DNA damage in astronauts induced by cosmic radiation poses a major barrier to human space exploration. Cellular responses and repair of the most lethal DNA double-strand breaks (DSBs) are crucial for genomic integrity and cell survival. Post-translational modifications (PTMs), including phosphorylation, ubiquitylation, and SUMOylation, are among the regulatory factors modulating a delicate balance and choice between predominant DSB repair pathways, such as non-homologous end joining (NHEJ) and homologous recombination (HR). In thiRes review, we earchers focused on the engagement of proteins in the DNA damage response (DDR) modulated by phosphorylation and ubiquitylation, including ATM, DNA-PKcs, CtIP, MDM2, and ubiquitin ligases. The involvement and function of acetylation, methylation, PARylation, and their essential proteins were also investigated, providing a repository of candidate targets for DDR regulators. However, there is a lack of radioprotectors in spite of their consideration in the discovery of radiosensitizers. We proposed new perspectives for the research and development of future agents against space radiation by the systematic integration and utilization of evolutionary strategies, including multi-omics analyses, rational computing methods, drug repositioning, and combinations of drugs and targets, which may facilitate the use of radioprotectors in practical applications in human space exploration to combat fatal radiation hazards.
- radiation protection
- space radiation
- DSB
- post-translational modification
- drug target
- drug discovery
1. Introduction
2. PTMs in the Choice of DNA Repair Pathways
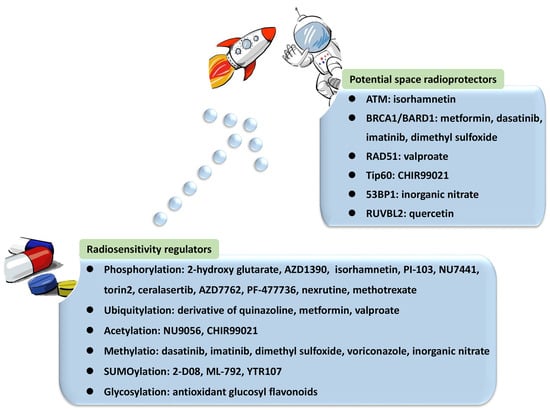
HR is a critical pathway for the error-free repair of DNA DSBs, while NHEJ always occurs in the absence of a sister chromatid, leading to error-prone repair and more mutations [42,43,44][42][43][44]. NHEJ was reported to be the predominant DNA repair pathway in mammalian cells [45]. The choice of the repair pathway was found to be tightly associated with the cell cycle, as NHEJ is the default repair pathway [46] usually executed in the G1 phase of the cell cycle in a rapid and high-capacity manner [42,47][42][47]. Unlike NHEJ, which may occur throughout the entire cell cycle, HR is largely limited to the S/G2 phases [42,48][42][48] and is conducted more slowly than NHEJ [47]. The underlying mechanism is that DSB repair is executed with higher efficiency during the S phase. DSB processing and checkpoint activation are much more efficient in the G2/M phase than in the G1 phase [49]. In general, the 5′-3′ degradation of DSB ends is needed for the loading of checkpoint and recombination proteins in all HR reactions [50,51][50][51]. The generation of long 3′ single-strand DNA (ssDNA) overhangs mediated by DNA helicases and exonuclease in DNA end resection was proven to be an essential committed process in HR [47,48,52,53,54][47][48][52][53][54]. In contrast, NHEJ requires DNA ends that have not been resected instead of 3′ ssDNA tails. DNA end resection is not needed, leading to the joining of two DNA ends with few references to the DNA sequence [47,48,55,56][47][48][55][56]. Therefore, controlling DNA end resection is one of the processes affecting whether DNA repair is conducted by NHEJ or HR [35,40][35][40]. For example, the 53BP1-RIF1-shieldin complex cooperates with the CTC1-STN1-TEN1 (CST)/Pol α-Prim complex in regulating the generation of 3′ overhangs, which are essential for DNA end protection and switching the DSB repair mode to NHEJ [53,57][53][57]. In contrast, BRCA1 promotes HR and antagonizes NHEJ by stimulating end resection [58,59][58][59]. Several key proteins and their complexes play regulatory roles in NHEJ. For instance, the Ku70-Ku80 heterodimer is central in initiating NHEJ by recognizing DSB ends and recruiting DNA-PKcs to DSB sites [51,60,61,62,63][51][60][61][62][63]. In addition, 53BP1 stimulates NHEJ by recruiting other DDR proteins such as ATM and inhibiting DNA end resection processing by protecting broken DNA ends with its co-factors PTIP, RIF1-shieldin, or REV7/MAD2L2 [48,56,61,62,64,65,66,67][48][56][61][62][64][65][66][67]. In contrast, the important factors in HR mainly include BRCA1/2, EXO1, MRE11 [47,48,64,68[47][48][64][68][69],69], and RAD51 and its paralogs [43,47,69,70][43][47][69][70]. Among them, BRCA1 directly affects the DSB repair pathway choice by regulating the initiation of end resection [52,59][52][59]. The preservation of long-term resection activity requires EXO1 exonuclease [71], the deficiency of which contributes to the accumulation of unprocessed DSBs and HR failure [72]. MRE11 exonuclease activity is needed for the assembly of a series of proteins to DSB sites to mediate extended-end resection for HR [73]. HR is orchestrated by several PTMs with elaborate primary mechanisms. The first one is phosphorylation. Switching the meiotic recombination mode of HR was reported to occur by the phosphorylation of RAD54 and HED1, downregulating RAD51 activity by suppressing Rad51/Rad54 complex formation [74,75][74][75]. Secondly, SUMOylation is important in HR. It affects all steps in HR and exerts various regulatory functions on substrates [76]. Evidence indicated that SUMOylation induced by topoisomerase 1-binding arginine/serine-rich protein (TOPORS) was essential for the recruitment of RAD51 to the damaged sites and the support of HR repair, maintaining genomic stability [77]. On the other hand, NHEJ might be associated with phosphorylation and methylation by DNA-PKcs and 53BP1, respectively. NHEJ and HR are competitive, and their balance is finely modulated by bioprocesses that include PTMs. Ubiquitination is the most vital PTM, playing a specific role in the recruitment and enrichment of DDR factors at DSB sites in chromosomes and governing DNA repair pathway choices between NHEJ and HR. DDR proteins are mainly assembled by ubiquitin E3 ligases RNF8 and RNF168, followed by accurate repair processes [35]. The ubiquitylation-dependent DSB repair pathway choice is frequently associated with DNA end resection. For example, Cullin3-KLHL15 ubiquitin ligase participates in CtIP protein turnover through the ubiquitin–proteasome pathway, fine-tuning DNA end resection and impacting the balance between HR and NHEJ [78]. RING domain-containing E3 ligase RNF138 is involved in the ubiquitination of Ku80 during the S phase and its removal from DSB sites, stimulating DSB end resection and promoting HR initiation [79]. In addition to DNA end resection, ubiquitylation also modulates the choice of DNA repair pathways by altering the expression of specific DDR proteins. CtIP, which is a target of anaphase-promoting complex/cyclosome (APC/C) ubiquitin ligase, is downregulated during G1 and G2 phases and reduces HR [80]. CtIP ubiquitylation and upregulation are stimulated by UBE2Ds and RNF138 at DNA damage sites, promoting DNA repair by HR [81]. Moreover, ubiquitination affects the DNA repair pathway choice by regulating histone H2A at Lys15 (H2AK15ub) and initiating downstream signaling events [82]. Phosphorylation is another major PTM involved in the balance of DNA repair pathways. The phosphorylation of ubiquitin at Thr12 (pUbT12) influences DDR by regulating the activity of 53BP1 in damaged chromosomes [83], and 53BP1 inhibits excessive DNA end resection and promotes repair by NHEJ through different phosphoprotein interactions [84]. RIF1 is prominent at DSB sites in the G1 phase of the cell cycle by the ATM-associated phosphorylation of 53BP1, ensuring the dominant position of NHEJ in this phase [58,59,85][58][59][85]. Collectively, a variety of proteins and their complexes were revealed to act in the complicated response mechanisms to DNA lesions induced by IR, participating in distinct PTMs and coordinating NHEJ, HR, and their balance in DNA repair (Figure 1). These essential factors could be properly categorized and investigated from the view of PTMs, including phosphorylation, ubiquitylation, acetylation, and methylation. Compounds targeting these factors influence DNA repair after IR, leading to radiosensitization or radioprotective effects (Table 1). Some of them have been approved for regulating DDR, and more candidates are under development. They provide resources in the discovery of future space radioprotectors (Figure 2).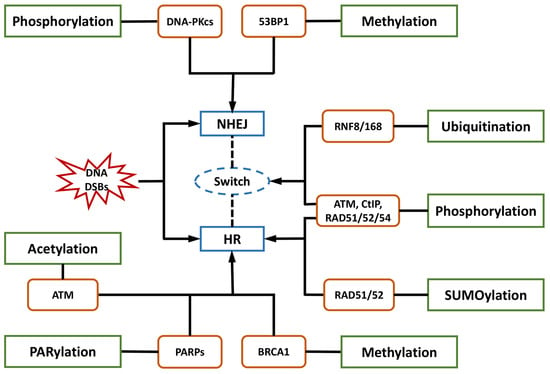
| Post-Translational Modification (PTM) | Essential Factor | Description | Application | Reference |
|---|---|---|---|---|
| Phosphorylation | ATM |
|
|
[86,87,88,89,90,91,92,93][86][87][88][89][90][91][92][93] |
| DNA-PKcs |
|
|
[88,94,95,96][88][94][95][96] | |
| CtIP |
|
|
[42,97,98,99][42][97][98][99] | |
| ATR |
|
|
[100,101][100][101] | |
| CHK1 |
|
|
[101,102,103][101][102][103] | |
| RAD51/52/54 |
|
|
[43,70,104,105,106,107][43][70][104][105][106][107] | |
| H2AX |
|
/ | [108,109][108][109] | |
| Ubiquitylation | RNF8 |
|
|
[110,111,112][110][111][112] |
| RNF168 |
|
|
[47,110,111,113,114,115,116][47][110][111][113][114][115][116] | |
| REV1 |
|
|
[117,118,119,120][117][118][119][120] | |
| MDM2 |
|
|
[89,121,122,123,124][89][121][122][123][124] | |
| BRCA1/BARD1 |
|
|
[83,125,126,127][83][125][126][127] | |
| RAD51 |
|
|
[128] | |
| Acetylation | Tip60 |
|
|
[90,129,130,[129][130131][90]][131] |
| Methylation | BRCA1 |
|
|
[132,133,134,135][132][133][134][135] |
| 53BP1 |
|
|
[136,137][136][137] | |
| PARylation | PARP1 |
|
|
[138,139][138][139] |
| Neddylation | NEDD8 |
|
|
[140,141,142,143][140][141][142][143] |
| SUMOylation | / |
|
|
[144] |
| MEIIL3 |
|
|
[145] | |
| NPM1 |
|
|
[146] | |
| Glycosylation | OGT |
|
|
[147,148][147][148] |
| NEIL3 |
|
|
[149,150,151,152][149][150][151][152] | |
| RUVBL1/2 |
|
|
[150,153][150][153] | |
| Kcr | CDYL1 |
|
/ | [154] |
| CBP/P300 |
|
|
[155,156,155][156]157,[157]158][[158] |
3. Targeting Essential Phosphorylation Factors for Regulating DDR
References
- Ramos, R.L.; Carante, M.P.; Ferrari, A.; Sala, P.; Vercesi, V.; Ballarini, F. A Mission to Mars: Prediction of GCR Doses and Comparison with Astronaut Dose Limits. Int. J. Mol. Sci. 2023, 24, 2328.
- Drago-Ferrante, R.; Di Fiore, R.; Karouia, F.; Subbannayya, Y.; Das, S.; Aydogan Mathyk, B.; Arif, S.; Guevara-Cerdán, A.P.; Seylani, A.; Galsinh, A.S.; et al. Extraterrestrial Gynecology: Could Spaceflight Increase the Risk of Developing Cancer in Female Astronauts? An Updated Review. Int. J. Mol. Sci. 2022, 23, 7465.
- Schroeder, M.K.; Liu, B.; Hinshaw, R.G.; Park, M.-A.; Wang, S.; Dubey, S.; Liu, G.G.; Shi, Q.; Holton, P.; Reiser, V.; et al. Long-Term Sex- and Genotype-Specific Effects of 56Fe Irradiation on Wild-Type and APPswe/PS1dE9 Transgenic Mice. Int. J. Mol. Sci. 2021, 22, 13305.
- Hamada, N.; Sato, T. Cataractogenesis following high-LET radiation exposure. Mutat. Res. 2016, 770, 262–291.
- Rudobeck, E.; Bellone, J.A.; Szücs, A.; Bonnick, K.; Mehrotra-Carter, S.; Badaut, J.; Nelson, G.A.; Hartman, R.E.; Vlkolinský, R. Low-dose proton radiation effects in a transgenic mouse model of Alzheimer’s disease—Implications for space travel. PLoS ONE 2017, 12, e0186168.
- Coleman, M.A.; Sasi, S.P.; Onufrak, J.; Natarajan, M.; Manickam, K.; Schwab, J.; Muralidharan, S.; Peterson, L.E.; Alekseyev, Y.O.; Yan, X.; et al. Low-dose radiation affects cardiac physiology: Gene networks and molecular signaling in cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1947–H1963.
- Barcellos-Hoff, M.H.; Blakely, E.A.; Burma, S.; Fornace, A.J.; Gerson, S.; Hlatky, L.; Kirsch, D.G.; Luderer, U.; Shay, J.; Wang, Y.; et al. Concepts and challenges in cancer risk prediction for the space radiation environment. Life Sci. Space Res. 2015, 6, 92–103.
- Cucinotta, F.A. Flying without a Net: Space Radiation Cancer Risk Predictions without a Gamma-ray Basis. Int. J. Mol. Sci. 2022, 23, 4324.
- Poignant, F.; Plante, I.; Patel, Z.S.; Huff, J.L.; Slaba, T.C. Geometrical Properties of the Nucleus and Chromosome Intermingling Are Possible Major Parameters of Chromosome Aberration Formation. Int. J. Mol. Sci. 2022, 23, 8638.
- Yachi, Y.; Matsuya, Y.; Yoshii, Y.; Fukunaga, H.; Date, H.; Kai, T. An Analytical Method for Quantifying the Yields of DNA Double-Strand Breaks Coupled with Strand Breaks by γ-H2AX Focus Formation Assay Based on Track-Structure Simulation. Int. J. Mol. Sci. 2023, 24, 1386.
- Hu, A.; Zhou, W.; Wu, Z.; Zhang, H.; Li, J.; Qiu, R. Modeling of DNA Damage Repair and Cell Response in Relation to p53 System Exposed to Ionizing Radiation. Int. J. Mol. Sci. 2022, 23, 11323.
- Hirose, E.; Noguchi, M.; Ihara, T.; Yokoya, A. Mitochondrial Metabolism in X-Irradiated Cells Undergoing Irreversible Cell-Cycle Arrest. Int. J. Mol. Sci. 2023, 24, 1833.
- Wang, F.; Bing, Z.; Zhang, Y.; Ao, B.; Zhang, S.; Ye, C.; He, J.; Ding, N.; Ye, W.; Xiong, J.; et al. Quantitative proteomic analysis for radiation-induced cell cycle suspension in 92-1 melanoma cell line. J. Radiat. Res. 2013, 54, 649–662.
- Albi, E.; Cataldi, S.; Lazzarini, A.; Codini, M.; Beccari, T.; Ambesi-Impiombato, F.S.; Curcio, F. Radiation and Thyroid Cancer. Int. J. Mol. Sci. 2017, 18, 911.
- Nie, Q.; Huan, X.; Kang, J.; Yin, J.; Zhao, J.; Li, Y.; Zhang, Z. MG149 Inhibits MOF-Mediated p53 Acetylation to Attenuate X-ray Radiation-Induced Apoptosis in H9c2 Cells. Radiat. Res. 2022, 198, 590–598.
- Tinganelli, W.; Luoni, F.; Durante, M. What can space radiation protection learn from radiation oncology? Life Sci. Space Res. 2021, 30, 82–95.
