Dendrimers are a class of three-dimensional nanosized synthetic macromolecules with a well-defined globular architecture. Due to their unique properties of monodispersity, biocompatibility, and excellent biodegradability, dendrimers themselves represent an intriguing class of nanovectors for the delivery of nucleic acid-based vaccines. In this entreview, wey, researchers have demonstrated the growing interest in dendrimers as carriers of DNA and mRNA vaccines, which can become an alternative to other delivery methods.
- dendrimer
- vaccine delivery
- DNA vaccine
- mRNA vaccine
Lyubov A. Kisakova 1†, Evgeny K. Apartsin 2 † *, Lily F. Nizolenko 1 and Larisa I. Karpenko 1,*
1. Introduction
1 State Research Center of Virology and Biotechnology VECTOR, Rospotrebnadzor, 630559 Koltsovo, Russia.
2 Institut de Chimie et Biologie des Membranes et Nano-Objets UMR5248 CNRS - Université de Bordeaux, 33600 Pessaacc, France
* Authors to whom correspondence should be addressed. E-mail: evgeny.apartsin@u-bordeaux.fr (E.K.A.), karpenko@vector.nsc.ru (L.I.K.)
† These es authors contributed equally to this work.
- Introduction
Vaccines are ae among the most effective means of achieving the epidemic well-being of the population. A good vaccine platform should ensure the formation of effective long-term immunity, be simple and fast to develop, reproducible, thermostable, and relatively inexpensive to manufacture and use, but should not cause un-desirable side reactions. Nucleic acid-based vaccines (mRNA and DNA vaccines) meet many of these requirements; they are promising tools compared to traditional vaccination platforms due to their unique properties [1,2][1][2]. Various forms of polycationic carriers are used for DNA and mRNA delivery, including lipids, polymeric and polypeptide systems, dendrimers, gold nanoparticles, and hybrid systems [3,4,5][3][4][5]. Therefore, research is being actively conducted to develop various alternative methods for the delivery of DNA and RNA vaccine constructions. A promising direction is the use of cationic dendrimers, hyperbranched three-dimensional macro-molecules with perfectly defined molecular structure. Here in, wresearchers review recent progress in the use of dendrimers for the delivery of DNA and mRNA vaccines against various diseases.
- Delivery of Nucleic Acid Using Dendrimers
2. Delivery of Nucleic Acid Using Dendrimers
2.1. Dendrimers for the Delivery of DNA Vaccines against Viral Infections
Ullas et al. [6] [6] focused on developing candidate vaccines against the rabies virus. The authors used the polyetherimine dendrimer (PETIM) to increase the immunogenicity of the pIRES-Rgp DNA vaccine, encoding the full-length rabies virus glycoprotein gene in order to increase its immunogenicity. PETIM is a fourth-generation amino-terminated dendrimer, a globular nanopolymer with an approximate diameter of 3.5 nm, and a low cytotoxicity profile (Figure 1).

Figure 1).

Constructing vaccine nanoformulations based on PETIM dendrimers.
The DNA vaccine pIRES-Rgp, in a complex with dendrimers, showed significantly higher immunogenicity when mice were immunized with either the naked plasmid pIRES-Rgp or the PETIM-pIRES-Rgp complex [6]. Rabies virus neutralizing antibody (RVNA) titers in immune sera were assessed using the rapid fluorescence focus inhibition test. Protective levels of RVNA titer (≥0.5 IU/mL) were observed by day 14 similar to in the animals immunized with naked pIRES-Rgp and its complex with dendriplex. However, immunization with the PETIM-pIRES-Rgp dendriplex induced a 4.5-fold higher RVNA titer compared to pIRES-Rgp, while the antibodies had virus-neutralizing activity. It is likely that the dendriplex size (~500 nm) facilitated its efficient uptake by antigen-presenting cells near the site of inoculation, which led to efficient release of the antigen and development of antibody responses. Sustained high levels of RVNA titers on day 90 in the groups immunized with the PETIM-pIRES-Rgp dendriplex provide additional evidence for the possibility of antigen retention and slow antigen release by the encapsulated nanospheres. Importantly, the protective efficacy provided by the PETIM-pIRES-Rgp complex was comparable to that of the inactivated virus vaccine (Rabipur). The authors believe that the PETIM-pIRES-Rgp vaccine will be easier and cheaper to manufacture, more stable and resistant to higher ambient temperatures during storage, and thus more suitable for resource-limited settings than the commercial Rabipur vaccine [6].
Dutta et al. [7] investigated the potential of dendrimers and dendrosomes in genetic immunization against hepatitis B using the pRc/CMV-HBs[S] DNA vaccine encoding the hepatitis B surface antigen (HBsAg) sequence (5.6 kb). Several complexes of pRc/CMV-HBs plasmid with G5 poly(propylene imine) dendrimer (PPI) were obtained, differing in the plasmid: dendrimer ratio (Figure 2).
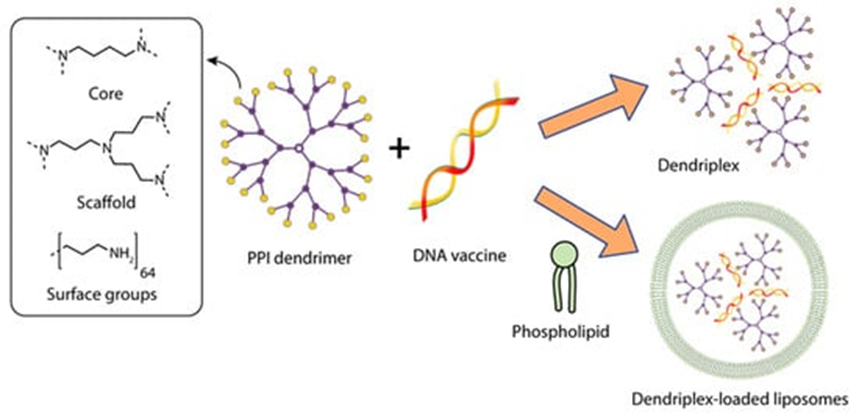
Figure 2 Constructing vaccine nanoformulations based on PPI dendrimers.
