Bladder cancer is one of the most common malignancies of the urinary tract and can be divided into non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC). Emerging evidence demonstrates that long noncoding RNAs play a crucial role in the carcinogenesis and progression of bladder cancer. Long intergenic noncoding RNAs (lincRNAs) are a subgroup of long noncoding RNAs (lncRNAs) that do not overlap protein-coding genes. Small nucleolar RNAs (snoRNAs) are a class of noncoding RNAs (ncRNAs) that mainly exist in the nucleolus, are approximately 60–300 nucleotides in length, and are hosted inside the introns of genes. Small nucleolar RNA host genes (SNHGs) have been associated with the origin and development of bladder cancer.
1. Introduction
Bladder cancer (BLCA) is the fifth most common malignancy and the second most common urological malignancy globally. With increasing incidence and mortality rates, BLCA was estimated to account for 81,180 new cases and 17,100 deaths in 2022
[1]. Approximately 70% of patients are diagnosed with non-muscle-invasive bladder cancer (NMIBC), most of whom are treated with transurethral resection of the bladder tumour (TURBT) and have a 5-year survival rate greater than 85%. The other 30% of patients are diagnosed with muscle-invasive BC (MIBC), need to receive comprehensive treatment, and have a low 5-year survival rate
[2].
Non-protein coding genes, which account for approximately 98% of the human genome, were long regarded as useless genes
[3][6]. However, with the development of next-generation sequencing technology, researchers have started to turn their attention to this unexplored area. Among ncRNAs, transport RNA (tRNA), ribosomal RNA (rRNA) and messenger RNA (mRNA) are well known for being widely involved in cellular processes. The remaining ncRNAs account for a small proportion with diverse classes, including small nuclear RNA (snRNA), small nucleolar RNA (snoRNA), microRNA (miRNA), PiWi-interacting RNA (piRNA) and long noncoding RNA (lncRNA)
[4][5][7,8].
SnoRNAs are small RNAs with a length of 60–300 nucleotides that are predominantly located on the nucleolus. SnoRNAs belong to the following two major families according to secondary structure and signature sequence: the C/D box snoRNA family and the H/ACA box snoRNA family. The major function of SnoRNAs is acting as guide RNAs for posttranscriptional modification of rRNA, tRNA and snRNAs. However, in the past few years, an increasing number of studies have demonstrated that the dysregulation of snoRNAs is also involved in regulating oncogenesis and cellular processes
[6][7][8][9][9,10,11,12].
The lncRNA family (>200 nt in length) is a large and multifunctional family. Most lncRNAs are located in the nucleus. LncRNAs can directly interact with DNA, therefore regulating gene transcription or influencing RNA splicing through binding with RNA binding proteins. In the cytoplasm, lncRNAs are also involved in multiple processes, including translation, RNA localization and posttranslational modification
[3][10][6,14]. To date, the function of most lncRNAs has not been identified; thus, the classification of lncRNAs relies on their location with respect to protein-coding genes, which are categorized as antisense, overlapping, intronic, host genes, intergenic lncRNAs (lincRNA) and enhancer RNAs. LincRNA is a subgroup of lncRNA and is transcribed from protein noncoding genomic regions
[11][15].
2. Pathophysiology of BLCA
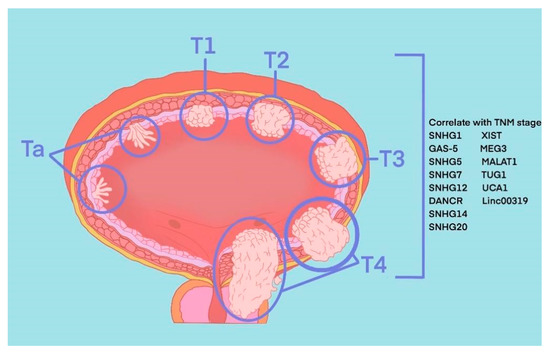
The most common pathological type of BLCA is bladder urothelial carcinoma. According to the depth of tumour invasion to bladder mucosa, BLCA can be divided into NMIBC and MIBC. Specifically, tumours at the Ta stage, T1 stage and carcinoma in situ (CIS) belong to NMIBC, and tumours at T3 and T4 stages belong to MIBC. These two types represent two periods of occurrence and development of BLCA, and their treatment methods and prognosis of them are quite different. TURBT and intravesical chemotherapy are the main treatment methods of the former, while the latter requires radical cystectomy or systemic treatment. Recently, in the published literature indicated 8 SNHGs (SNHG1, SNHG2, SNHG5, SNHG7, SNHG12-14 and SNHG20) and 6 lincRNAs (linc0001, linc00023, linc00047, linc00080, linc00178 and linc00319) that correlate with the TNM stage of BLCA (
Figure 1).
