the addition of hydroxyl groups, and finally, after more than one exposure, the occurrence of defects and breakdown of the gold film. On the other hand, oxygen plasma has been shown to remove organic contaminants just as well as the piranha mixture, and it can be used more than once
[20]. It also led to a smoother surface, with a uniform structure. After activation, linker molecules are deposited onto gold. Several common linkers serve different purposes, as will be shown further.
3.2. Thiol Anchoring
It is well known that gold possesses a high affinity for thiols, and the deposition of thiols on the surface is followed by the formation of self-assembled monolayers (SAMs). SAMs are usually the result of the spontaneous alignment of alkanethiols on gold. Different types of alkanethiol can be used, depending on the nature of terminal groups (-CH
3; -COOH; -NH
2; -OH), the number of methyl groups, and the aromatic or aliphatic structure
[21]; 11-mercaptoundecanoic acid (11-MUA), is the most used linker because of its hydrophilic nature. Moreover, the carboxyl end group can easily react with primary amine groups from antibodies
[22] and proteins
[23] via EDC (1-Ethyl-3-(3-dimethyl aminopropyl)carbodiimide)/NHS (N-hydroxysuccinimide) esters. A viable strategy for obtaining a larger surface contact with bioreceptors is the use of mixtures of 11-MUA and short-chain thiols such as 1-octane thiol
[22] or 3-mercaptopropionic acid
[24]. Ataman Sadik et al. reported a mixed self-assembled monolayer composed of 3,3′-dithiodipropionic acid di(N-hydroxysuccinimide ester) (DSP) and 6-mercapto-1-hexanol (MCH) that reduced the steric hindrance and minimized the non-specific interactions
[25]. Even if DSP and MCH have the same length, the binding degree of bovine serum albumin (BSA) and lysozyme to DSP was higher. The same research group has developed an immunosensor for thrombin by immobilizing anti-thrombin antibodies on the DSP/MCH layer. The linear range was 1.0–500.0 nM
[25][26]. However, there are some disadvantages of metal coating with thiol-SAMs. First, SAMs are not stable when they are stored for a long period at room temperature. There is also a risk that the thiol groups may be oxidized. Furthermore, thiol anchoring is a time-consuming process, requiring at least 12 h.
Another approach is the direct attachment of a biomolecule of already modified thiol group at one end to the gold chip. Wang et al.
[27] studied the direct attachment of a specific aptamer for detecting
Escherichia coli (
E. coli) and
Staphylococcus aureus (
S. aureus). They studied the thickness of the immobilized short-chain aptamer in the presence of different concentrations of NaCl and observed that at a lower concentration, the aptamer thickness increased. Moreover, the addition of NaBr led to an increase in the aptamer density. Droz et al. studied three different strategies to immobilize ssDNA on gold: thiol (DNA-SH) chemosorption, dithiocarbamate (DNA-DTC) chemisorption, and physical absorption. MCH was added afterwards to reduce the non-specific adsorption and block the pin-hole defects. From all three, DNA-SH showed the highest stability in both homogeneous and MCH mixed layers, while DNA-DTC was prone to desorption in the presence of MCH
[28]. Simon et al. deposited a peptide-nucleic acid (PNA) layer by micro-spotting thiolated PNA directly to bare Au. They monitored the high-affinity interaction between PNA and microRNA. It was observed, by comparing the PNA strands prehybridized with a complementary DNA strand and the ssPNA, that the prehybridized PNA strands exhibited a wide dynamic range for microRNA and the lowest amount obtained was 140 fmol
[29]. Direct attachment of biomolecules on gold surfaces showed some disadvantages such as non-specific interactions and physical adsorption, but the one-step immobilization is less time-consuming and involves fewer reagents.
3.3. Dextran-Based Hydrogel Attachment
Carboxymethylated dextran (CMD) is often used for surface modification, especially for protein immobilization. Compared with SAMs, carboxymethylated dextrans are not strictly oriented, offering a higher degree of freedom for interactions with bioreceptors. Dextran is a branched polysaccharide modified with carboxymethyl groups that can easily react with free-amino functional groups of a biomolecule. CMD hydrogels present some interesting properties. Because of their ramified structure, more functional groups are available for interaction. Moreover, they have a 3D structure, which can be correlated to a larger surface and more binding sites. Another advantage associated with their structure is the minimization of non-specific binding
[30]. Compared with SAMs, which are thin layers (less than 2 nm), the thickness of CMDs is between 100 and 200 nm, which can lower the SPR response and sensitivity
[31]. Another drawback is that the functional groups from the CMD are not distributed uniformly, and this can hinder the linkage with the bioreceptor. The most common CMD-modified chips are available commercially from Biacore (CM5)
[32][33].
3.4. Silane Attachment
Silicon-based biosensors are based on alkoxysilane SAMs. The silanization reaction takes place only if the surface has been previously treated to contain hydroxyl groups. Silane layers can be added to different surfaces such as glass, silicon, or gold
[34][35][36]. SPR substrates covered with silicon oxides (SiO
2) or silicon nitrides are preferred because of the ease and cost-effectiveness of their processing. First, the surface needs to be activated to obtain hydroxyl groups for further reactions with silanes. Afterwards, the silane is added to bind (3-aminopropyl)triethoxysilane (APTES), which is a silane coupling agent
[37][38]. APTES has one end-functionalized with an amino group, while the other end is a silane group. The thickness of the silane layer grows with the concentration of added APTES
[36]. Moreover, different end groups can be attached to the silane such as aldehydes, cyano (C=N) groups, thiols, poly(ethylenglicol) (PEG), carboxylic acids and others
[39].