. Constructing vaccine nanoformulations based on PPI dendrimers.
The complex with a DNA: dendrimer ratio of 1:50 (PPI 50), which was subsequently chosen to obtain dendrosomes, had the highest statistically significant transfection efficiency in vitro. To obtain dendrosomes, phosphatidylcholine (PC) and cholesterol (C) were dissolved in diethyl ether at various molar ratios; a solution of the optimized dendrimer-DNA complex in PBS was added, sonicated, and homogenized to obtain particles sized ~200 nm. The authors showed that the optimal capture efficiency (the ratio between the amount of captured PPI-DNA complex and the amount of lipid complex added, expressed as a percentage) of 46.79 ± 1.33% was achieved at a 7:3 molar ratio of phosphatidylcholine to cholesterol (PC:C) in dendrosome composition DF3. DF3 was shown to have the optimal vesicle size, zeta potential, and uptake efficiency during the transfection of CHO cells, which was determined by the production of HBsAg [7].
The immunogenicity of the vaccine was studied using Balb/c mice as a model. The first group of animals received a single intramuscular injection of 10 μg of the naked plasmid pRc/CMV-HBs (DNA vaccines); the second group of animals was immunized with the PPI 50 dendrimer complex; and the third group was immunized with the DF3 dendrosome. The immunogenicity test was based on anti-HBsAg antibody titer assessment. It was shown that the DF3 complex elicited the maximum immune response of both total IgG and its studied subclasses (IgG1, IgG2a, and IgG2b) compared to naked DNA vaccine and PPI 50. The lowest antibody titer was observed in the animals immunized with naked plasmid pRc/CMV-HBs. In addition, the animals immunized with pRc/CMV-HBs and PPI 50 showed an abrupt decrease in antibody titer after six weeks, while the DF3 group had stable levels of IgG1, IgG2a, and IgG2b after six weeks, thus proving its effectiveness in maintaining antibody titer and protection against hepatitis B [7].
The animals immunized with the dendrimer and dendrosome formulations exhibited a Th1 immune response, further evidenced by higher IgG2a/IgG1 ratios. Cellular immune response was measured according to the content of endogenous IFN-γ in the spleen of mice immunized with pRc/CMV-HBs, PPI 50, and DF3, which were injected intravenously with 1 mg of the encoded antigen 24 hours before harvesting. The animals immunized with PPI 50 and DF3 complexes produced a significantly higher level of IFN-γ, which indicates the formation of a Th1-cell response [7].
Karpenko et al. [8] studied the delivery of a DNA vaccine against the Ebola virus using the fourth-generation polyamidoamine dendrimers (PAMAM G4) and a polyglucin:spermidine (PG) conjugate (Figure 3).
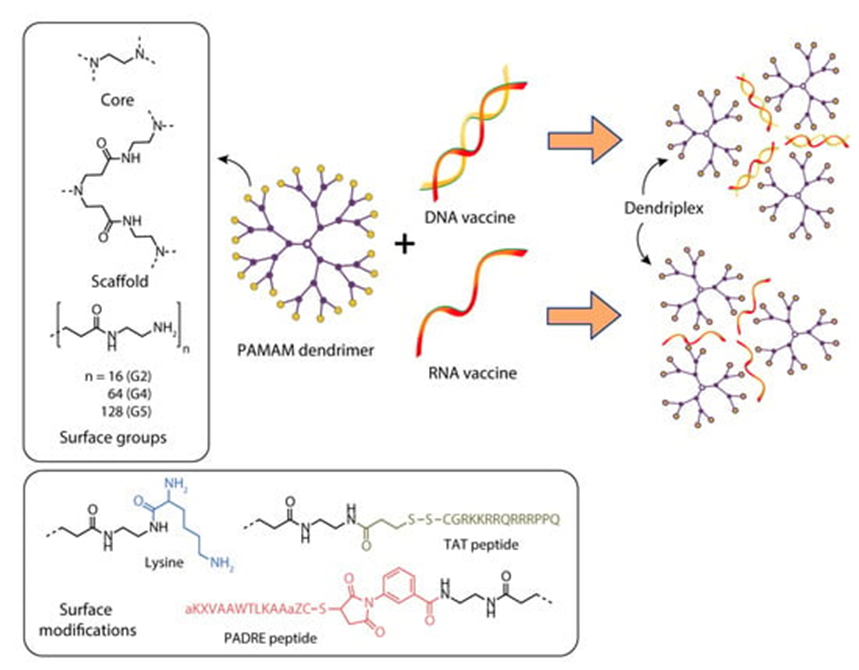
Figure 3).
. Constructing vaccine nanoformulations based on PAMAM dendrimers.

Figure 3 Constructing vaccine nanoformulations based on PAMAM dendrimers.
DNA vaccines are present among plasmids encoding artificial multi-epitope T-cell antigens EV.CTL and EV.Th, consisting of conserved epitopes of the EBOV GP, VP24, VP30, VP35, VP40, NP, and L Ebola virus proteins [8].
The physicochemical properties of complexes of polycationic polymers with DNA vaccines at different plasmid:polymer ratios were studied. Taking into account both the completeness of DNA binding and the size of the resulting complexes, the following ratio was chosen for biological tests: 3:1 for PAMAM. Since the DNA was taken in a charge excess, the surface charge of the resulting constructs remained negative after complex formation. The resulting complexes are nanosized structures with sizes of 100–300 nm for PG and <100 nm for PAMAM [8].
The study focusing on immunogenicity of the resulting constructs showed no statistically significant differences in the ability to elicit virus-specific T-cell responses between the groups of mice immunized with naked DNA vaccines and in combination with PAMAM G4. At the 3:1 ratio, PAMAM used as a packaging agent did not significantly improve the immunogenicity of the DNA vaccine. The authors believe that the complex formation of PAMAM with plasmids needs further optimization [8].
