Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Jessie Wu and Version 2 by Jessie Wu.
Avian influenza (AI) is a contagious disease among the poultry population with high avian mortality, which generates significant economic losses and elevated costs for disease control and outbreak eradication. AI is caused by an RNA virus part of the Orthomyxoviridae family; however, only Influenzavirus A is capable of infecting birds. AI pathogenicity is based on the lethality, signs, and molecular characteristics of the virus. Low pathogenic avian influenza (LPAI) virus has a low mortality rate and ability to infect, whereas the highly pathogenic avian influenza (HPAI) virus can cross respiratory and intestinal barriers, diffuse to the blood, damage all tissues of the bird, and has a high mortality rate.
- virus
- control
- poultry
- avian influenza
1. Avian Influenza Virus
1.1. Etiology
The influenza virus is an RNA virus part of the Orthomyxoviridae family with seven genera, namely Influenzavirus A, Influenzavirus B, Influenzavirus C, Influenzavirus D, Thogotovirus, and Isavirus and Quarajavirus [1][2]; moreover, Influenzavirus A has been identified in a wide range of hosts with the highest genetic variability and is the only one capable of infecting birds [3][4]. Moreover, due to the segmented nature of the viral genome, new strains can emerge through genetic reassortment and antigenic drift, further increasing the difficulty in its control and prevention [5].
The avian influenza (AI) virus subtypes depend on the antigen present on the surface of the influenza A virus; there are 16 hemagglutinin subtypes and 9 neuraminidase subtypes [6][7]. However, recent scientific studies reported new HA subtypes (18 in total) and NA (11 in total), which were isolated in bats [8].
Additionally, there are two specific lineages of the HA subtype that phylogenetically divide into the Eurasian and North American lineages; these lineages have progressively evolved independently due to limited intercontinental contact between avian populations [9].
1.2. Pathogenicity and Virulence
AI viruses generally cause gastrointestinal disturbances in birds with minimal clinical signs and are classified as LPAI viruses. LPAI virus subtypes H5 and H7 circulate naturally in domestic birds but can evolve and become highly pathogenic [10].
Pathogenicity results from the accumulation of multiple basic amino acids at the HA cleavage site (termed the polybasic cleavage site or polybasic motif), allowing the HA molecule to develop outside the gastrointestinal tract and establish a systemic infection, causing an outbreak of highly pathogenic avian influenza (HPAI) that is characterized by rapid disease onset and progression associated with high mortality rates [9][11][12]. Moreover, some H5 and H7 viruses of low and high pathogenicity show virulence in mammals, and the highly pathogenic viruses can cause systemic infection in animal models [9][13][14].
1.2.1. Low Pathogenic Avian Influenza Virus (LPAI)
LPAI has a low mortality rate and ability to infect, causing little to no disease in birds, because they can only replicate in tracheal tissues and the small intestine [1]. However, the H5/H7 subtypes of low pathogenicity (common in poultry and wild waterfowl) [15] can mutate by insertion and recombination processes in the proteolytic cleavage site of HA [16] until becoming HPAI viruses [17].
1.2.2. Highly Pathogenic Avian Influenza Virus (HPAI)
The HPAI virus can cross respiratory and intestinal barriers, diffuse to the blood, and damage all tissues of the bird [15]. HPAI refers to strains with an “intravenous pathogenicity index” (IVPI) greater than 1.2 or a mortality rate equal to or higher than 75% of the total number of poultry over a period of 10 days [18].
The HPAI pathogenic strains of avian influenza belong to the H5 and H7 subtypes, with bird mortality that exceeds 90–100% during the 48 h after disease onset [18][19].
To date, subtypes H5 and H7 have been recognized as HPAI viruses capable of generating acute and considerable diseases in chickens, turkeys, and other economically significant birds. Moreover, H9 has been included as another subtype with pandemic risk because their high mutability could favor the evolution of viruses that allow sustained transmission in the human species, and H9 can cause zoonotic infections [15].