Figure 1.
SNHGs and lincRNAs that correlate with TNM stage of BLCA.
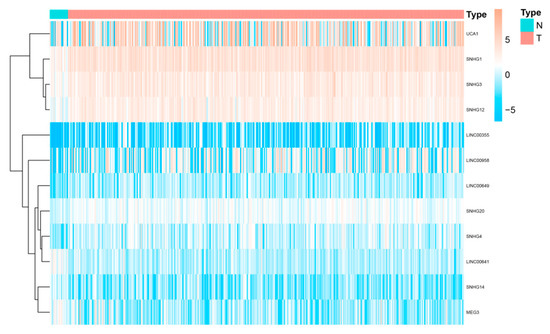
We downloaded RNA-seq data from The Cancer Genome Atlas bladder cancer (TCGA-BLCA) dataset. Then “limma” package was used to explore necroptosis-related differentially expressed genes (DEGs) between normal and tumour samples under a false discovery rate (FDR) < 0.05 and |log2fold change (FC) >1|. SNHG14, linc00023 and linc00641 were high expressed in BLCA samples, while SNHG1, SNHG3, SNHG4, SNHG12, SMHG209, linc00355, linc00649, linc00958 and linc00178 were expressed at low levels (
Figure 2).
Figure 2. Differentially expressed SNHGs and lincRNAs between BLCA and paired normal samples.
3. SnoRNA and lincRNA
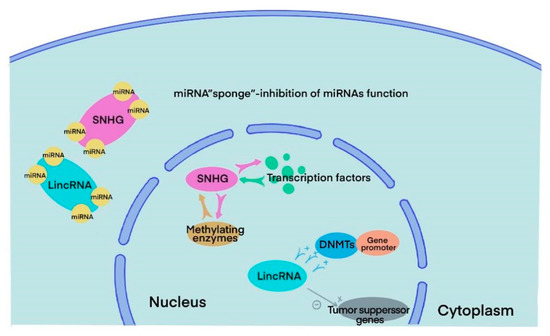
SnoRNAs are highly conserved RNAs that can be traced back to archaea and eukaryotes 20–30 billion years ago. They are mainly localized in the nucleus and have a length of 60–300 nt [12]. The main functions of snoRNAs include guiding methylation and pseudouridylation of rRNA, alternative splicing of mRNA, telomere synthesis and other unknown cell processes [3]. There are the following two main families of snoRNAs according to their secondary structure and signature sequence: C/D boxes and H/ACA boxes. All snoRNAs from the two families need to bind to highly conserved core proteins to form snoRNPs and function in vivo. In the C/D box family, the core proteins NOP5, NOP5 and NHPX form a bridge that links C/D guide RNA and the methyltransferase fibrillarin. The C/D RNP guides 2′-O-ribose methylation. In the H/ACA box family, RNA can directly interact with the pseudouridine synthase dyskerin and three core proteins, GAR1, NOP10 and NHP2, forming H/ACA BNP and guiding pseudouridylation [6][7][13]. In humans, snoRNAs are generated from host genes, especially SNHGs. SNHGs can act in several ways with different subcellular compartment localizations. Inside the nucleus, it can directly interact with transcription factors and methylating enzymes to modulate gene transcription. In the cytoplasm, it can sponge miRNA, directly bind to mRNA or interact with proteins to regulate translation [14] ( Differentially expressed SNHGs and lincRNAs between BLCA and paired normal samples.