The use of native dendrimers and their conjugates with various proteins for the delivery of DNA vaccines is currently being studied.
Bahadoran et al. [9] studied the efficiency of delivery of a DNA vaccine against the H5N1 influenza virus (pBud-H5-GFP-IRF3) as part of a complex with PAMAM polyamidoamine dendrimer or a dendrimer conjugated with the TAT-PAMAM transcription transactivator TAT Figure 3.
The TAT–PAMAM polyplexes showed approximately 10 mV higher zeta potential values than the PAMAM polyplexes. A comparison of the transfection efficiencies of eukaryotic cells in vitro revealed that the pDNA–TAT–PAMAM complex is more efficient and less toxic than pDNA–PAMAM. The authors suggest that TAT-conjugated dendrimers have a higher charge density and, therefore, can form positively charged polyplexes, which are considered important for their adsorption on negatively charged cell membranes followed by an uptake by cells through internalization mechanisms [9].
To evaluate the immune response, mice were immunized with pBud-H5-GFP, PAMAM/pBud-H5-GFP, TAT-PAMAM/pBud-H5-GFP, and TAT-PAMAM/pBud-H5-GFP-IRF3 constructs. The expression levels of the H5 gene in the blood were determined on days 3 and 7 after immunization. Significantly higher expression of the H5 gene was detected in the group of animals immunized with the DNA vaccine in combination with the dendrimer-TAT conjugate. In the same group of mice, a higher titer of specific antibodies was observed compared to the level of antibodies in animals immunized with pDNA–PAMAM [10].
Similar results were obtained in the study of cellular response, which was assessed using flow cytometry. The largest cellular response was induced by the TAT-PAMAM/pBud-H5-GFP-IRF3 construct.
The use of the TAT peptide resulted in more than a doubling of the number of CD8+ T lymphocytes. The effect of the TAT peptide on CD4+ T cells was not as significant as on CD8+ T cells [10].
Thus, taking into account such properties as high transfection efficiency with relatively low cytotoxicity and ease of preparation, the TAT-PAMAM dendrimer is a promising non-viral vector together with IRF3 as a genetic adjuvant to induce appropriate immune responses. Further research is needed to determine whether improved immune responses can protect mice after a lethal dose of the H5N1 virus [10].
2.2. Dendrimers for mRNA Delivery of Vaccines against Viral Infections
There is a very limited number of studies on mRNA vaccines against viral diseases using dendrimers.
Chahal et al. [11] reported the results of studies on the use of a modified PAMAM dendrimer bearing multiple aliphatic chains for the delivery of mRNA replicons against three viral infections (Ebola virus, H1N1 influenza, and Toxoplasma gondii) and their delivery using a modified dendrimer molecule (Figure 4) as follows: an ionizable dendrimer-based nanomaterial, lipid-anchored PEG, and RNA are combined to form the final vaccine-modified dendrimer nanoparticle (MDNP). The vaccine constructs were self-replicating RNAs derived from Venezuelan equine encephalitis virus (VEEV) as a vector, with sequences encoding the Ebola virus, H1N1 influenza, or Toxoplasma gondii antigens inserted into its genome. The resulting mRNA replicons were encapsulated in MDNP, and their activities were examined both in vitro and in vivo.
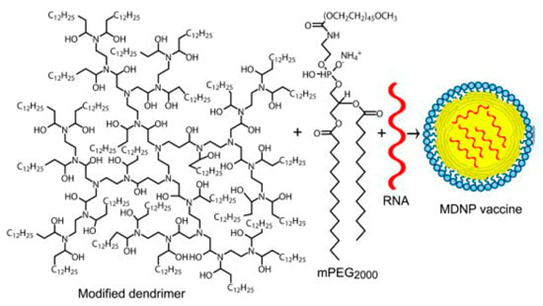
Figure 4 The structure of the amphiphilic dendrimer used for preparing MDNP [11].
. The structure of the amphiphilic dendrimer used for preparing MDNP [11].
MDNP-encapsulated RNAs have been successfully expressed in a wide range of cell culture types, including the HeLa human epithelial cell line, mouse and human primary fibroblasts, a mouse dendritic cell line, etc.
C57BL mice were immunized with MDNP VEEV with the Ebola virus glycoprotein gene. Animals were challenged with a lethal dose of mouse-adapted EBOV (ma-EBOV) 28 days after immunization. All control animals succumbed to EBOV infection by day 7, with 100% survival achieved by a single immunization with 40 μg MDNP, and no clinical pathological findings of EBOV were observed during the study. Protection was only attenuated at doses of MDNP vaccine below 40 µg, but 60% of the animals survived after immunization with 40 µg MDNP. Comparable humoral and protective reactions were caused by the introduction of a “naked” replicon, that is, a 100-fold increase in the total amount of RNA [11].
A similar experiment was carried out for influenza. BALB/c mice were immunized with MDNP VEEV encoding influenza H1N1 hemagglutinin (A/WSN/33) and challenged with a lethal dose of virus 14 days later. Control mice died from the infection by day 7, while animals immunized with the nanoparticles survived the challenge and showed complete recovery of body weight by day 11.
A multiplex vaccine against Toxoplasma gondii was created using the same principle. No toxoplasmosis vaccine currently exists, so it is impossible to provide absolute prevention [12].
Six self-replicating VEEV mRNAs, each encoding one parasite antigen (GRA6, ROP2A, ROP18, SAG1, SAG2A, and AMA1), were pooled at equal molar ratios using a monodisperse ionizable dendrimer nanoparticle. Selected antigens appear at several stages of the life cycle of the parasite and are common to several strains. BALB/c mice were immunized intramuscularly with a single 40 μg vaccine dose and exposed to lethal doses of the parasite 30 days post-immunization. The animals were followed up for clinical symptoms of the disease. On day 12, all the immunized mice survived the infection. Using the model of transgenic mice OT-1/Rag1−/−, the authors showed that this system is capable of inducing responses not only of antibodies, but also of CD8+ T cells. It is also interesting that such a vaccine does not require an adjuvant and is administered once [11].