- Putt, K.S.; Du, Y.; Fu, H.; Zhang, Z.-Y. High-throughput screening strategies for space-based radiation countermeasure discovery. Life Sci. Space Res. 2022, 35, 88–104.
- Cheema, A.K.; Mehta, K.Y.; Fatanmi, O.O.; Wise, S.Y.; Hinzman, C.P.; Wolff, J.; Singh, V.K. A Metabolomic and Lipidomic Serum Signature from Nonhuman Primates Administered with a Promising Radiation Countermeasure, Gamma-Tocotrienol. Int. J. Mol. Sci. 2017, 19, 79.
- Gan, L.; Wang, Z.; Si, J.; Zhou, R.; Sun, C.; Liu, Y.; Ye, Y.; Zhang, Y.; Liu, Z.; Zhang, H. Protective effect of mitochondrial-targeted antioxidant MitoQ against iron ion 56Fe radiation induced brain injury in mice. Toxicol. Appl. Pharmacol. 2018, 341, 1–7.
- Yang, P.; Luo, X.; Li, J.; Zhang, T.; Gao, X.; Hua, J.; Li, Y.; Ding, N.; He, J.; Zhang, Y.; et al. Ionizing Radiation Upregulates Glutamine Metabolism and Induces Cell Death via Accumulation of Reactive Oxygen Species. Oxid. Med. Cell. Longev. 2021, 2021, 5826932.
- Moreno-Villanueva, M.; Wong, M.; Lu, T.; Zhang, Y.; Wu, H. Interplay of space radiation and microgravity in DNA damage and DNA damage response. NPJ Microgravity 2017, 3, 14.
- Li, W.; Ge, C.; Yang, L.; Wang, R.; Lu, Y.; Gao, Y.; Li, Z.; Wu, Y.; Zheng, X.; Wang, Z.; et al. CBLB502, an Agonist of Toll-Like Receptor 5, has Antioxidant and Scavenging Free Radicals Activities in vitro. Int. J. Biol. Macromol. 2016, 82, 97–103.
- Hosseinimehr, S.J. The protective effects of trace elements against side effects induced by ionizing radiation. Radiat. Oncol. J. 2015, 33, 66–74.
- Burns, F.J.; Tang, M.; Frenkel, K.; Nádas, A.; Wu, F.; Uddin, A.; Zhang, R. Induction and prevention of carcinogenesis in rat skin exposed to space radiation. Radiat. Environ. Biophys. 2007, 46, 195–199.
- Li, X.M.; Tan, Y.; Huang, C.Q.; Xu, M.C.; Li, Q.; Pan, D.; Zhao, B.Q.; Hu, B.R. MMP Inhibitor Ilomastat Improves Survival of Mice Exposed to γ-Irradiation. Biomed. Environ. Sci. BES 2018, 31, 467–472.
- Clemente, A.; Sonnante, G.; Domoney, C. Bowman-Birk inhibitors from legumes and human gastrointestinal health: Current status and perspectives. Curr. Protein Pept. Sci. 2011, 12, 358–373.
- Kennedy, A.R.; Zhou, Z.; Donahue, J.J.; Ware, J.H. Protection against adverse biological effects induced by space radiation by the Bowman-Birk inhibitor and antioxidants. Radiat. Res. 2006, 166, 327–332.
- Garg, S.; Garg, T.K.; Wise, S.Y.; Fatanmi, O.O.; Miousse, I.R.; Savenka, A.V.; Basnakian, A.G.; Singh, V.K.; Hauer-Jensen, M. Effects of Gamma-Tocotrienol on Intestinal Injury in a GI-Specific Acute Radiation Syndrome Model in Nonhuman Primate. Int. J. Mol. Sci. 2022, 23, 4643.
- Singh, V.K.; Hauer-Jensen, M. γ-Tocotrienol as a Promising Countermeasure for Acute Radiation Syndrome: Current Status. Int. J. Mol. Sci. 2016, 17, 663.
- Lulli, M.; Witort, E.; Papucci, L.; Torre, E.; Schiavone, N.; Dal Monte, M.; Capaccioli, S. Coenzyme Q10 protects retinal cells from apoptosis induced by radiation in vitro and in vivo. J. Radiat. Res. 2012, 53, 695–703.
- Li, J.; Xu, J.; Xu, W.; Qi, Y.; Lu, Y.; Qiu, L.; Hu, Z.; Chu, Z.; Chai, Y.; Zhang, J. Protective Effects of Hong Shan Capsule against Lethal Total-Body Irradiation-Induced Damage in Wistar Rats. Int. J. Mol. Sci. 2015, 16, 18938–18955.
- Li, J.; Feng, L.; Xing, Y.; Wang, Y.; Du, L.; Xu, C.; Cao, J.; Wang, Q.; Fan, S.; Liu, Q.; et al. Radioprotective and antioxidant effect of resveratrol in hippocampus by activating Sirt1. Int. J. Mol. Sci. 2014, 15, 5928–5939.
- Sekiguchi, M.; Matsushita, N. DNA Damage Response Regulation by Histone Ubiquitination. Int. J. Mol. Sci. 2022, 23, 8187.
- Casari, E.; Rinaldi, C.; Marsella, A.; Gnugnoli, M.; Colombo, C.V.; Bonetti, D.; Longhese, M.P. Processing of DNA Double-Strand Breaks by the MRX Complex in a Chromatin Context. Front. Mol. Biosci. 2019, 6, 43.
- Uckelmann, M.; Sixma, T.K. Histone ubiquitination in the DNA damage response. DNA Repair 2017, 56, 92–101.
- Mladenov, E.; Paul-Konietzko, K.; Mladenova, V.; Stuschke, M.; Iliakis, G. Increased Gene Targeting in Hyper-Recombinogenic LymphoBlastoid Cell Lines Leaves Unchanged DSB Processing by Homologous Recombination. Int. J. Mol. Sci. 2022, 23, 9180.
- Argunhan, B.; Iwasaki, H.; Tsubouchi, H. Post-translational modification of factors involved in homologous recombination. DNA Repair 2021, 104, 103114.
- Yu, F.; Wei, J.; Cui, X.; Yu, C.; Ni, W.; Bungert, J.; Wu, L.; He, C.; Qian, Z. Post-translational modification of RNA m6A demethylase ALKBH5 regulates ROS-induced DNA damage response. Nucleic. Acids Res. 2021, 49, 5779–5797.
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705.
- Tang, M.; Li, S.; Chen, J. Ubiquitylation in DNA double-strand break repair. DNA Repair 2021, 103, 103129.
- Huang, Y.-C.; Yuan, W.; Jacob, Y. The Role of the TSK/TONSL-H3.1 Pathway in Maintaining Genome Stability in Multicellular Eukaryotes. Int. J. Mol. Sci. 2022, 23, 9029.
- Yun, M.H.; Hiom, K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature 2009, 459, 460–463.
- Suwaki, N.; Klare, K.; Tarsounas, M. RAD51 paralogs: Roles in DNA damage signalling, recombinational repair and tumorigenesis. Semin. Cell Dev. Biol. 2011, 22, 898–905.
- Cannan, W.J.; Pederson, D.S. Mechanisms and Consequences of Double-Strand DNA Break Formation in Chromatin. J. Cell Physiol. 2016, 231, 3–14.
- Audebert, M.; Salles, B.; Calsou, P. Involvement of poly (ADP-ribose) polymerase-1 and XRCC1/DNA ligase III in an alternative route for DNA double-strand breaks rejoining. J. Biol. Chem. 2004, 279, 55117–55126.
- Shibata, A.; Conrad, S.; Birraux, J.; Geuting, V.; Barton, O.; Ismail, A.; Kakarougkas, A.; Meek, K.; Taucher-Scholz, G.; Löbrich, M.; et al. Factors determining DNA double-strand break repair pathway choice in G2 phase. EMBO J. 2011, 30, 1079–1092.
- Scully, R.; Panday, A.; Elango, R.; Willis, N.A. DNA double-strand break repair-pathway choice in somatic mammalian cells. Nat. Rev. Mol. Cell Biol. 2019, 20, 698–714.