1.3. Transmission Mechanisms
Bird-to-Bird Transmission
Wild waterfowl are natural reservoirs of the AI virus and play a role in spreading through their long-distance migratory routes [20], infecting land birds and domesticated waterfowl via contaminated water sources or food [21]. However, the oral–fecal path is the main transmission route between birds due to the high viral levels in the fecal matter of infected birds, and it can be transmissible for approximately 21 days [2][22].
Chatziprodromidou et al. described proximity to water as a significant risk factor for virus transmission because there may be a close interaction between migratory birds and commercial poultry activities, increasing disease transmission [23].
AI virus can also be transmitted through secretions and body fluids, such as saliva, mucus, and urine [24]. In the production systems, these fluids and feces contaminate the clothing and footwear of operators, cages, implements, and mechanical equipment for egg collection, among others. This route has been considered the principal vehicle for disease dissemination within flocks [25], making commercial poultry responsible for epidemics registered worldwide [26].
1.4. Interspecies Transmission
1.4.1. Transmission to Mammals
Direct contact is the main route of transmission because it has not been demonstrated that the virus can effectively infect mammals through aerosols [27]. Transmission to other species generally occurs after virus circulation in densely populated infected avian species, indicating that AI viruses can adapt to promote the spread [21]. For effective transmission and replication in mammals, the virus must evolve and mutate until it reaches compatibility with the new host environment; this is known as viral reassortment, which has been responsible for the appearance of almost all pandemic viruses in the past [28][29][30].
Infections with avian influenza virus have been reported in cats, mice, and pigs with AI subtype H5N6 [31][32][33]; in canines with subtype H3N8 [34]; and in tigers and leopards with subtype H1N1 [35][36]. All of them have been epidemiologically related to avian influenza outbreaks. Furthermore, avian influenza subtypes have been isolated in ferrets and laboratory animals to evaluate their pathogenicity [37][38].