3. SnoRNA and lincRNA
SnoRNAs are highly conserved RNAs that can be traced back to archaea and eukaryotes 20–30 billion years ago. They are mainly localized in the nucleus and have a length of 60–300 nt [18]. The main functions of snoRNAs include guiding methylation and pseudouridylation of rRNA, alternative splicing of mRNA, telomere synthesis and other unknown cell processes [6]. There are the following two main families of snoRNAs according to their secondary structure and signature sequence: C/D boxes and H/ACA boxes. All snoRNAs from the two families need to bind to highly conserved core proteins to form snoRNPs and function in vivo. In the C/D box family, the core proteins NOP5, NOP5 and NHPX form a bridge that links C/D guide RNA and the methyltransferase fibrillarin. The C/D RNP guides 2′-O-ribose methylation. In the H/ACA box family, RNA can directly interact with the pseudouridine synthase dyskerin and three core proteins, GAR1, NOP10 and NHP2, forming H/ACA BNP and guiding pseudouridylation [9,10,19]. In humans, snoRNAs are generated from host genes, especially SNHGs. SNHGs can act in several ways with different subcellular compartment localizations. Inside the nucleus, it can directly interact with transcription factors and methylating enzymes to modulate gene transcription. In the cytoplasm, it can sponge miRNA, directly bind to mRNA or interact with proteins to regulate translation [13] ().
Figure 3.
Major functions of SNHGs and lincRNAs in BLCA cells.
LincRNAs are transcribed between two protein-coding genes with a length of more than 200 nt. Although lincRNAs do not code proteins directly, they play an important role in multiple cellular processes like other ncRNAs. Most lincRNAs are located in the nucleus rather than the cytoplasm and may impact malignant progression in various ways. Several lincRNAs can control gene expression by regulating chromatin in cis and in trans. In the nucleus, lincRNAs could not only facilitate the bind between DNA methyltransferases (DNMTs) and gene promoters, inhibiting the expression of specific genes but also directly silence tumour suppressor genes.
4. The Role of snoRNA in BLCA
SNHG1, which is located at chromosome 11q12.3, is overexpressed in several types of tumours, including pancreatic, prostate, non-small-cell lung and BLCA [15][16][17][18]. It is widely considered an oncogene that promotes cancer proliferation, migration, invasion and tumorigenesis. SNHG1 can bind and coregulate with the PP2A catalytic subunit (PP2A-c) to promote c-Jun phosphorylation. Then, activated c-Jun increases matrix metalloproteinase 2 (MMP2) transcription, which induces cancer cell invasion and metastasis. Additionally, transcription of miR-34a was downregulated by SNHG1 via autophagy, thus maintaining MM2 mRNA stabilization [15].
SNHG1, which is located at chromosome 11q12.3, is overexpressed in several types of tumours, including pancreatic, prostate, non-small-cell lung and BLCA [21,22,23,24]. It is widely considered an oncogene that promotes cancer proliferation, migration, invasion and tumorigenesis. SNHG1 can bind and coregulate with the PP2A catalytic subunit (PP2A-c) to promote c-Jun phosphorylation. Then, activated c-Jun increases matrix metalloproteinase 2 (MMP2) transcription, which induces cancer cell invasion and metastasis. Additionally, transcription of miR-34a was downregulated by SNHG1 via autophagy, thus maintaining MM2 mRNA stabilization [21].
SNHG2, which is also known as growth arrest-specific transcript 5 (GAS-5), is located at chromosome 1q25 and acts as a tumour suppressor in multiple cancers [19][20][21][22]. Studies have found that SNHG2 is expressed at low levels in BLCA cells and tissues and that the expression level of SNHG2 is markedly correlated with the clinical characteristics and prognosis of BLCA. High expression of SNHG2 promotes BLCA cell apoptosis and inhibits tumour proliferation. SNHG2 directly interacts with E2F transcription factor 4 (E2F4) and recruits it to the enhancer of the zeste homolog 2 (EZH2) promoter, thereby downregulating transcription of the EZH2 oncogene [19]. In the BLCA cell line HTB-9, SNHG2 sponges miR-21, thus promoting transcription of its downstream gene phosphatase and tensin homolog (PTEN), leading to the downregulation of antiapoptotic proteins and cell cycle-associated proteins, which suppresses proliferation and increases apoptosis of bladder cancer cells [20]. According to previous studies, downregulation of SNHG2 could also directly arrest the cell cycle at the S phase in a cyclin-dependent kinase 6 (CDK6)-dependent manner [21].