- Xu, Y.; Xu, D. Repair pathway choice for double-strand breaks. Essays Biochem. 2020, 64, 765–777.
- Zierhut, C.; Diffley, J.F.X. Break dosage, cell cycle stage and DNA replication influence DNA double strand break response. EMBO J. 2008, 27, 1875–1885.
- Costelloe, T.; Louge, R.; Tomimatsu, N.; Mukherjee, B.; Martini, E.; Khadaroo, B.; Dubois, K.; Wiegant, W.W.; Thierry, A.; Burma, S.; et al. The yeast Fun30 and human SMARCAD1 chromatin remodellers promote DNA end resection. Nature 2012, 489, 581–584.
- Chanut, P.; Britton, S.; Coates, J.; Jackson, S.P.; Calsou, P. Coordinated nuclease activities counteract Ku at single-ended DNA double-strand breaks. Nat. Commun. 2016, 7, 12889.
- Daley, J.M.; Niu, H.; Miller, A.S.; Sung, P. Biochemical mechanism of DSB end resection and its regulation. DNA Repair 2015, 32, 66–74.
- Mirman, Z.; Lottersberger, F.; Takai, H.; Kibe, T.; Gong, Y.; Takai, K.; Bianchi, A.; Zimmermann, M.; Durocher, D.; de Lange, T. 53BP1-RIF1-shieldin counteracts DSB resection through CST- and Polα-dependent fill-in. Nature 2018, 560, 112–116.
- Peterson, S.E.; Li, Y.; Wu-Baer, F.; Chait, B.T.; Baer, R.; Yan, H.; Gottesman, M.E.; Gautier, J. Activation of DSB processing requires phosphorylation of CtIP by ATR. Mol. Cell 2013, 49, 657–667.
- Zimmermann, M.; de Lange, T. 53BP1: Pro choice in DNA repair. Trends Cell Biol. 2014, 24, 108–117.
- Panier, S.; Boulton, S.J. Double-strand break repair: 53BP1 comes into focus. Nat. Rev. Mol. Cell Biol. 2014, 15, 7–18.
- Ghezraoui, H.; Oliveira, C.; Becker, J.R.; Bilham, K.; Moralli, D.; Anzilotti, C.; Fischer, R.; Deobagkar-Lele, M.; Sanchiz-Calvo, M.; Fueyo-Marcos, E.; et al. 53BP1 cooperation with the REV7-shieldin complex underpins DNA structure-specific NHEJ. Nature 2018, 560, 122–127.
- Escribano-Díaz, C.; Orthwein, A.; Fradet-Turcotte, A.; Xing, M.; Young, J.T.F.; Tkáč, J.; Cook, M.A.; Rosebrock, A.P.; Munro, M.; Canny, M.D.; et al. A cell cycle-dependent regulatory circuit composed of 53BP1-RIF1 and BRCA1-CtIP controls DNA repair pathway choice. Mol. Cell 2013, 49, 872–883.
- Chapman, J.R.; Barral, P.; Vannier, J.-B.; Borel, V.; Steger, M.; Tomas-Loba, A.; Sartori, A.A.; Adams, I.R.; Batista, F.D.; Boulton, S.J. RIF1 is essential for 53BP1-dependent nonhomologous end joining and suppression of DNA double-strand break resection. Mol. Cell 2013, 49, 858–871.
- Spagnolo, L.; Rivera-Calzada, A.; Pearl, L.H.; Llorca, O. Three-dimensional structure of the human DNA-PKcs/Ku70/Ku80 complex assembled on DNA and its implications for DNA DSB repair. Mol. Cell 2006, 22, 511–519.
- Lee, J.-H.; Paull, T.T. Cellular functions of the protein kinase ATM and their relevance to human disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 796–814.
- Bartek, J.; Lukas, C.; Lukas, J. Checking on DNA damage in S phase. Nat. Rev. Mol. Cell Biol. 2004, 5, 792–804.
- Liang, L.; Deng, L.; Nguyen, S.C.; Zhao, X.; Maulion, C.D.; Shao, C.; Tischfield, J.A. Human DNA ligases I and III, but not ligase IV, are required for microhomology-mediated end joining of DNA double-strand breaks. Nucleic Acids Res. 2008, 36, 3297–3310.
- Chapman, J.R.; Sossick, A.J.; Boulton, S.J.; Jackson, S.P. BRCA1-associated exclusion of 53BP1 from DNA damage sites underlies temporal control of DNA repair. J. Cell Sci. 2012, 125, 3529–3534.
- Fradet-Turcotte, A.; Canny, M.D.; Escribano-Díaz, C.; Orthwein, A.; Leung, C.C.Y.; Huang, H.; Landry, M.-C.; Kitevski-LeBlanc, J.; Noordermeer, S.M.; Sicheri, F.; et al. 53BP1 is a reader of the DNA-damage-induced H2A Lys 15 ubiquitin mark. Nature 2013, 499, 50–54.
- Zhou, Y.; Caron, P.; Legube, G.; Paull, T.T. Quantitation of DNA double-strand break resection intermediates in human cells. Nucleic Acids Res. 2014, 42, e19.
- Barazas, M.; Annunziato, S.; Pettitt, S.J.; de Krijger, I.; Ghezraoui, H.; Roobol, S.J.; Lutz, C.; Frankum, J.; Song, F.F.; Brough, R.; et al. The CST Complex Mediates End Protection at Double-Strand Breaks and Promotes PARP Inhibitor Sensitivity in BRCA1-Deficient Cells. Cell Rep. 2018, 23, 2107–2118.
- Turan, V.; Oktay, K. BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging. Hum. Reprod. Update 2020, 26, 43–57.
- Pishvaian, M.J.; Blais, E.M.; Brody, J.R.; Rahib, L.; Lyons, E.; De Arbeloa, P.; Hendifar, A.; Mikhail, S.; Chung, V.; Sohal, D.P.S.; et al. Outcomes in Patients With Pancreatic Adenocarcinoma With Genetic Mutations in DNA Damage Response Pathways: Results From the Know Your Tumor Program. JCO Precis. Oncol. 2019, 3, 1–10.
- Zhao, Y.; Chen, S. Targeting DNA Double-Strand Break (DSB) Repair to Counteract Tumor Radio-resistance. Curr. Drug Targets 2019, 20, 891–902.
- Zhu, Z.; Chung, W.-H.; Shim, E.Y.; Lee, S.E.; Ira, G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell 2008, 134, 981–994.
- Mimitou, E.P.; Symington, L.S. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature 2008, 455, 770–774.
- Truong, L.N.; Li, Y.; Shi, L.Z.; Hwang, P.Y.-H.; He, J.; Wang, H.; Razavian, N.; Berns, M.W.; Wu, X. Microhomology-mediated End Joining and Homologous Recombination share the initial end resection step to repair DNA double-strand breaks in mammalian cells. Proc. Natl. Acad. Sci. USA 2013, 110, 7720–7725.
- Niu, H.; Wan, L.; Busygina, V.; Kwon, Y.; Allen, J.A.; Li, X.; Kunz, R.C.; Kubota, K.; Wang, B.; Sung, P.; et al. Regulation of meiotic recombination via Mek1-mediated Rad54 phosphorylation. Mol. Cell 2009, 36, 393–404.
- Tsubouchi, H.; Roeder, G.S. Budding yeast Hed1 down-regulates the mitotic recombination machinery when meiotic recombination is impaired. Genes. Dev. 2006, 20, 1766–1775.
- Dhingra, N.; Zhao, X. Advances in SUMO-based regulation of homologous recombination. Curr. Opin. Genet. Dev. 2021, 71, 114–119.
- Hariharasudhan, G.; Jeong, S.-Y.; Kim, M.-J.; Jung, S.M.; Seo, G.; Moon, J.-R.; Lee, S.; Chang, I.-Y.; Kee, Y.; You, H.J.; et al. TOPORS-mediated RAD51 SUMOylation facilitates homologous recombination repair. Nucleic Acids Res. 2022, 50, 1501–1516.
- Ferretti, L.P.; Himmels, S.-F.; Trenner, A.; Walker, C.; von Aesch, C.; Eggenschwiler, A.; Murina, O.; Enchev, R.I.; Peter, M.; Freire, R.; et al. Cullin3-KLHL15 ubiquitin ligase mediates CtIP protein turnover to fine-tune DNA-end resection. Nat. Commun. 2016, 7, 12628.