1.4.2. Zoonotic Transmission
Avian influenza viruses have demonstrated the capacity to cross the barrier between species for multifactorial reasons that have favored transmission. Certain mammals, such as bats [39], pigs [40], cats, dogs, horses, ferrets, sea lions, and bats [41], can act as reservoirs, which allow genetic mixing between viruses that intend to infect humans and birds [18]. Moreover, host susceptibility, exposure level to infected birds, viral mutations, and favorable environmental conditions form an ideal scenario for the zoonotic transmission of the avian influenza virus [42].
The main route of transmission between birds and humans is direct contact with the feces or secretions of infected animals and exposure to contaminated or virus-infected environments (Figure 1) [43][44]. There is no evidence of human-to-human infection [21][24]. People within the poultry production chain (from farm to table) are at a higher infection risk than the general population due to prolonged exposure to the infectious agent [11].
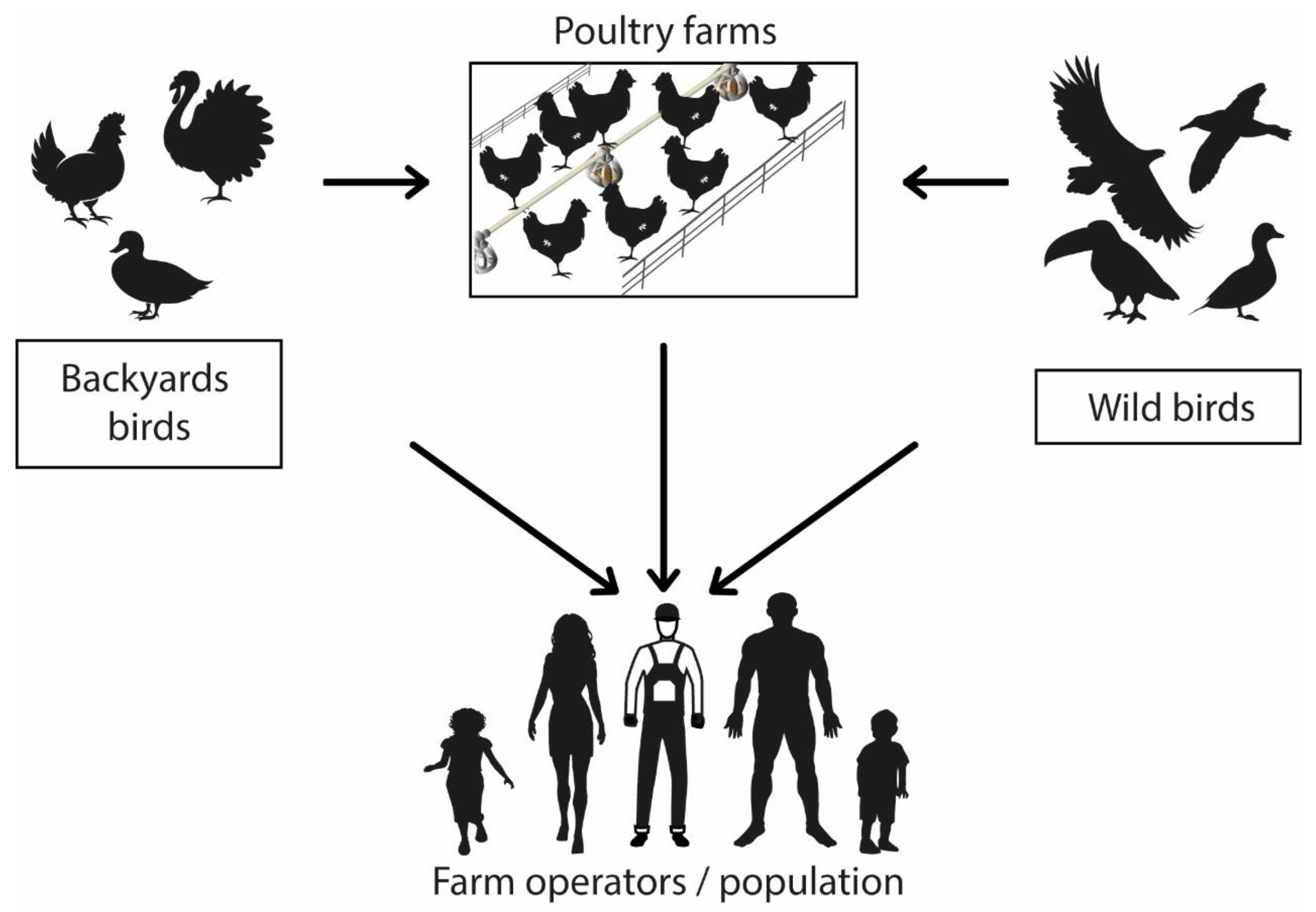
Figure 1. Avian influenza virus transmission mechanism. Graphic representation of virus zoonotic potential.
1.5. Virus Reservoirs
The avian influenza virus ecological niche or natural reservoir are waterfowl belonging to Anseriformes (waterfowl, ducks, geese, and swans) and Charadriiformes (gulls and shorebirds), which include more than 100 species of wild birds belonging to about 25 different families, indicating the global distribution of the virus in free-living waterfowl [12][45].
Subtype AI viruses (H5, H7, H6, and H9) can be found in both waterfowl and poultry [46], and several papers have described avian influenza viruses identified in different mammals. For instance, the AI subtype (H3, H7) in equines, the AI subtype (H1, H3) in swine, and the AI subtype in aquatic mammals (H10, H4, H7, and H13) originate from the genetics inherent to viruses naturally found in wild waterfowl (H1–H16) [2][47].