Thus, a fully synthetic single-dose delivery platform has been created; it requires adjuvants and allows the use of vaccine constructs with multiple replicons expressing different antigens. After a single immunization, these contaminant-free, rapid-production vaccines elicit vital CD8+ T cell and antibody responses that provide complete protection against exposure to even lethal pathogens. According to the authors, this technology may allow one to create rapid-response vaccines with broad efficacy, which will reduce the number and frequency of vaccinations [11].
The dendrimer vaccine technology developed by the same group of researchers was used to create a candidate RNA vaccine against the Zika virus [13]. The vector replicon RNA encoding the premembrane (prM) and envelope (E) proteins of the ZIKV Z1106033 isolate as a single open reading frame was compiled and packaged using the MDNP technology. Mice were immunized on day 0 and boosted 5 weeks later with doses of 40 μg (based on RNA weight) of the nanoparticle vaccine by intramuscular injection. Immunization induced the formation of a high titer of specific IgG to the ZIKV protein and CD8+ cells, specifically recognizing the conserved T-cell epitope of IGVSNRDFV. The authors emphasize that their approach can be used to evaluate new candidate antigens and identify immune correlates without using the live Zika virus [13].
2.3. Dendrimers for the Delivery of DNA Vaccines against Bacterial Infection
A number of publications have focused on the delivery of DNA vaccines encoding antigens of various bacterial infectious agents.
Ribeiro et al. [14] [14] encapsulated dendriplexes containing a dendrimer:plasmid complex encoding the Bacillus anthracis protective antigen (PA) gene into poly(lactide-co-glycolide) (PLGA) particles using the double emulsion method. They used two types of dendrons: a dendron with three C18 chains (C18 dendron) and a dendron without any hydrocarbon chains attached (C0 dendron) (Figure 5).
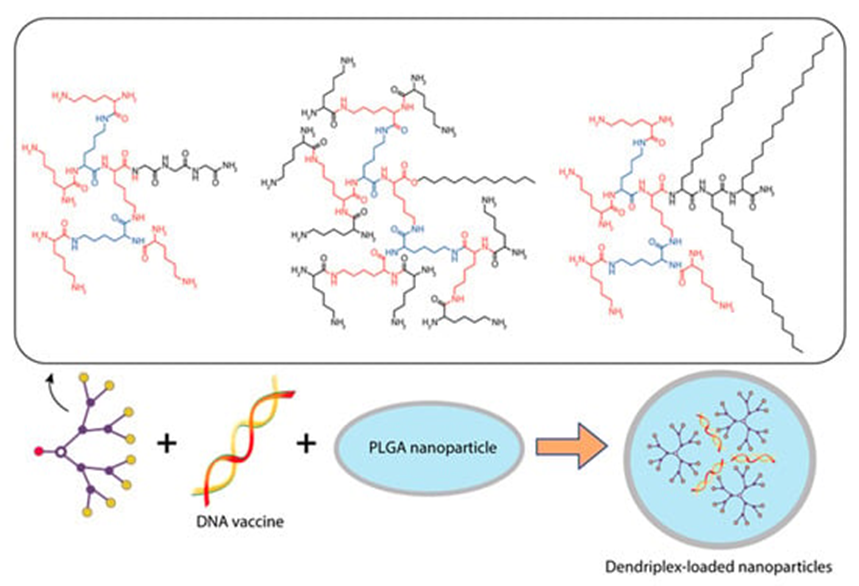
Figure 5 Constructing vaccine nanoformulations based on glycine-polylysine dendrons and their amphiphilic variants.
. Constructing vaccine nanoformulations based on glycine-polylysine dendrons and their amphiphilic variants.
Three types of particles were investigated, namely PLGA-C18 dendriplexes, PLGA-C0 dendriplexes, and a pDNA control system without PLGA. Dendron:pDNA complexes were encapsulated in PLGA particles at a ratio of 10:1 to assess antibody production, primarily because they have the smallest diameter at low dendron concentrations, which also minimizes their toxicity [14].
Research into the immunogenicity of dendriplexes showed that the antibody response to both PLGA PA-C18 and PLGA PA-C0 particles gradually increased over a 9-week period. It was likely to occur due to the booster effect in addition to the delayed release of DNA from the PLGA particles and, therefore, transfection of muscle cells to promote antigen presentation [14].
Mice that had received PLGA particles containing naked DNA-PA were only able to generate a weak response against PA. Animals in both groups that were immunized with PLGA PA-C0 and PLGA PA-C18 had higher anti-PA IgG titers than those immunized with PLGA-PA. The highest level of IgG antibodies against PA was observed in the serum of animals in the PLGA-C18 group. However, none of the sera of animals immunized with PA-C18, PA-C0, or PA-PLGA were able to neutralize the toxin. Thus, the mice lacked protection against a lethal dose of the toxin and infection with Bacillus anthracis. The authors believe that further studies are needed to optimize the composition of DNA vaccines and increase the level of antibodies against the lethal toxin as well as their functionality [14].
Verminnen et al. [15] [15] focused on developing an aerosolized DNA vaccine to protect turkeys against Chlamydophila psittaci infection. pcDNA1/MOMPopt–EGFP plasmids were constructed to test the efficiency of transfection, and pcDNA1/MOMPopt containing optimized sequences of the transgene and regulatory elements. For packaging the DNA vaccine, the authors used plasmid complexes with various cationic polymers, such as G2 and G5 PAMAM dendrimers (Figure 4), linear and branched polyethyleneimine (lPEI and brPEI), and lipoplexes with DOTAP/DOPE cationic liposomes [15].
An evaluation of the efficiency of in vitro transfection with the plasmid pcDNA1/MOMPopt–EGFP, in combination with different polymers, showed that, although the plasmid:lPEI complex had the best results, it was completely destroyed in the nebulizer, and PAMAM did not increase the transfection ability of the plasmid. The efficiency of lipoplexes were also statistically unreliable. Therefore, a plasmid complex with a branched polyethyleneimine (brPEI) resembling dendrimers but lacking their regular structure was chosen for further work [15].