SNHG2, which is also known as growth arrest-specific transcript 5 (GAS-5), is located at chromosome 1q25 and acts as a tumour suppressor in multiple cancers [26,27,28,29]. Studies have found that SNHG2 is expressed at low levels in BLCA cells and tissues and that the expression level of SNHG2 is markedly correlated with the clinical characteristics and prognosis of BLCA. High expression of SNHG2 promotes BLCA cell apoptosis and inhibits tumour proliferation. SNHG2 directly interacts with E2F transcription factor 4 (E2F4) and recruits it to the enhancer of the zeste homolog 2 (EZH2) promoter, thereby downregulating transcription of the EZH2 oncogene [26]. In the BLCA cell line HTB-9, SNHG2 sponges miR-21, thus promoting transcription of its downstream gene phosphatase and tensin homolog (PTEN), leading to the downregulation of antiapoptotic proteins and cell cycle-associated proteins, which suppresses proliferation and increases apoptosis of bladder cancer cells [27]. According to previous studies, downregulation of SNHG2 could also directly arrest the cell cycle at the S phase in a cyclin-dependent kinase 6 (CDK6)-dependent manner [28].
SNHG3 is also located at chromosome 1 but is generally considered an oncogene. Studies have demonstrated that SNHG3 is upregulated in BLCA cells and tissue and is positively correlated with poor clinicopathological characteristics and prognosis [23]. Overexpression of SNHG3 in the BLCA cell line facilitated cell growth, metastasis and tumorigenesis through the SNHG3/c-MYC/BMI1 axis. The knockdown of SNHG3 significantly suppressed the proliferation of BLCA both in vivo and in vitro. Interestingly, the knockdown of SNHG3 barely induced lower transcription of c-MYC in vivo [24]. Furthermore, SNHG3 also promotes the expression of Go-Ichi-Ni-San2 (GINS2), a subunit of the cell cycle regulating complex GINS, by acting as a sponge of miR-515-5p, thus promoting tumour proliferation and epithelial-mesenchymal transition (EMT) [25]. SNHG3 is also located at chromosome 1 but is generally considered an oncogene. Studies have demonstrated that SNHG3 is upregulated in BLCA cells and tissue and is positively correlated with poor clinicopathological characteristics and prognosis [30]. Overexpression of SNHG3 in the BLCA cell line facilitated cell growth, metastasis and tumorigenesis through the SNHG3/c-MYC/BMI1 axis. The knockdown of SNHG3 significantly suppressed the proliferation of BLCA both in vivo and in vitro. Interestingly, the knockdown of SNHG3 barely induced lower transcription of c-MYC in vivo [31]. Furthermore, SNHG3 also promotes the expression of Go-Ichi-Ni-San2 (GINS2), a subunit of the cell cycle regulating complex GINS, by acting as a sponge of miR-515-5p, thus promoting tumour proliferation and epithelial-mesenchymal transition (EMT) [32].
SNHG5 and SNHG6 are oncogenes located at 6q14.3 and 8q13.1, respectively. The dysregulation of these two genes was found to be closely related to tumour growth and metastasis in multiple cancers [26][27]. Nevertheless, there is still a lack of research on the functions of SNHG5 and SNHG6 in bladder cancer. Ma et al. noted that SNHG5 was upregulated in both BLCA tissues and cell lines, and higher expression of SNHG5 was markedly correlated with poorer tumour clinicopathological features and overall survival (OS). SNHG5 and SNHG6 are oncogenes located at 6q14.3 and 8q13.1, respectively. The dysregulation of these two genes was found to be closely related to tumour growth and metastasis in multiple cancers [33,34]. Nevertheless, there is still a lack of research on the functions of SNHG5 and SNHG6 in bladder cancer. Ma et al. noted that SNHG5 was upregulated in both BLCA tissues and cell lines, and higher expression of SNHG5 was markedly correlated with poorer tumour clinicopathological features and overall survival (OS).