1.6. Virus Survival
Studies have found that the virus is more resistant to low temperatures (below 28 °C) [25][48]. The avian influenza virus can survive for up to 200 days in the body fluids of infected birds, four days in feces at animal body temperature, 35 days in feces at temperatures below 4 °C, and about five weeks in the environment of the infected poultry house [25][48]. The virus can survive in carcasses, meat, and eggs (especially at low temperatures); therefore, upon suspicion or confirmation of positive cases of avian influenza, the products generated should be eliminated [48]
2. Containment Measures for Confirmed Cases
2.1. Sanitary Slaughter of Infected Poultry
Within 24 to 48 h after confirmation of a positive case [25], all birds within a 3 km radius of the infected area should be monitored and traced to establishments with direct or indirect links to the infected premises. A tracing period should be considered, and AI high-risk establishment identification should be performed to opt for stamping out [19]. Sanitary slaughter could be an option for AI eradication, but it involves complex economic, ethical, environmental, and public health considerations due to the risk of zoonosis. However, if this procedure is chosen, animal welfare must be guaranteed [49] and be under the current legislation [50]. There are different procedures to be followed.
2.1.1. Environment Saturation with CO2
Slaughtering by releasing carbon dioxide is one of the most recommended methods because large animal volumes can be euthanized with minimal contact by the responsible personnel. For example, in optimum conditions, a 50 L tank is enough to sacrifice 20–30 thousand birds [25]. This process can be carried out inside the same sheds where the animals are kept or by placing them in vans, cages, or airtight containers that ensure CO2 concentration remains stable [25]. Birds should first be exposed to concentrations below 40% CO2 and once they lose consciousness due to the anesthetic and central nervous system depressant gas effect, the concentration values can be raised to above 60% [51].
2.1.2. Carbon Dioxide Foam
The animals must be placed at ground level, and the foam should be concentrated at 1% CO2 in water. The entire bird should be covered at approximately 1.50 m in height or 30 cm above their heads [25][52][53]. This is a very efficient method to deal with emerging outbreaks because total depopulation can be carried out quickly by inducing hypoxia in the animals [52][53].
2.1.3. Cervical Dislocation
In case the previously mentioned methods are not available, euthanasia should be performed by manual or mechanical separation of the skull from the vertebral column after dislocating the neck of the bird [54]. Cervical dislocation is a non-invasive method; however, it requires trained personnel and is inefficient for large flocks or birds, in addition to the long exposure time of the responsible personnel [55].
2.2. Disposal
2.2.1. Burial
For the disposal of the slaughtered birds and all the implements that have been in contact with the infected area (protective clothing, bedding, feed, and eggs), burial is a cost-effective and efficient option [25]. Heavy machinery and sufficient space should be available to dig the pits, occupying approximately 200–300 birds per cubic meter [25]. Long trenches that are not too large should be considered, and the channels should be covered with a layer of soil of about 40 cm, followed by an even layer of calcium dioxide and, finally, another layer of soil [19].
2.2.2. Incineration
This method is not recommended for large poultry populations due to its high costs, environmental contamination, and lack of certainty in the effectiveness of the disposal method due to the volatility of the remains [25]. It is suggested to dig an 8 m long, 2 m wide, and 1 m deep trench; use firewood to completely burn the dead birds; and then add a layer of lime [56]. The channels should be covered with a layer of soil, a layer of lime, and finally, another layer of soil [19].
2.3. Infected Poultry Farms’ Disinfection
After slaughter and disposal of carcasses and other contaminated products, recommendations indicate that a three-step cleaning and disinfection protocol must be carried out [25][57]:
-
The first step is to spray all surfaces that may have been in contact with the affected birds with cationic surfactants, oxidizing agents, aldehydes, or acids and leave them for one day [57].
-
The second step is general cleaning with hot water or steam using degreasing and sulfating agents, finishing with disinfectant application for one week.
-
The third step involves the same procedure as the second step but, optimally, leaving the disinfectant for 21 days [25].
References
- Nuñez, I.A.; Ross, T.M. A Review of H5Nx Avian Influenza Viruses. Therapeutic Advances in Vaccines and Immunotherapy; SAGE Publications Ltd.: New York, NY, USA, 2019; Volume 7, p. 2515135518821625.
- Wahlgren, J. Influenza A viruses: An ecology review. Infect. Ecol. Epidemiol. 2011, 1, 6004.