DNA vaccine immunogenicity testing was performed in SPF turkey models that had been vaccinated either intramuscularly (IM) or nebulised, followed by challenge with 108 TCID50 of Cp. psittaci avian genotype D. Turkeys were divided into three groups: groups one and two were immunized intramuscularly with naked pcDNA/MOMPopt or brPEI-pcDNA/MOMPopt complex, respectively, and group three was immunized aerogenically by spraying brPEI pcDNA/MOMPopt through a nebulizer [15].
For the IM administration of the brPEI-pcDNA1/MOMPopt complexes, the immune response was higher than for the case when these complexes were introduced by aerosol (the average titers of MOMP-specific total serum antibodies per group were 85.0 ± 83.6 and 45.0 ± 17.3). However, the immunogenicity of the aerosolized complex was higher than that of the naked pcDNA1/MOMPopt injected IM (30.0 ± 24.5). Meanwhile, a significant level of protection against infection was observed in all the immunized turkeys. Severe clinical signs and lesions occurred only in intact control animals. However, turkeys immunized with brPEI-pcDNA1/MOMPopt IM appeared to be more protected than turkeys aerosolized with this complex. The authors hypothesized that the birds simply inhaled only a fraction of the 500 micrograms administered per bird. Accordingly, the method of delivering the vaccine construct must be adapted to obtain a more homogeneous distribution of the vaccine in the upper and lower respiratory tract of birds, and to reduce the vaccine dose. At the same time, there were no statistically significant differences in tissue lesions, the presence of chlamydial antigen, or isolation of chlamydia in turkeys immunized intramuscularly with naked pcDNA1/MOMPopt (group 1) and turkeys immunized aerogenically with brPEI-pcDNA1/MOMPopt (group 3). Protection correlated with a high B-cell response upon immunization, with an “early” secondary serum antibody response upon challenge, and with a high proliferative response, especially of CD4+ T cells [15].
2.4. Dendrimers for the Delivery of DNA Vaccines against Parasitic Infections
Creating anti-parasitic vaccines is not an easy task. It is no coincidence that a number of researchers have high hopes for new types of vaccines, such as DNA vaccines. Of course, the question inevitably continues to arise here about finding ways to effectively deliver them.
The aim of the research by Wang et al. [16] [16] was to evaluate the effectiveness of lysine-modified PAMAM for delivering DNA vaccines against Japanese schistosomiasis and assess its ability to enhance protective effects against Schistosoma japonicum infection. The PAMAM-Lys dendrimer based on PAMAM G4, which was modified with lysine, was synthesized (Figure 6).
Using PAMAM-Lys, complexes with a DNA vaccine against Schistosoma japonicum encoding the SjC23 membrane protein gene were obtained and characterized. To determine the most appropriate charge ratio (R+/2) of plasmid DNA and PAMAM-Lys/plasmid, complexes with various R+/2 ratios ranging from 0.5 to 10 were prepared, and the samples were analyzed by 1% agarose gel electrophoresis. The results showed that plasmid DNA exhibited complete retardation at a charge ratio of two with PAMAM G4 or four with PAMAM-Lys. Electron microscopy showed that the PAMAM-Lys/DNA complex forms particles with a diameter of 50 to 100 nm. It is important to note that PAMAM-Lys showed significantly higher transfection efficiency in 293T cells compared to native PMAM; in addition, the cytotoxicity of PAMAM-Lys was lower compared to that of PAMAM G5 [16].
An immunogenicity study showed that antibody titers in mice immunized with the combined PAMAM-Lys-SjC23 DNA vaccine were significantly higher than those in mice immunized with the naked SjC23 DNA vaccine. PAMAM-Lys elicited a predominantly humoral IgG2a response and dramatically increased IL-2 and IFN-γ production [16].
Immunization of mice with the SjC23 DNA vaccine in combination with PAMAM-Lys resulted in a 45–50% decrease in the number of helminths and a 59–62% decrease in the number of liver eggs, which is significantly higher than the effectiveness of the vaccine without SjC23 DNA. A 50–60% improvement in protection in the mouse model, as measured according to the reduction in the number of adult worms and eggs in the liver with a single antigen vaccine, is considered very encouraging. Thus, the SjC23 DNA vaccine with PAMAM-Lys provided an acceptable level of protective efficacy in the mouse model. It has been demonstrated that PAMAM-Lys dendrimers can increase the immunoreactivity of the DNA vaccine and enhance the protective effect of the SjC23 DNA vaccine against S. japonicum infection. The authors suggest that this new vaccine delivery vector could be an efficient and safe delivery vector for S. japonicum vaccine research and could also be used to develop other vaccines [16].
2.5. Dendrimers for the Delivery of DNA Vaccines against Cancer
Oncology is an area where dendrimers are widely used. They are employed both to deliver plasmids for cancer gene therapy [17] [17] and as drug or small interfering RNA (siRNA) carriers, and can combine both payloads in a single preparation [18].
An original approach for delivery of antitumor DNA vaccines into anti-gen-presenting cells was suggested by Daftarian et al. [19]. This approach is based on the conjugation of fifth-generation polyamidoamine dendrimers (G5-PAMAM) with MHC class II–targeting peptides that can selectively deliver these dendrimers to APCs (Figure 6) [19].
The best results were obtained with the PADRE peptide conjugated to PAMAM. This peptide has a high affinity for more than 95% of all dietary leukocyte-D (HLA-DR) and murine H2-I-Ab antigens. PADRE-conjugated PAMAM dendrimer (PPD) was used to deliver the DNA vaccine pcDNA3-TRP2, encoding tyrosine-related protein-2, a melanocyte self-differentiation antigen. TRP2 is the main antigenic target of the immune response induced in mice by immunization with genetically modified B16 melanoma vaccines [19].