SNHG7 and SNHG20 are novel host genes located at chromosome 9q34.3 and 17q25.2, respectively. Upregulation of SNHG7 and SNHG20 has been connected to the progression of several cancers, including BLCA, and linked to increased invasive capacity along with poor OS [28][29]. Proliferation, proapoptotic and EMT-related protein levels were regulated when SNHG7 was knocked down. The levels of PCNA and Ki-67 (proliferation-related), Bax and cleaved caspase 3 (proapoptotic-related) were elevated, while MMP2, MMP7 and E-cadherin (EMT-related) were reduced [30]. SNHG7 and SNHG20 are novel host genes located at chromosome 9q34.3 and 17q25.2, respectively. Upregulation of SNHG7 and SNHG20 has been connected to the progression of several cancers, including BLCA, and linked to increased invasive capacity along with poor OS [36,37]. Proliferation, proapoptotic and EMT-related protein levels were regulated when SNHG7 was knocked down. The levels of PCNA and Ki-67 (proliferation-related), Bax and cleaved caspase 3 (proapoptotic-related) were elevated, while MMP2, MMP7 and E-cadherin (EMT-related) were reduced [38].
SNHG13 (also known as DANCR) is located at chromosome 4q12 and was first identified in primary human keratinocytes in 2012 [31]. Recent studies have found that SNHG13 is aberrantly upregulated in BLCA tissues and cells and accounts for the proliferation and metastasis of BLCA cells. In the cytoplasm, SNHG13 can directly interact with leucine-rich pentatricopeptide repeat containing (LRPPRC) and stabilize interleukin 11 (IL-11), plasminogen activator urokinase (PLAU) and CCND1 mRNAs. The enhanced expression of IL-11 led to the activation of the IL-11/JAK/STAT3 axis and drove the proliferation, invasion and migration of BLCA cells. PLAU and CCND1 play important roles in tumour cell migration and the cell cycle, respectively [32].
SNHG13 (also known as DANCR) is located at chromosome 4q12 and was first identified in primary human keratinocytes in 2012 [40]. Recent studies have found that SNHG13 is aberrantly upregulated in BLCA tissues and cells and accounts for the proliferation and metastasis of BLCA cells. In the cytoplasm, SNHG13 can directly interact with leucine-rich pentatricopeptide repeat containing (LRPPRC) and stabilize interleukin 11 (IL-11), plasminogen activator urokinase (PLAU) and CCND1 mRNAs. The enhanced expression of IL-11 led to the activation of the IL-11/JAK/STAT3 axis and drove the proliferation, invasion and migration of BLCA cells. PLAU and CCND1 play important roles in tumour cell migration and the cell cycle, respectively [41].
5. The Role of lincRNA in BLCA
Linc00001, also known as X-inactive specific transcript (XIST), is located on chromosome Xq13.2. It was first found in the process of X chromosome inactivation, which occurs at the early development stage of mammalian females, and was later shown to act as an oncogene and to be upregulated in multiple types of cancers, including BLCA [33][50]. Recent studies have revealed that XIST can regulate cancer cell progression by acting as a sponge of miRNAs and binding to proteins [34][35][36][37][51,52,53,54]. Specifically, the expression of XIST was elevated in BLCA tissues and cell lines, including T24, 253J, RT112 and HT-1376. XIST silencing induced the loss of proliferation, metastasis and stemness capability combined with the overexpression of miR-200c and miR-133a in vitro [36][37][53,54].
Linc00023 is located on chromosome 14q32.2 within the DLK-MEG3 locus and is also widely known as the maternally expressed 3 gene (MEG3). The deregulation of linc00023 was reported to be involved in the progression of various tumours, including meningioma, hepatocellular cancer, breast cancer and BLCA [38][39][55,56]. Similarly, MEG3 can also act as a ceRNA to regulate tumour cell proliferation and apoptosis. In bladder cancer, MEG3 was found to function as a ceRNA for PTEN by competitively binding miR-494, thus repressing the proliferation, migration and invasion and promoting the apoptosis of tumour cells [40][57].