- Alexander, D.J. An overview of the epidemiology of avian influenza. Vaccine 2007, 25, 5637–5644.
- Stallknecht, D.E.; Shane, S.M. Host range of avian influenza virus in free-living birds. Vet. Res. Commun. 1988, 12, 125–141.
- Parvin, R.; Hossain, I.; Hasan, A.; Afrin, S.Z.; Shehata, A.A. Influenza and coronavirus zoonoses: An overview on pandemic events, viral genome, replication and emergency preparedness. Ger. J. Microbiol. 2022, 2, 1–11.
- Sendor, A.; Weerasuriya, D.; Sapra, A. Influenza Aviar; National Library of Medicine: Ottawa, IL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553072/#article-18048.s4 (accessed on 6 December 2022).
- Fouchier, R.A.M.; Munster, V.; Wallensten, A.; Bestebroer, T.M.; Herfst, S.; Smith, D.; Rimmelzwaan, G.F.; Olsen, B.; Osterhaus, A.D. Characterization of a Novel Influenza A Virus Hemagglutinin Subtype (H16) Obtained from Black-Headed Gulls. J. Virol. 2005, 79, 2814.
- Tong, S.; Li, Y.; Rivailler, P.; Conrardy, C.; Castillo, D.A.; Chen, L.M.; Recuenco, S.; Ellison, J.A.; Davis, C.T.; York, I.A.; et al. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. USA 2012, 109, 4269–4274.
- Sutton, T.C. The Pandemic Threat of Emerging H5 and H7 Avian Influenza Viruses. Viruses 2018, 10, 461.
- Swayne, D.E.; Pantin-Jackwood, M. Pathogenicity of avian influenza viruses in poultry. Dev. Biol. 2006, 124, 61–67.
- Organización Panamericana de la Salud. Influenza Aviar; OPS/OMS: Buenos Aires, Argentina, 2023; Available online: https://www.paho.org/en/topics/avian-influenza (accessed on 15 January 2023).
- Lee, D.-H.; Bertran, K.; Kwon, J.-H.; Swayne, D.E. Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4. J. Vetereinary Sci. 2017, 18, 269–280.
- Zitzow, L.A.; Rowe, T.; Morken, T.; Shieh, W.-J.; Zaki, S.; Katz, J.M. Pathogenesis of Avian Influenza A (H5N1) Viruses in Ferrets. J. Virol. 2002, 76, 4420.
- Belser, J.A.; Lu, X.; Maines, T.R.; Smith, C.; Li, Y.; Donis, R.O.; Katz, J.M.; Tumpey, T.M. Pathogenesis of Avian Influenza (H7) Virus Infection in Mice and Ferrets: Enhanced Virulence of Eurasian H7N7 Viruses Isolated from Humans. J. Virol. 2007, 81, 11139.
- Oficina Internacional de Epizootias (OIE). Influenza Aviar (Incluida la Infección por los Virus de la Influenza Aviar Altamente Patógenos). Capítulo 3.3.4. 2021. Available online: https://www.woah.org/fileadmin/Home/esp/Health_standards/tahm/3.03.04_AI.pdf (accessed on 22 February 2023).
- Lee, D.H.; Criado, M.F.; Swayne, D.E. Pathobiological Origins and Evolutionary History of Highly Pathogenic Avian Influenza Viruses. Cold Spring Harb. Perspect. Med. 2021, 11, a038679.
- Rabadan, R.; Robins, H. Evolution of the Influenza A Virus: Some New Advances. Evol. Bioinform. 2007, 3, 299.
- To, K.K.W.; Chan, J.F.W.; Chen, H.; Li, L.; Yuen, K.Y. The emergence of influenza A H7N9 in human beings 16 years after influenza A H5N1: A tale of two cities. Lancet Infect. Dis. 2013, 13, 809.