A comparative experiment was conducted in which C57BL/6 mice were immunized with pcDNA3-TRP2 or pcDNA3 using PPD or non-conjugated dendrimers. The immunization was performed using subcutaneous electroporation [19].
Daftaryan et al. showed that conjugation of PADRE peptide to dendrimers enhances the immune response obtained by the dendrimer, generating high affinity memory T cells capable of recognizing not only TRP2-pulsed MBL2 but also the rare MHC class I molecules endogenously loaded with TRP2 in the B16 melanoma [19].
Subcutaneous injection of DNA-peptide-dendrimer complexes in vivo preferentially transfected dendritic cells (DCs) in draining lymph nodes, promoted generation of highly effective T cells and induced rejection of established tumors. The delivery of pcDNA3-TRP2 via PPD leads to B16 tumor regression, and mice survival is roughly 50%. The results demonstrated that PADRE-PAMAM dendrimer complexes with DNA vaccines can be used for high transfection efficiency and effective targeting of APCs in vivo, conferring properties essential to generate effective DNA vaccines [19].
2.6. Dendrimers for the Delivery of RNA Vaccines for Treating Protein Metabolism Disorders
Messenger RNA can also be used as a platform for treating protein metabolism disorders.
Cheng et al. [20] [20] studied the possibility of delivering mRNA encoding the fumarylacetoacetate hydrolase (FAH) protein gene using dendrimeric lipid nanoparticles (mDLNPs). In order to improve the therapeutic delivery of FAH mRNA to the liver, the authors obtained non-toxic dendrimeric lipid nanoparticles (mDLNPs) that should be well tolerated by mice with impaired liver function, as well as to ensure efficient packaging of long mRNAs.
They used a systematic orthogonal array design methodology designed to elucidate the functional contribution of each component of mDLNP nanoparticles for efficient mRNA delivery. 5A2-SC8 was chosen as an ionizable cationic dendrimer from a large library (Figure 6 Figure 6) because it had been successfully used previously by the authors to deliver siRNA to the liver to study gene functionality and showed very low toxicity. The incorporation of ionizable cationic lipids was necessary for RNA delivery because they bind RNA at low pH during mixing and promote intracellular release at lower pH during endosomal maturation. The phospholipid DOPE, which enhances mRNA loading and can form unstable hexagonal phases contributing to LDL disassembly and destabilization of the endosome membrane, was chosen [20].

Figure 6 Constructing vaccine nanoformulations based on amphiphilic dendron 5A2-SC8.
Figure 6. Constructing vaccine nanoformulations based on amphiphilic dendron 5A2-SC8.
In order to understand at what ratio each component of the nanoparticle composition should be taken, Cheng et al. used several rounds of optimization with testing of 44 mDLNP, which cover the theoretical space of 500 formulations. They found that LNPs optimized for mRNA delivery should contain significantly less ionizable cationic lipid and more zwitterionic phospholipids compared to standard siRNA formulations. Ultimately, the systematic optimization process allowed the development of a non-toxic, degradable delivery system that improved mRNA delivery to liver hepatocytes. mDLNPs were monodisperse particles with a diameter of ~100 nm and an almost neutral surface charge (−3.58 mV). Due to the high transfection efficiency in vivo, mDLNP 5A2-SC8 was able to extend the lives of FAH−/− knockout mice. The FAH mRNA treatment normalized body weight and liver function throughout the 30-day experiment. The authors believe that the ability of mDLNP 5A2-SC8 to deliver FAH mRNA to the diseased liver without carrier toxicity makes this system suitable for treating a wide range of liver diseases [20].
2.7. The Use of Complexes of Dendrimers with Metal Nanoparticles for mRNA Delivery
Recently, several studies have used the remarkable properties of dendrimers as stabilizers of metal nanoparticles (NPs) [21,22,23,24,25,26][21][22][23][24][25][26]. This strategy combines the unique properties of metal NPs with those of cationic dendrimers to create safe and highly efficient non-viral gene delivery systems. Gold nanoparticles (AuNPs) are among the most commonly used metal NPs today due to their ease of synthesis, biocompatibility, favorable surface-to-volume ratio, ability to be modified, and low cytotoxicity [27,28][27][28].
Mbatha et al. [29] [29] studied the original complexes of PAMAM G5D dendrimers with gold nanoparticles (AuNPs). The objective of their research was to generate folic-acid-(FA)-modified, poly-amidoamine-generation-5 (PAMAM G5D)-grafted gold nanoparticles (AuNPs) and evaluate their cytotoxicity and efficacy profiles for in vitro mRNA delivery (Figure 7).
Figure 7).

. Constructing vaccine nanoformulations based on dendrimer-functionalized gold nanoparticles.
Figure 7 Constructing vaccine nanoformulations based on dendrimer-functionalized gold nanoparticles.
The mRNA encoding the luciferase gene (FLuc-mRNA) was used as a model. Mbatha et al. obtained a number of nanocomplexes that contained a constant amount of FLuc-mRNA (0.05 µg) together with increasing amounts of G5D, Au:G5D, G5D:FA, and Au:G5D:FA NPs. The NPs appeared spherical with a uniform distribution and hydrodynamic diameters ranging from 65 to 128 nm. Nanocomplexes with mRNA prepared at the optimum binding ratios (w/w) are presented as clusters of smaller particles with hydrodynamic diameters ranging from 101 nm to 265 nm. The zeta potentials generally ranged from 20.9 to 87.2 mV for the NPs and from −21.0 to −65 mV for the nanocomplexes, indicating good colloidal stability. Au:G5D and Au:G5D:FA nanocomplexes had the highest zeta potentials of −37.3 mV and −65.7 mV, respectively. The polydispersity indices (PDI) revealed that all the NPs and nanocomplexes were highly monodisperse and uniform in size, with PDI values below 0.2, suggesting that these NPs and nanocomplexes have a lower tendency to agglomerate [29].