- Ismail, I.H.; Gagné, J.-P.; Genois, M.-M.; Strickfaden, H.; McDonald, D.; Xu, Z.; Poirier, G.G.; Masson, J.-Y.; Hendzel, M.J. The RNF138 E3 ligase displaces Ku to promote DNA end resection and regulate DNA repair pathway choice. Nat. Cell Biol. 2015, 17, 1446–1457.
- Lafranchi, L.; de Boer, H.R.; de Vries, E.G.E.; Ong, S.-E.; Sartori, A.A.; van Vugt, M.A.T.M. APC/C(Cdh1) controls CtIP stability during the cell cycle and in response to DNA damage. EMBO J. 2014, 33, 2860–2879.
- Schmidt, C.K.; Galanty, Y.; Sczaniecka-Clift, M.; Coates, J.; Jhujh, S.; Demir, M.; Cornwell, M.; Beli, P.; Jackson, S.P. Systematic E2 screening reveals a UBE2D-RNF138-CtIP axis promoting DNA repair. Nat. Cell Biol. 2015, 17, 1458–1470.
- Becker, J.R.; Clifford, G.; Bonnet, C.; Groth, A.; Wilson, M.D.; Chapman, J.R. BARD1 reads H2A lysine 15 ubiquitination to direct homologous recombination. Nature 2021, 596, 433–437.
- Walser, F.; Mulder, M.P.C.; Bragantini, B.; Burger, S.; Gubser, T.; Gatti, M.; Botuyan, M.V.; Villa, A.; Altmeyer, M.; Neri, D.; et al. Ubiquitin Phosphorylation at Thr12 Modulates the DNA Damage Response. Mol. Cell 2020, 80, 423–436.e9.
- Callen, E.; Di Virgilio, M.; Kruhlak, M.J.; Nieto-Soler, M.; Wong, N.; Chen, H.-T.; Faryabi, R.B.; Polato, F.; Santos, M.; Starnes, L.M.; et al. 53BP1 mediates productive and mutagenic DNA repair through distinct phosphoprotein interactions. Cell 2013, 153, 1266–1280.
- Feng, L.; Fong, K.-W.; Wang, J.; Wang, W.; Chen, J. RIF1 counteracts BRCA1-mediated end resection during DNA repair. J. Biol. Chem. 2013, 288, 11135–11143.
- Burma, S.; Chen, B.P.; Murphy, M.; Kurimasa, A.; Chen, D.J. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J. Biol. Chem. 2001, 276, 42462–42467.
- Zha, S.; Guo, C.; Boboila, C.; Oksenych, V.; Cheng, H.-L.; Zhang, Y.; Wesemann, D.R.; Yuen, G.; Patel, H.; Goff, P.H.; et al. ATM damage response and XLF repair factor are functionally redundant in joining DNA breaks. Nature 2011, 469, 250–254.
- Chen, B.P.C.; Uematsu, N.; Kobayashi, J.; Lerenthal, Y.; Krempler, A.; Yajima, H.; Löbrich, M.; Shiloh, Y.; Chen, D.J. Ataxia telangiectasia mutated (ATM) is essential for DNA-PKcs phosphorylations at the Thr-2609 cluster upon DNA double strand break. J. Biol. Chem. 2007, 282, 6582–6587.
- Cheng, Q.; Chen, J. Mechanism of p53 stabilization by ATM after DNA damage. Cell Cycle Georget. Tex. 2010, 9, 472–478.
- Sun, Y.; Xu, Y.; Roy, K.; Price, B.D. DNA damage-induced acetylation of lysine 3016 of ATM activates ATM kinase activity. Mol. Cell Biol. 2007, 27, 8502–8509.
- Kery, M.; Papandreou, I. Emerging strategies to target cancer metabolism and improve radiation therapy outcomes. Br. J. Radiol. 2020, 93, 20200067.
- Durant, S.T.; Zheng, L.; Wang, Y.; Chen, K.; Zhang, L.; Zhang, T.; Yang, Z.; Riches, L.; Trinidad, A.G.; Fok, J.H.L.; et al. The brain-penetrant clinical ATM inhibitor AZD1390 radiosensitizes and improves survival of preclinical brain tumor models. Sci. Adv. 2018, 4, eaat1719.
- Nishiyama, Y.; Morita, A.; Tatsuta, S.; Kanamaru, M.; Sakaue, M.; Ueda, K.; Shono, M.; Fujita, R.; Wang, B.; Hosoi, Y.; et al. Isorhamnetin Promotes 53BP1 Recruitment through the Enhancement of ATM Phosphorylation and Protects Mice from Radiation Gastrointestinal Syndrome. Genes 2021, 12, 1514.
- Djuzenova, C.S.; Fischer, T.; Katzer, A.; Sisario, D.; Korsa, T.; Steussloff, G.; Sukhorukov, V.L.; Flentje, M. Opposite effects of the triple target (DNA-PK/PI3K/mTOR) inhibitor PI-103 on the radiation sensitivity of glioblastoma cell lines proficient and deficient in DNA-PKcs. BMC Cancer 2021, 21, 1201.
- Zhang, B.; Wu, H.; Hao, J.; Wu, Y.; Yang, B. Inhibition of DNA-PKcs activity re-sensitizes uveal melanoma cells to radio- and chemotherapy. Biochem. Biophys. Res. Commun. 2020, 522, 639–646.
- Ciszewski, W.M.; Tavecchio, M.; Dastych, J.; Curtin, N.J. DNA-PK inhibition by NU7441 sensitizes breast cancer cells to ionizing radiation and doxorubicin. Breast Cancer Res. Treat. 2014, 143, 47–55.
- Huertas, P.; Jackson, S.P. Human CtIP mediates cell cycle control of DNA end resection and double strand break repair. J. Biol. Chem. 2009, 284, 9558–9565.
- Zhang, Y.; Lai, J.; Du, Z.; Gao, J.; Yang, S.; Gorityala, S.; Xiong, X.; Deng, O.; Ma, Z.; Yan, C.; et al. Targeting radioresistant breast cancer cells by single agent CHK1 inhibitor via enhancing replication stress. Oncotarget 2016, 7, 34688–34702.
- Udayakumar, D.; Pandita, R.K.; Horikoshi, N.; Liu, Y.; Liu, Q.; Wong, K.-K.; Hunt, C.R.; Gray, N.S.; Minna, J.D.; Pandita, T.K.; et al. Torin2 Suppresses Ionizing Radiation-Induced DNA Damage Repair. Radiat. Res. 2016, 185, 527–538.
- Chen, J. Ataxia telangiectasia-related protein is involved in the phosphorylation of BRCA1 following deoxyribonucleic acid damage. Cancer Res. 2000, 60, 5037–5039.
- Hu, S.; Hui, Z.; Duan, J.; Garrido, C.; Xie, T.; Ye, X.-Y. Discovery of small-molecule ATR inhibitors for potential cancer treatment: A patent review from 2014 to present. Expert Opin. Ther. Pat. 2022, 32, 401–421.
- Choi, C.; Cho, W.K.; Park, S.; Shin, S.-W.; Park, W.; Kim, H.; Choi, D.H. Checkpoint Kinase 1 (CHK1) Inhibition Enhances the Sensitivity of Triple-Negative Breast Cancer Cells to Proton Irradiation via Rad51 Downregulation. Int. J. Mol. Sci. 2020, 21, 2691.
- Hussain, S.S.; Huang, S.-B.; Bedolla, R.G.; Rivas, P.; Basler, J.W.; Swanson, G.P.; Hui-Ming Huang, T.; Narayanasamy, G.; Papanikolaou, N.; Miyamoto, H.; et al. Suppression of ribosomal protein RPS6KB1 by Nexrutine increases sensitivity of prostate tumors to radiation. Cancer Lett. 2018, 433, 232–241.
- Sun, H.; Fan, G.; Deng, C.; Wu, L. Mir-4429 sensitized cervical cancer cells to irradiation by targeting RAD51. J. Cell Physiol. 2020, 235, 185–193.