- Kanaujia, R.; Bora, I.; Ratho, R.K.; Thakur, V.; Mohi, G.K.; Thakur, P. Avian influenza revisited: Concerns and constraints. VirusDisease 2022, 33, 456–465.
- Su, S.; Bi, Y.; Wong, G.; Gray, G.C.; Gao, G.F.; Li, S. Epidemiology, Evolution, and Recent Outbreaks of Avian Influenza Virus in China. J. Virol. 2015, 89, 8671–8676.
- Yamaji, R.; Saad, M.D.; Davis, C.T.; Swayne, D.E.; Wang, D.; Wong, F.Y.; McCauley, J.W.; Peiris, J.M.; Webby, R.J.; Fouchier, R.A.; et al. Pandemic potential of highly pathogenic avian influenza clade 2.3.4.4 A(H5) viruses. Rev. Med. Virol. 2020, 30, e2099.
- Bui, C.; Bethmont, A.; Chughtai, A.A.; Gardner, L.; Sarkar, S.; Hassan, S.; Seale, H.; MacIntyre, C.R. A Systematic Review of the Comparative Epidemiology of Avian and Human Influenza A H5N1 and H7N9—Lessons and Unanswered Questions. Transbound. Emerg. Dis. 2016, 63, 602–620.
- Chatziprodromidou, I.P.; Arvanitidou, M.; Guitian, J.; Apostolou, T.; Vantarakis, G.; Vantarakis, A. Global avian influenza outbreaks 2010–2016: A systematic review of their distribution, avian species and virus subtype. Syst. Rev. 2018, 7, 17.
- Swayne, D.E. Avian Influenza; Blackwell Publishing: Ames, IO, USA, 2008; Available online: https://books.google.com.ec/books?hl=es&lr=&id=eZjFavPmuxAC&oi=fnd&pg=PP2&ots=jyiRjnKa6Q&sig=tbhpc0dDVC45dPzGpFokIANkasU&redir_esc=y#v=onepage&q&f=false (accessed on 21 February 2023).
- Caría, D.; Ferrer, M.E.; Chuard, N. Manual de Procedimientos para la Contingencia de la Influenza Aviar; SENASA: Buenos Aires, Argentina, 2017. Available online: https://www.argentina.gob.ar/sites/default/files/manual_de_procedimientos_-_plan_de_contingencia_de_ia_res._ndeg_73.2010.pdf (accessed on 10 January 2023).
- Horimoto, T.; Kawaoka, Y. Pandemic Threat Posed by Avian Influenza A Viruses. Clin. Microbiol. Rev. 2001, 14, 129.
- Herfst, S.; Mok, C.K.P.; van den Brand, J.M.A.; van der Vliet, S.; Rosu, M.E.; Spronken, M.I.; Yang, Z.; de Meulder, D.; Lexmond, P.; Bestebroer, T.M.; et al. Human Clade 2.3.4.4 A/H5N6 Influenza Virus Lacks Mammalian Adaptation Markers and Does Not Transmit via the Airborne Route between Ferrets. mSphere 2018, 3, e00405.17.
- Gambaryan, S.-A.; Matrosovich, M.-N. What Adaptative is Hemagglutinin and Neuraminidase are Necessary for Emergence of Pandemic Influenza Virus from Its Avian Precursor? Biochemistry 2015, 80, 872–880.
- Kuiken, T.; Holmes, E.C.; McCauley, J.; Rimmelzwaan, G.F.; Williams, C.S.; Grenfell, B.T. Host Species Barriers to Influenza Virus Infections. Science 2006, 312, 394–397.
- Cáceres, C.J.; Rajao, D.S.; Perez, D.R. Airborne Transmission of Avian Origin H9N2 Influenza A Viruses in Mammals. Viruses 2021, 13, 1919.
- Yu, Z.; Gao, X.; Wang, T.; Li, Y.; Li, Y.; Xu, Y.; Chu, D.; Sun, H.; Wu, C.; Li, S.; et al. Fatal H5N6 Avian Influenza Virus Infection in a Domestic Cat and Wild Birds in China. Sci. Rep. 2015, 5, 10704.