The authors demonstrated that nanocomplexes at optimum nanoparticle:mRNA (w/w) binding ratios showed good protection of the bound mRNA against nucleases and were well tolerated in all tested cell lines. The transfection efficiency and luciferase gene expression levels were significantly higher with FA-targeted, dendrimer-grafted AuNPs (Au:G5D:FA) in FA receptors overexpressing MCF-7 and KB cells compared to the G5D and G5D:FA NPs, decreasing significantly in the presence of excess competing FA ligand, which confirmed the nanocomplex uptake via receptor mediation [30]. The level of Luc gene expression was higher when Au:G5D and Au:G5D:FA nanocomplexes with mRNA were used to transfect cells compared to when G5D and G5D:FA nanocomplexes were employed; this fact indicates the important role played by AuNP in this delivery system [29].
3. Conclusion
In this entreview, wey, researchers set out to demonstrate the growing interest in dendrimers as carriers of DNA and mRNA vaccines, which can become an alternative to other delivery methods.
Dendrimers as delivery systems have a number of useful properties, such as monodispersity, biocompatibility, low reactogenicity, high biodegradability, water solubility, simplicity, and a relatively low cost of synthesis [31,32,33,34][31][32][33][34]. Dendrimers often exhibit significant adjuvant effects in vaccine delivery because they can be readily taken up by antigen-presenting cells. In addition, cationic dendrimers form more stable complexes with NA, providing greater protection during cellular transport than cationic lipids [35].
Thus, dendrimers represent a useful platform for the development of safe vaccines with new properties and application potential and will also be useful for basic research on the mechanisms underlying the induction and control of immunity.
[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35]
References
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef][Green Version]
- Liu, M.A. A Comparison of Plasmid DNA and mRNA as Vaccine Technologies. Vaccines 2019, 7, 37. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aldosari, B.N.; Alfagih, I.M.; Almurshedi, A.S. Lipid Nanoparticles as Delivery Systems for RNA-Based Vaccines. Pharmaceutics 2021, 13, 206. [Google Scholar] [CrossRef]
- Islam, M.A.; Reesor, E.K.G.; Xu, Y.; Zope, H.R.; Zetter, B.R.; Shi, J. Biomaterials for mRNA delivery. Biomater. Sci. 2015, 3, 1519–1533. [Google Scholar] [CrossRef][Green Version]
- Karpenko, L.I.; Rudometov, A.P.; Sharabrin, S.V.; Shcherbakov, D.N.; Borgoyakova, M.B.; Bazhan, S.I.; Volosnikova, E.A.; Rudometova, N.B.; Orlova, L.A.; Pyshnaya, I.A.; et al. Delivery of mrna vaccine against SARS-CoV-2 using a polyglucin:Spermidine conjugate. Vaccines 2021, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ullas, P.T.; Madhusudana, S.N.; Desai, A.; Sagar, B.K.C.; Jayamurugan, G.; Rajesh, Y.B.R.D.; Jayaraman, N. Enhancement of immunogenicity and efficacy of a plasmid DNA rabies vaccine by nanoformulation with a fourth-generation amine-terminated poly(ether imine) dendrimer. Int. J. Nanomed. 2014, 9, 627–634. [Google Scholar] [CrossRef][Green Version]
- Dutta, T.; Garg, M.; Jain, N.K. Poly(propyleneimine) dendrimer and dendrosome mediated genetic immunization against hepatitis B. Vaccine 2008, 26, 3389–3394. [Google Scholar] [CrossRef] [PubMed]
- Karpenko, L.I.; Apartsin, E.K.; Dudko, S.G.; Starostina, E.V.; Kaplina, O.N.; Antonets, D.V.; Volosnikova, E.A.; Zaitsev, B.N.; Bakulina, A.Y.; Venyaminova, A.G.; et al. Cationic Polymers for the Delivery of the Ebola DNA Vaccine Encoding Artificial T-Cell Immunogen. Vaccines 2020, 8, 718. [Google Scholar] [CrossRef]
- Bahadoran, A.; Moeini, H.; Bejo, M.H.; Hussein, M.Z.; Omar, A.R. Development of Tat-Conjugated Dendrimer for Transdermal DNA Vaccine Delivery. J. Pharm. Pharm. Sci. 2016, 19, 325–338. [Google Scholar] [CrossRef][Green Version]
- Bahadoran, A.; Ebrahimi, M.; Yeap, S.K.; Safi, N.; Moeini, H.; Hair-Bejo, M.; Hussein, M.Z.; Omar, A.R. Induction of a robust immune response against avian influenza virus following transdermal inoculation with H5-DNA vaccine formulated in modified dendrimer-based delivery system in mouse model. Int. J. Nanomed. 2017, 12, 8573–8585. [Google Scholar] [CrossRef][Green Version]
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S.; et al. Dendrimer-RNA nanoparticles generate protective immunity against lethal Ebola, H1N1 influenza, and Toxoplasma gondii challenges with a single dose. Proc. Natl. Acad. Sci. USA 2016, 113, e4133–e4142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Folliero, V.; Zannella, C.; Chianese, A.; Stelitano, D.; Ambrosino, A.; De Filippis, A.; Galdiero, M.; Franci, G.; Galdiero, M. Application of Dendrimers for Treating Parasitic Diseases. Pharmaceutics 2021, 13, 343. [Google Scholar] [CrossRef]