- Liu, M.; Chen, H.; Chen, X.; Xiong, J.; Song, Z. Silencing UCHL3 enhances radio-sensitivity of non-small cell lung cancer cells by inhibiting DNA repair. Aging 2021, 13, 14277–14288.
- Du, L.-Q.; Du, X.-Q.; Bai, J.-Q.; Wang, Y.; Yang, Q.-S.; Wang, X.-C.; Zhao, P.; Wang, H.; Liu, Q.; Fan, F.-Y. Methotrexate-mediated inhibition of RAD51 expression and homologous recombination in cancer cells. J. Cancer Res. Clin. Oncol. 2012, 138, 811–818.
- Gemenetzidis, E.; Gammon, L.; Biddle, A.; Emich, H.; Mackenzie, I.C. Invasive oral cancer stem cells display resistance to ionising radiation. Oncotarget 2015, 6, 43964–43977.
- Tsukuda, T.; Fleming, A.B.; Nickoloff, J.A.; Osley, M.A. Chromatin remodelling at a DNA double-strand break site in Saccharomyces cerevisiae. Nature 2005, 438, 379–383.
- Zgheib, O.; Pataky, K.; Brugger, J.; Halazonetis, T.D. An oligomerized 53BP1 tudor domain suffices for recognition of DNA double-strand breaks. Mol. Cell Biol. 2009, 29, 1050–1058.
- Mattiroli, F.; Vissers, J.H.A.; van Dijk, W.J.; Ikpa, P.; Citterio, E.; Vermeulen, W.; Marteijn, J.A.; Sixma, T.K. RNF168 ubiquitinates K13–15 on H2A/H2AX to drive DNA damage signaling. Cell 2012, 150, 1182–1195.
- Thorslund, T.; Ripplinger, A.; Hoffmann, S.; Wild, T.; Uckelmann, M.; Villumsen, B.; Narita, T.; Sixma, T.K.; Choudhary, C.; Bekker-Jensen, S.; et al. Histone H1 couples initiation and amplification of ubiquitin signalling after DNA damage. Nature 2015, 527, 389–393.
- Kao, C.-N.; Moi, S.-H.; Hou, M.-F.; Luo, C.-W.; Chen, F.-M.; Pan, M.-R. RNF8-CDH1 Co-Expression Predicts Clinical Benefit of Chemoradiotherapy in Triple-Negative Breast Cancer. J. Pers. Med. 2021, 11, 655.
- Schmid, J.A.; Berti, M.; Walser, F.; Raso, M.C.; Schmid, F.; Krietsch, J.; Stoy, H.; Zwicky, K.; Ursich, S.; Freire, R.; et al. Histone Ubiquitination by the DNA Damage Response Is Required for Efficient DNA Replication in Unperturbed S Phase. Mol. Cell 2018, 71, 897–910.e8.
- Doil, C.; Mailand, N.; Bekker-Jensen, S.; Menard, P.; Larsen, D.H.; Pepperkok, R.; Ellenberg, J.; Panier, S.; Durocher, D.; Bartek, J.; et al. RNF168 binds and amplifies ubiquitin conjugates on damaged chromosomes to allow accumulation of repair proteins. Cell 2009, 136, 435–446.
- Stewart, G.S.; Panier, S.; Townsend, K.; Al-Hakim, A.K.; Kolas, N.K.; Miller, E.S.; Nakada, S.; Ylanko, J.; Olivarius, S.; Mendez, M.; et al. The RIDDLE Syndrome Protein Mediates a Ubiquitin-Dependent Signaling Cascade at Sites of DNA Damage. Cell 2009, 136, 420–434.
- Wang, F.-C.; Peng, B.; Ren, T.-T.; Liu, S.-P.; Du, J.-R.; Chen, Z.-H.; Zhang, T.-T.; Gu, X.; Li, M.; Cao, S.-L.; et al. A 1,2,3-Triazole Derivative of Quinazoline Exhibits Antitumor Activity by Tethering RNF168 to SQSTM1/P62. J. Med. Chem. 2022, 65, 15028–15047.
- Ross, A.-L.; Simpson, L.J.; Sale, J.E. Vertebrate DNA damage tolerance requires the C-terminus but not BRCT or transferase domains of REV1. Nucleic Acids Res. 2005, 33, 1280–1289.
- Saha, P.; Mandal, T.; Talukdar, A.D.; Kumar, D.; Kumar, S.; Tripathi, P.P.; Wang, Q.-E.; Srivastava, A.K. DNA polymerase eta: A potential pharmacological target for cancer therapy. J. Cell Physiol. 2021, 236, 4106–4120.
- Yang, Y.; Liu, Z.; Wang, F.; Temviriyanukul, P.; Ma, X.; Tu, Y.; Lv, L.; Lin, Y.-F.; Huang, M.; Zhang, T.; et al. FANCD2 and REV1 cooperate in the protection of nascent DNA strands in response to replication stress. Nucleic Acids Res. 2015, 43, 8325–8339.
- Guo, C.; Tang, T.-S.; Bienko, M.; Parker, J.L.; Bielen, A.B.; Sonoda, E.; Takeda, S.; Ulrich, H.D.; Dikic, I.; Friedberg, E.C. Ubiquitin-binding motifs in REV1 protein are required for its role in the tolerance of DNA damage. Mol. Cell Biol. 2006, 26, 8892–8900.
- Alimova, I.; Wang, D.; Danis, E.; Pierce, A.; Donson, A.; Serkova, N.; Madhavan, K.; Lakshmanachetty, S.; Balakrishnan, I.; Foreman, N.K.; et al. Targeting the TP53/MDM2 axis enhances radiation sensitivity in atypical teratoid rhabdoid tumors. Int. J. Oncol. 2022, 60, 32.
- Sun, D.; Zhu, Y.; Zhu, J.; Tao, J.; Wei, X.; Wo, Y.; Hou, H. Primary resistance to first-generation EGFR-TKIs induced by MDM2 amplification in NSCLC. Mol. Med. Camb. Mass 2020, 26, 66.
- Kim, B.H.; Kim, Y.J.; Kim, M.-H.; Na, Y.R.; Jung, D.; Seok, S.H.; Kim, J.; Kim, H.J. Identification of FES as a Novel Radiosensitizing Target in Human Cancers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 265–273.
- Decaudin, D.; Frisch Dit Leitz, E.; Nemati, F.; Tarin, M.; Naguez, A.; Zerara, M.; Marande, B.; Vivet-Noguer, R.; Halilovic, E.; Fabre, C.; et al. Preclinical evaluation of drug combinations identifies co-inhibition of Bcl-2/XL/W and MDM2 as a potential therapy in uveal melanoma. Eur. J. Cancer 2020, 126, 93–103.
- Zhao, W.; Steinfeld, J.B.; Liang, F.; Chen, X.; Maranon, D.G.; Jian Ma, C.; Kwon, Y.; Rao, T.; Wang, W.; Sheng, C.; et al. BRCA1-BARD1 promotes RAD51-mediated homologous DNA pairing. Nature 2017, 550, 360–365.
- Tarsounas, M.; Sung, P. The antitumorigenic roles of BRCA1-BARD1 in DNA repair and replication. Nat. Rev. Mol. Cell Biol. 2020, 21, 284–299.
- Park, J.-W.; Park, J.-E.; Kim, S.-R.; Sim, M.-K.; Kang, C.-M.; Kim, K.S. Metformin alleviates ionizing radiation-induced senescence by restoring BARD1-mediated DNA repair in human aortic endothelial cells. Exp. Gerontol. 2022, 160, 111706.
- Liu, G.; Lim, D.; Cai, Z.; Ding, W.; Tian, Z.; Dong, C.; Zhang, F.; Guo, G.; Wang, X.; Zhou, P.; et al. The Valproate Mediates Radio-Bidirectional Regulation Through RFWD3-Dependent Ubiquitination on Rad51. Front. Oncol. 2021, 11, 646256.
- Sun, Y.; Jiang, X.; Xu, Y.; Ayrapetov, M.K.; Moreau, L.A.; Whetstine, J.R.; Price, B.D. Histone H3 methylation links DNA damage detection to activation of the tumour suppressor Tip60. Nat. Cell Biol. 2009, 11, 1376–1382.