- Li, X.; Fu, Y.; Yang, J.; Guo, J.; He, J.; Guo, J.; Weng, S.; Jia, Y.; Liu, B.; Li, X.; et al. Genetic and biological characterization of two novel reassortant H5N6 swine influenza viruses in mice and chickens. Infect. Genet. Evol. 2015, 36, 462–466.
- Cao, X.; Yang, F.; Wu, H.; Xu, L. Genetic characterization of novel reassortant H5N6-subtype influenza viruses isolated from cats in eastern China. Arch. Virol. 2017, 162, 3501–3505.
- Yoon, K.J.; Cooper, V.L.; Schwartz, K.J.; Harmon, K.M.; Kim, W.I.; Janke, B.H.; Strohbehn, J.; Butts, D.; Troutman, J. Influenza Virus Infection in Racing Greyhounds. Emerg. Infect. Dis. 2005, 11, 1974.
- Amonsin, A.; Payungporn, S.; Theamboonlers, A.; Thanawongnuwech, R.; Suradhat, S.; Pariyothorn, N.; Tantilertcharoen, R.; Damrongwantanapokin, S.; Buranathai, C.; Chaisingh, A.; et al. Genetic characterization of H5N1 influenza A viruses isolated from zoo tigers in Thailand. Virology 2006, 344, 480–491.
- Keawcharoen, J.; Oraveerakul, K.; Kuiken, T.; Fouchier, R.A.; Amonsin, A.; Payungporn, S.; Noppornpanth, S.; Wattanodorn, S.; Theambooniers, A.; Tantilertcharoen, R.; et al. Avian Influenza H5N1 in Tigers and Leopards. Emerg. Infect. Dis. 2004, 10, 2189.
- Marchenko, V.; Goncharova, N.; Susloparov, I.; Kolosova, N.; Gudymo, A.; Svyatchenko, S.; Danilenko, A.; Durymanov, A.; Gavrilova, E.; Maksyutov, R.; et al. Isolation and characterization of H5Nx highly pathogenic avian influenza viruses of clade 2.3.4.4 in Russia. Virology 2018, 525, 216–223.
- Kwon, H.I.; Kim, E.H.; Kim, Y.I.; Park, S.J.; Si, Y.J.; Lee, I.W.; Nguyen, H.D.; Yu, K.M.; Yu, M.A.; Jung, J.H.; et al. Comparison of the pathogenic potential of highly pathogenic avian influenza (HPAI) H5N6, and H5N8 viruses isolated in South Korea during the 2016–2017 winter season. Emerg. Microbes Infect. 2018, 7, 29.
- Nabi, G.; Wang, Y.; Lü, L.; Jiang, C.; Ahmad, S.; Wu, Y.; Li, D. Bats and birds as viral reservoirs: A physiological and ecological perspective. Sci. Total Environ. 2021, 754, 142372.
- Zhang, H.; Li, H.; Wang, W.; Wang, Y.; Han, G.Z.; Chen, H.; Wang, X. A unique feature of swine ANP32A provides susceptibility to avian influenza virus infection in pigs. PLoS Pathog. 2020, 16, e1008330.
- Roguski, K.; Fry, A. Travel-related infectious diseasesChapter 4; Travelers’ HealthCenters for Disease Control and Prevention: Atlanta, GA, USA, 2019; Available online: https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/influenza#:~:text=The (accessed on 25 February 2023).
- Chan, J.F.W.; To, K.K.W.; Tse, H.; Jin, D.Y.; Yuen, K.Y. Interspecies transmission and emergence of novel viruses: Lessons from bats and birds. Trends Microbiol. 2013, 21, 544.
- Organización Mundial de la Salud. Gripe (Aviar y Otras Zoonóticas); OMS: Geneva, Switzerland, 2018; Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(avian-and-other-zoonotic) (accessed on 25 February 2023).