- Chahal, J.S.; Fang, T.; Woodham, A.W.; Khan, O.F.; Ling, J.; Anderson, D.G.; Ploegh, H.L. An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model. Sci. Rep. 2017, 7, 252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ribeiro, S.; Rijpkema, S.G.; Durrani, Z.; Florence, A.T. PLGA-dendron nanoparticles enhance immunogenicity but not lethal antibody production of a DNA vaccine against anthrax in mice. Int. J. Pharm. 2007, 331, 228–232. [Google Scholar] [CrossRef]
- Verminnen, K.; Beeckman, D.S.A.; Sanders, N.N.; De Smedt, S.; Vanrompay, D.C.G. Vaccination of turkeys against Chlamydophila psittaci through optimised DNA formulation and administration. Vaccine 2010, 28, 3095–3105. [Google Scholar] [CrossRef]
- Wang, X.; Dai, Y.; Zhao, S.; Tang, J.; Li, H.; Xing, Y.; Qu, G.; Li, X.; Dai, J.; Zhu, Y.; et al. PAMAM-Lys, a Novel Vaccine Delivery Vector, Enhances the Protective Effects of the SjC23 DNA Vaccine against Schistosoma japonicum Infection. PLoS ONE 2014, 9, e86578. [Google Scholar] [CrossRef][Green Version]
- Singh, M.; Briones, M.; Ott, G.; O’Hagan, D. Cationic microparticles: A potent delivery system for DNA vaccines. Proc. Natl. Acad. Sci. USA 2000, 97, 811–816. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huang, Q.; Zeng, J.; Yan, J. COVID-19 mRNA vaccines. J. Genet. Genomics 2021, 48, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Daftarian, P.; Kaifer, A.E.; Li, W.; Blomberg, B.B.; Frasca, D.; Roth, F.; Chowdhury, R.; Berg, E.A.; Fishman, J.B.; Sayegh, H.A.A.; et al. Peptide-Conjugated PAMAM Dendrimer as a Universal DNA Vaccine Platform to Target Antigen-Presenting Cells. Cancer Res. 2011, 71, 7452–7462. [Google Scholar] [CrossRef][Green Version]
- Cheng, Q.; Wei, T.; Jia, Y.; Farbiak, L.; Zhou, K.; Zhang, S.; Wei, Y.; Zhu, H.; Siegwart, D.J. Dendrimer-Based Lipid Nanoparticles Deliver Therapeutic FAH mRNA to Normalize Liver Function and Extend Survival in a Mouse Model of Hepatorenal Tyrosinemia Type I. Adv. Mater. 2018, 30, e1805308. [Google Scholar] [CrossRef]
- Pillay, N.S.; Daniels, A.; Singh, M. Folate-Targeted Transgenic Activity of Dendrimer Functionalized Selenium Nanoparticles In Vitro. Int. J. Mol. Sci. 2020, 21, 7177. . [Google Scholar] [CrossRef]
- Mbatha, L.S.; Maiyo, F.C.; Singh, M. Dendrimer functionalized folate-targeted gold nanoparticles for luciferase gene silencing in vitro: A proof of principle study. Acta Pharm. 2018, 69, 49–61. [Google Scholar] [CrossRef][Green Version]
- Mbatha, L.S.; Singh, M. Starburst Poly(amidoamine) Dendrimer Grafted Gold Nanoparticles as a Scaffold for Folic Acid-Targeted Plasmid DNA Delivery In Vitro. J. Nanosci. Nanotechnol. 2019, 19, 1959–1970. [Google Scholar] [CrossRef]
- Shan, Y.; Luo, T.; Peng, C.; Sheng, R.; Cao, A.; Cao, X.; Shen, M.; Guo, R.; Tomás, H.; Shi, X. Gene delivery using dendrimer-entrapped gold nanoparticles as nonviral vectors. Biomaterials 2012, 33, 3025–3035. [Google Scholar] [CrossRef]
- Yuan, X.; Wen, S.; Shen, M.; Shi, X. Dendrimer-stabilized silver nanoparticles enable efficient colorimetric sensing of mercury ions in aqueous solution. Anal. Methods 2013, 5, 5486–5492. [Google Scholar]
- Figueroa, E.R.; Lin, A.Y.; Yan, J.; Luo, L.; Foster, A.E.; Drezek, R.A. Optimization of PAMAM-gold nanoparticle conjugation for gene therapy. Biomaterials 2014, 35, 1725–1734. [Google Scholar] [CrossRef][Green Version]
- Oladimeji, O.; Akinyelu, J.; Singh, M. Co-Polymer Functionalised Gold Nanoparticles Show Efficient Mitochondrial Targeted Drug Delivery in Cervical Carcinoma Cells. J. Biomed. Nanotechnol. 2020, 16, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Akinyelu, J.; Oladimeji, O.; Singh, M. Lactobionic acid-chitosan functionalised gold-coated poly(lactide-co-glycolide) nanoparticles for hepatocyte targeted gene delivery. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 045017. [Google Scholar] [CrossRef]
- Mbatha, L.S.; Maiyo, F.; Daniels, A.; Singh, M. Dendrimer-Coated Gold Nanoparticles for Efficient Folate-Targeted mRNA Delivery In Vitro. Pharmaceutics 2021, 13, 900. [Google Scholar] [CrossRef] [PubMed]
- A Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of OP-101 after Intravenous Administration in Healthy Volunteers. Available online: https://clinicaltrials.gov/ct2/show/NCT03500627 (accessed on 29 January 2023).
- Kalomiraki, M.; Thermos, K.; Chaniotakis, N.A. Dendrimers as tunable vectors of drug delivery systems and biomedical and ocular applications. Int. J. Nanomed. 2016, 11, 1–12. [Google Scholar] [CrossRef][Green Version]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Amin, M.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef]
- Chowdhury, S.; Toth, I.; Stephenson, R.J. Dendrimers in vaccine delivery: Recent progress and advances. Biomaterials 2022, 280, 121303. [Google Scholar] [CrossRef]
- Beg, S.; Samad, A.; Alam, M.I.; Nazish, I. Dendrimers as Novel Systems for Delivery of Neuropharmaceuticals to the Brain. CNS Neurol. Disord. Drug Targets 2011, 10, 576–588. [Google Scholar] [CrossRef]
- Bolhassani, A.; Javanzad, S.; Saleh, T.; Hashemi, M.; Aghasadeghi, M.R.; Sadat, S.M. Polymeric nanoparticles Potent vectors for vaccine delivery targeting cancer and infectious diseases. Human Vaccines Immunother. 2014, 10, 321–332. [Google Scholar] [CrossRef][Green Version]