- Coffey, K.; Blackburn, T.J.; Cook, S.; Golding, B.T.; Griffin, R.J.; Hardcastle, I.R.; Hewitt, L.; Huberman, K.; McNeill, H.V.; Newell, D.R.; et al. Characterisation of a Tip60 specific inhibitor, NU9056, in prostate cancer. PLoS ONE 2012, 7, e45539.
- Wang, X.; Wei, L.; Cramer, J.M.; Leibowitz, B.J.; Judge, C.; Epperly, M.; Greenberger, J.; Wang, F.; Li, L.; Stelzner, M.G.; et al. Pharmacologically blocking p53-dependent apoptosis protects intestinal stem cells and mice from radiation. Sci. Rep. 2015, 5, 8566.
- Huang, J.; Lin, C.; Dong, H.; Piao, Z.; Jin, C.; Han, H.; Jin, D. Targeting MALAT1 induces DNA damage and sensitize non-small cell lung cancer cells to cisplatin by repressing BRCA1. Cancer Chemother. Pharmacol. 2020, 86, 663–672.
- Classen, S.; Rahlf, E.; Jungwirth, J.; Albers, N.; Hebestreit, L.P.; Zielinski, A.; Poole, L.; Groth, M.; Koch, P.; Liehr, T.; et al. Partial Reduction in BRCA1 Gene Dose Modulates DNA Replication Stress Level and Thereby Contributes to Sensitivity or Resistance. Int. J. Mol. Sci. 2022, 23, 13363.
- Affandi, T.; Ohm, A.M.; Gaillard, D.; Haas, A.; Reyland, M.E. Tyrosine kinase inhibitors protect the salivary gland from radiation damage by increasing DNA double-strand break repair. J. Biol. Chem. 2021, 296, 100401.
- Huang, Z.; Peng, R.; Yu, H.; Chen, Z.; Wang, S.; Wang, Z.; Dong, S.; Li, W.; Jiang, Q.; Li, F.; et al. Dimethyl Sulfoxide Attenuates Radiation-Induced Testicular Injury through Facilitating DNA Double-Strand Break Repair. Oxid. Med. Cell Longev. 2022, 2022, 9137812.
- Chang, S.; Hu, L.; Xu, Y.; Li, X.; Ma, L.; Feng, X.; Wang, J.; Zhang, C.; Wang, S. Inorganic Nitrate Alleviates Total Body Irradiation-Induced Systemic Damage by Decreasing Reactive Oxygen Species Levels. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 945–957.
- Lee, V.; Gober, M.D.; Bashir, H.; O’Day, C.; Blair, I.A.; Mesaros, C.; Weng, L.; Huang, A.; Chen, A.; Tang, R.; et al. Voriconazole enhances UV-induced DNA damage by inhibiting catalase and promoting oxidative stress. Exp. Dermatol. 2020, 29, 29–38.
- Liu, C.; Vyas, A.; Kassab, M.A.; Singh, A.K.; Yu, X. The role of poly ADP-ribosylation in the first wave of DNA damage response. Nucleic Acids Res. 2017, 45, 8129–8141.
- Prokhorova, E.; Zobel, F.; Smith, R.; Zentout, S.; Gibbs-Seymour, I.; Schützenhofer, K.; Peters, A.; Groslambert, J.; Zorzini, V.; Agnew, T.; et al. Serine-linked PARP1 auto-modification controls PARP inhibitor response. Nat. Commun. 2021, 12, 4055.
- Brown, J.S.; Lukashchuk, N.; Sczaniecka-Clift, M.; Britton, S.; le Sage, C.; Calsou, P.; Beli, P.; Galanty, Y.; Jackson, S.P. Neddylation promotes ubiquitylation and release of Ku from DNA-damage sites. Cell Rep. 2015, 11, 704–714.
- Zhou, L.; Zhang, W.; Sun, Y.; Jia, L. Protein neddylation and its alterations in human cancers for targeted therapy. Cell Signal. 2018, 44, 92–102.
- Yu, Q.; Hu, Z.; Shen, Y.; Jiang, Y.; Pan, P.; Hou, T.; Pan, Z.-Q.; Huang, J.; Sun, Y. Gossypol inhibits cullin neddylation by targeting SAG-CUL5 and RBX1-CUL1 complexes. Neoplasia 2020, 22, 179–191.
- Zhang, S.; You, X.; Xu, T.; Chen, Q.; Li, H.; Dou, L.; Sun, Y.; Xiong, X.; Meredith, M.A.; Sun, Y. PD-L1 induction via the MEK-JNK-AP1 axis by a neddylation inhibitor promotes cancer-associated immunosuppression. Cell Death Dis. 2022, 13, 844.
- Zhou, L.; Zheng, L.; Hu, K.; Wang, X.; Zhang, R.; Zou, Y.; Zhong, L.; Wang, S.; Wu, Y.; Kang, T. SUMOylation stabilizes hSSB1 and enhances the recruitment of NBS1 to DNA damage sites. Signal Transduct. Target. Ther. 2020, 5, 80.
- Liu, Q.; Huang, Q.; Liu, H.; He, F.-J.; Liu, J.-H.; Zhou, Y.-Y.; Zeng, M.-T.; Pei, Q.; Zhu, H. SUMOylation of methyltransferase-like 3 facilitates colorectal cancer progression by promoting circ_0000677 in an m6 A-dependent manner. J. Gastroenterol. Hepatol. 2022, 37, 700–713.
- Traver, G.; Sekhar, K.R.; Crooks, P.A.; Keeney, D.S.; Freeman, M.L. Targeting NPM1 in irradiated cells inhibits NPM1 binding to RAD51, RAD51 foci formation and radiosensitizes NSCLC. Cancer Lett. 2021, 500, 220–227.
- Ping, X.; Stark, J.M. O-GlcNAc transferase is important for homology-directed repair. DNA Repair 2022, 119, 103394.
- He, N.; Ma, D.; Tan, Y.; Liu, M. Upregulation of O-GlcNAc transferase is involved in the pathogenesis of acute myeloid leukemia. Asia Pac. J. Clin. Oncol. 2022, 18, e318–e328.
- Semlow, D.R.; Zhang, J.; Budzowska, M.; Drohat, A.; Walter, J.C. Replication-Dependent Unhooking of DNA Interstrand Cross-Links by the NEIL3 Glycosylase. Cell 2016, 167, 498–511.e14.
- Li, N.; Wang, J.; Wallace, S.S.; Chen, J.; Zhou, J.; D’Andrea, A.D. Cooperation of the NEIL3 and Fanconi anemia/BRCA pathways in interstrand crosslink repair. Nucleic Acids Res. 2020, 48, 3014–3028.
- Wang, Q.; Li, Z.; Yang, J.; Peng, S.; Zhou, Q.; Yao, K.; Cai, W.; Xie, Z.; Qin, F.; Li, H.; et al. Loss of NEIL3 activates radiotherapy resistance in the progression of prostate cancer. Cancer Biol. Med. 2021, 19, 1193–1210.
- Wang, Y.; Xu, L.; Shi, S.; Wu, S.; Meng, R.; Chen, H.; Jiang, Z. Deficiency of NEIL3 Enhances the Chemotherapy Resistance of Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 4098.
- Yu, H.; Haskins, J.S.; Su, C.; Allum, A.; Haskins, A.H.; Salinas, V.A.; Sunada, S.; Inoue, T.; Aizawa, Y.; Uesaka, M.; et al. In vitro screening of radioprotective properties in the novel glucosylated flavonoids. Int. J. Mol. Med. 2016, 38, 1525–1530.
- Machour, F.E.; Ayoub, N. Transcriptional Regulation at DSBs: Mechanisms and Consequences. Trends Genet. TIG 2020, 36, 981–997.
- Liu, X.; Wei, W.; Liu, Y.; Yang, X.; Wu, J.; Zhang, Y.; Zhang, Q.; Shi, T.; Du, J.X.; Zhao, Y.; et al. MOF as an evolutionarily conserved histone crotonyltransferase and transcriptional activation by histone acetyltransferase-deficient and crotonyltransferase-competent CBP/p300. Cell Discov. 2017, 3, 17016.