- Chin, J. El control de las enfermedades transmisibles. Organ. Panam. De La Salud 2001, 17, 1–673. Available online: www.paho.org (accessed on 25 February 2023).
- Daoust, P.Y.; van de Bildt, M.; van Riel, D.; van Amerongen, G.; Bestebroer, T.; Vanderstichel, R.; Fouchier, R.A.; Kuiken, T. Replication of 2 Subtypes of Low-Pathogenicity Avian Influenza Virus of Duck and Gull Origins in Experimentally Infected Mallard Ducks. Vet. Pathol. 2013, 50, 548–559.
- Capua, I.; Alexander, D.J. Avian influenza: Recent developments. Avian Pathol. 2004, 33, 393–404.
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152.
- Blagodatski, A.; Trutneva, K.; Glazova, O.; Mityaeva, O.; Shevkova, L.; Kegeles, E.; Onyanov, N.; Fede, K.; Maznina, A.; Khavina, E.; et al. Avian Influenza in Wild Birds and Poultry: Dissemination Pathways, Monitoring Methods, and Virus Ecology. Pathogens 2021, 10, 630.
- Agencia de Regulación y Control Fito y zoosanitario. Bienestar Animal Faenamiento de Animales de Producción; Lineamientos Técnicos—Agrocalidad: Quito, Ecuador, 2020; pp. 1–52. Available online: https://www.agrocalidad.gob.ec/wp-content/uploads/2020/05/ll3.pdf (accessed on 9 December 2022).
- Ministerio de Agricultura y Ganadería—Ecuador. Reglamento General de la Ley Orgánica de Sanidad Agropecuaria; Ministerio de Agricultura y Ganadería: Quito, Ecuador, 2019. Available online: http://www.epmrq.gob.ec/images/servicios/Reglamento_LOSA.pdf (accessed on 9 December 2022).
- Berg, C.; Raj, A.B.M. Procesado de Carne Métodos de Aturdido para las Aves: Aspectos del Bienestar Animal. Selecciones Avícolas 2014, 1, 142–152.
- Benson, E.R.; Alphin, R.L.; Rankin, M.K.; Caputo, M.P.; Hougentogler, D.P.; Johnson, A.L. Mass Emergency Water-Based Foam Depopulation of Poultry. Avian Diseases. 2012, 56, 891–896.
- Alphin, R.L.; Rankin, M.K.; Johnson, K.J.; Benson, E.R. Comparison of Water-Based Foam and Inert-Gas Mass Emergency Depopulation Methods. Avian Diseases. 2010, 54, 757–762.
- Organización Mundial de Sanidad Animal. Código Sanitario Para Los Animales Terrestres. Capítulo 7.6. 2011. Available online: https://www.woah.org/fileadmin/Home/esp/Health_standards/tahc/2011/es_chapitre_1.7.6.htm (accessed on 9 December 2022).
- Fuenzalida, S.E. Análisis del sacrificio de pollos de carne desde el punto de vista del Bienestar Animal; Universidad De Las Américas: Santiago, Chile, 2017; Available online: https://repositorio.udla.cl/xmlui/bitstream/handle/udla/283/a40840.pdf?sequence=1&isAllowed=y (accessed on 9 December 2022).
- Aziz, J. Manual for the Control of Highly Pathogenic Avian Influenza (HPAI) Malaysia; MOA-INCORPORATED: Compton, CA, USA, 2005; pp. 1–55. Available online: https://www.woah.org/fileadmin/database/ASIA/Malaysia/Manual_for_the_control_of_Highly_Pathogenic_Avian_Influenza_(HPAI)_Malaysia.pdf (accessed on 9 December 2022).
- Jesus, R. Bioseguridad, Vacío sanitario. In Programas 3D (Desinfección, Desinsectación y Desratización); Ceva Salud Animal S. A.: Barcelona, Spain, 2017; pp. 1–18. Available online: https://www.wpsa-aeca.es/aeca_imgs_docs/09_04_46_Bioseguridad.pdf (accessed on 10 December 2022).
More