Linc00047 is also known as metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) and has a length of 8.5 kb. It is located on chromosome 11q13, is involved in the regulation of several molecular signalling pathways and has been shown to be a potential biomarker in bladder cancer, nasopharyngeal carcinoma and osteosarcoma [41][42][61,62]. The expression of MALAT1 was significantly elevated in BLCA tissues and cell lines and was positively correlated with advanced clinical stage and poor prognosis [43][63]. In addition, MALAT1 sponged miR-101-3p and suppressed the expression of VEGF-C in BLCA 5637 and EJ-M3 cell lines, enhancing cisplatin sensitivity. Furthermore, the knockdown of MALAT1 in cisplatin-resistant 5637 and EJ-M3 cells could reverse drug resistance [44][64].
Linc00080, also known as taurine-upregulated gene 1 (TUG1), is located on chromosome 22q12 and is almost twice as elevated in bladder tissues compared to adjacent normal tissues. It is broadly considered an oncogene in several tumours, such as colorectal cancer, oesophageal cancer, osteosarcoma and bladder cancer [45][66]. In BLCA, TUG1 is involved in tumour proliferation, metastasis and apoptosis, as well as radioresistance and chemotherapy resistance [46][47][48][49][50][67,68,69,70,71]. Silencing TUG1 in BLCA cell lines restrains the tendency of EMT by acting as a ceRNA of ZEB2 by sponging miR-145 and leading to radioresistance [46][67]. It also promotes the expression of high mobility group box 1 protein (HMGB1), thus enhancing bladder cancer radioresistance in vivo and in vitro, yet the mechanism needs further exploration [47][68].
Linc00178 is located on 19p13.12 and was first identified in bladder transitional cell carcinoma; thus, linc00178 is also known as urothelial cancer associated 1 (UCA1). Recent studies have verified the overexpression and oncogene functions of UCA1 in different tumours [51][72]. Specifically, UCA1 can serve as a sponge of miRNAs to regulate tumour growth, metastasis, drug resistance and mitochondrial functions. Li et al. found that knockdown of UCA1 in the BLCA cell line 5637 decreased mitochondrial DNA copy number by more than half with accompanying decreased ATP production. The results of a UCA1 overexpression experiment on the BLCA cell line UMUC2 were highly consistent with those of the knockdown experiments.
Linc00319 is a novel lncRNA located on chromosome 21q22.3 that has been implicated in the tumorigenesis and progression of cervical cancer, gastric cancer, osteosarcoma and laryngeal squamous cell carcinoma in a miRNA-dependent manner [52][53][54][55][77,78,79,80]. Moreover, linc00319 expression levels were remarkably higher in BLCA tissues than in adjacent normal tissues. Patients with higher linc00319 levels had higher clinical stages and lower recurrence-free survival rates [56][81].
Linc00355 is located on chromosome 13q21.31 and has been validated to function as a ceRNA by sponging miRNAs in lung squamous cell carcinoma, glioma and hepatocellular carcinoma [57][58][59][83,84,85]. In BLCA, linc00355 could act as a sponge of miR-424-5p to modulate high mobility group AT-hook 2 (HMGA2) expression, which could regulate the EMT-related proteins ZEB1, E-cadherin and vimentin and finally contribute to BLCA EMT and lung metastasis [60][86].
Linc00649 is located on 21q22.11 and is widely considered an oncogene in multiple tumours, such as lung squamous cell carcinoma, breast cancer and gastric cancer [61][62][63][94,95,96]. A recent study found that linc00649 is a basement membrane-related lncRNA and is correlated with clinical prognosis based on analysis of transcriptional and clinical data of bladder cancer from the TCGA, GEO and BM-BASE databases, revealing that linc00649 is a potential biomarker of BLCA. Additionally, a model containing eight lncRNAs, including linc00649, was constructed and used to accurately predict the prognosis of BLCA patients [64][97].
Linc00958 is located on 11p15.3 and was found to be substantially expressed in bladder cancer tissues compared to normal bladder epithelial tissues. Increasingly, studies have demonstrated that linc00958 is involved in the malignant progression of various cancers, such as hepatocellular carcinoma, colorectal cancer, osteosarcoma and endometrial cancer [65][100]. Anna et al. identified 72 BLCA tissues and 8 normal bladder epithelial tissues by RNA sequencing and selected five significantly dysregulated lncRNAs, including linc00958, for further analysis. They found that the knockdown of linc00958 led to a loss of cell mobility in vitro.