- Sabari, B.R.; Tang, Z.; Huang, H.; Yong-Gonzalez, V.; Molina, H.; Kong, H.E.; Dai, L.; Shimada, M.; Cross, J.R.; Zhao, Y.; et al. Intracellular crotonyl-CoA stimulates transcription through p300-catalyzed histone crotonylation. Mol. Cell 2015, 58, 203–215.
- Mooring, S.R.; Jin, H.; Devi, N.S.; Jabbar, A.A.; Kaluz, S.; Liu, Y.; Van Meir, E.G.; Wang, B. Design and synthesis of novel small-molecule inhibitors of the hypoxia inducible factor pathway. J. Med. Chem. 2011, 54, 8471–8489.
- He, Z.-X.; Wei, B.-F.; Zhang, X.; Gong, Y.-P.; Ma, L.-Y.; Zhao, W. Current development of CBP/p300 inhibitors in the last decade. Eur. J. Med. Chem. 2021, 209, 112861.
- Bensimon, A.; Schmidt, A.; Ziv, Y.; Elkon, R.; Wang, S.-Y.; Chen, D.J.; Aebersold, R.; Shiloh, Y. ATM-dependent and -independent dynamics of the nuclear phosphoproteome after DNA damage. Sci. Signal. 2010, 3, rs3.
- Bian, L.; Meng, Y.; Zhang, M.; Guo, Z.; Liu, F.; Zhang, W.; Ke, X.; Su, Y.; Wang, M.; Yao, Y.; et al. ATM Expression Is Elevated in Established Radiation-Resistant Breast Cancer Cells and Improves DNA Repair Efficiency. Int. J. Biol. Sci. 2020, 16, 1096–1106.
- Huang, R.-X.; Zhou, P.-K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 2020, 5, 60.
- Wengner, A.M.; Scholz, A.; Haendler, B. Targeting DNA Damage Response in Prostate and Breast Cancer. Int. J. Mol. Sci. 2020, 21, 8273.
- Pilié, P.G.; Tang, C.; Mills, G.B.; Yap, T.A. State-of-the-art strategies for targeting the DNA damage response in cancer. Nat. Rev. Clin. Oncol. 2019, 16, 81–104.
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082.
- Cleary, J.M.; Aguirre, A.J.; Shapiro, G.I.; D’Andrea, A.D. Biomarker-Guided Development of DNA Repair Inhibitors. Mol. Cell 2020, 78, 1070–1085.
- Zhou, Y.; Lee, J.-H.; Jiang, W.; Crowe, J.L.; Zha, S.; Paull, T.T. Regulation of the DNA Damage Response by DNA-PKcs Inhibitory Phosphorylation of ATM. Mol. Cell 2017, 65, 91–104.
- Dylgjeri, E.; Kothari, V.; Shafi, A.A.; Semenova, G.; Gallagher, P.T.; Guan, Y.F.; Pang, A.; Goodwin, J.F.; Irani, S.; McCann, J.J.; et al. A Novel Role for DNA-PK in Metabolism by Regulating Glycolysis in Castration-Resistant Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 1446–1459.
- Liu, Y.; Efimova, E.V.; Ramamurthy, A.; Kron, S.J. Repair-independent functions of DNA-PKcs protect irradiated cells from mitotic slippage and accelerated senescence. J. Cell Sci. 2019, 132, jcs229385.
- Biehs, R.; Steinlage, M.; Barton, O.; Juhász, S.; Künzel, J.; Spies, J.; Shibata, A.; Jeggo, P.A.; Löbrich, M. DNA Double-Strand Break Resection Occurs during Non-homologous End Joining in G1 but Is Distinct from Resection during Homologous Recombination. Mol. Cell 2017, 65, 671–684.e5.
- Gupta, P.; Saha, B.; Chattopadhyay, S.; Patro, B.S. Pharmacological targeting of differential DNA repair, radio-sensitizes WRN-deficient cancer cells in vitro and in vivo. Biochem. Pharmacol. 2021, 186, 114450.
- Quennet, V.; Beucher, A.; Barton, O.; Takeda, S.; Löbrich, M. CtIP and MRN promote non-homologous end-joining of etoposide-induced DNA double-strand breaks in G1. Nucleic Acids Res. 2011, 39, 2144–2152.
- Klomp, J.E.; Lee, Y.S.; Goodwin, C.M.; Papke, B.; Klomp, J.A.; Waters, A.M.; Stalnecker, C.A.; DeLiberty, J.M.; Drizyte-Miller, K.; Yang, R.; et al. CHK1 protects oncogenic KRAS-expressing cells from DNA damage and is a target for pancreatic cancer treatment. Cell Rep. 2021, 37, 110060.
- Liu, Q.; Guntuku, S.; Cui, X.S.; Matsuoka, S.; Cortez, D.; Tamai, K.; Luo, G.; Carattini-Rivera, S.; DeMayo, F.; Bradley, A.; et al. Chk1 is an essential kinase that is regulated by Atr and required for the G(2)/M DNA damage checkpoint. Genes Dev. 2000, 14, 1448–1459.
- Jazayeri, A.; Falck, J.; Lukas, C.; Bartek, J.; Smith, G.C.M.; Lukas, J.; Jackson, S.P. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat. Cell Biol. 2006, 8, 37–45.
- Qiu, Z.; Oleinick, N.L.; Zhang, J. ATR/CHK1 inhibitors and cancer therapy. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2018, 126, 450–464.
- Karukonda, P.; Odhiambo, D.; Mowery, Y.M. Pharmacologic inhibition of ataxia telangiectasia and Rad3-related (ATR) in the treatment of head and neck squamous cell carcinoma. Mol. Carcinog. 2022, 61, 225–238.
- Chughtai, A.A.; Pannhausen, J.; Dinger, P.; Wirtz, J.; Knüchel, R.; Gaisa, N.T.; Eble, M.J.; Rose, M. Effective Radiosensitization of Bladder Cancer Cells by Pharmacological Inhibition of DNA-PK and ATR. Biomedicines 2022, 10, 1277.
- Lim, G.; Chang, Y.; Huh, W.-K. Phosphoregulation of Rad51/Rad52 by CDK1 functions as a molecular switch for cell cycle-specific activation of homologous recombination. Sci. Adv. 2020, 6, eaay2669.
- Toma, M.; Sullivan-Reed, K.; Śliwiński, T.; Skorski, T. RAD52 as a Potential Target for Synthetic Lethality-Based Anticancer Therapies. Cancers 2019, 11, 1561.
- Bugreev, D.V.; Mazina, O.M.; Mazin, A.V. Rad54 protein promotes branch migration of Holliday junctions. Nature 2006, 442, 590–593.
- Li, X.; Heyer, W.-D. RAD54 controls access to the invading 3′-OH end after RAD51-mediated DNA strand invasion in homologous recombination in Saccharomyces cerevisiae. Nucleic Acids Res. 2009, 37, 638–646.
- Goyal, N.; Rossi, M.J.; Mazina, O.M.; Chi, Y.; Moritz, R.L.; Clurman, B.E.; Mazin, A.V. RAD54 N-terminal domain is a DNA sensor that couples ATP hydrolysis with branch migration of Holliday junctions. Nat. Commun. 2018, 9, 34.
- Paull, T.T.; Rogakou, E.P.; Yamazaki, V.; Kirchgessner, C.U.; Gellert, M.; Bonner, W.M. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. CB 2000, 10, 886–895.
- Roos, W.P.; Kaina, B. DNA damage-induced cell death: From specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013, 332, 237–248.
- Ramsden, D.A.; Carvajal-Garcia, J.; Gupta, G.P. Mechanism, cellular functions and cancer roles of polymerase-theta-mediated DNA end joining. Nat. Rev. Mol. Cell Biol. 2022, 23, 125–140.
- Riballo, E.; Kühne, M.; Rief, N.; Doherty, A.; Smith, G.C.M.; Recio, M.-J.; Reis, C.; Dahm, K.; Fricke, A.; Krempler, A.; et al. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol. Cell 2004, 16, 715–724.
- Bhattacharjee, S.; Nandi, S. DNA damage response and cancer therapeutics through the lens of the Fanconi Anemia DNA repair pathway. Cell Commun. Signal. CCS 2017, 15, 41.
