2.2.1. Wnt/β-Catenin Signaling Pathway
The Wnt/β-catenin signaling pathway, also known as the canonical Wnt signaling pathway, plays a crucial role in numerous biological processes, such as embryonic development, the regulation of the cell cycle, apoptosis and many others. On the other hand, the dysregulation of the Wnt/β-catenin pathway is often associated with a variety of diseases, including cancer
[47][48][62,63]. In colorectal cancers, the Wnt/β-catenin pathway is one of the most important signaling pathways
[49][64]; therefore, the Wnt/β-catenin signaling pathway is an attractive target for cancer treatment and several small inhibitors targeting the Wnt/β-catenin pathway have been examined in preclinical and clinical studies
[50][51][52][65,66,67].
2.2.2. Nuclear Factor Kappa B Signaling Pathway
The nuclear factor kappa B (NF-
kB) signaling pathway plays a key role in the regulation of numerous genes and proteins involved in broad spectrum biological activities, such as inflammation, immune response and cell death and survival. The abnormal activation of the NF-
kB signaling has been reported in different tumor types
[53][74].
Licochalcone A has been reported to suppress the proliferation of colon cancer cells. Further Western blot and Rt-PCR analyzes have shown that LiA negatively modulates NF-
kB signaling and that this effect is closely related to the inhibition of p65 phosphorylation. It is important to mention that p65 phosphorylation is crucial for the transcriptional activity of NF-κB
[54][75]. Recently, Papierska et al.
[55][76] documented the potential of newly synthesized thioderivative chalcones to modulate NF-κB activity. This effect was associated with a decrease in the p65 nuclear fraction in chalcone-treated HCT116 cells. Moreover, the expression of the gene encoding p65 also significantly decreased in treated cells. The anticancer effect associated with the inhibition of NF-κB signaling and p65 translocation has also been observed in vivo in cardamonin-treated mice
[56][77].
2.2.3. Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway
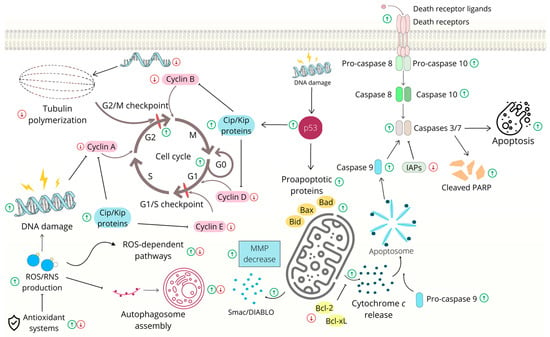
The mitogen-activated protein kinase (MAPK) pathway is involved in the regulation of different biological processes, such as gene expression and cell proliferation and survival. In cancer, the aberrant activation of MAPK signaling is often associated with the uncontrolled proliferation of cells and defective apoptosis
[57][85]. There are several components in MAPK signaling, although extracellular regulated kinases (ERK1/2), c-Jun N-terminal kinase (JNK) and p38 have been studied the most.
Cardamonin has also been reported to be an inhibitor of β-catenin and NF-κB signaling. Another mechanism of its antiproliferative effect is related to its ability to activate JNK via the stimulation of p53. As many authors have suggested, the activation of the p53/JNK pathway is essential for cardamonin-induced autophagy and the suppression of HCT116 colon cancer cells
[58][86]. Furthermore, the antiproliferative effect of isoliquiritigenin (ISL) has also been associated with the increased phosphorylation of JNK and ERK as a result of the increased production of reactive oxygen species (ROS)
[59][87].
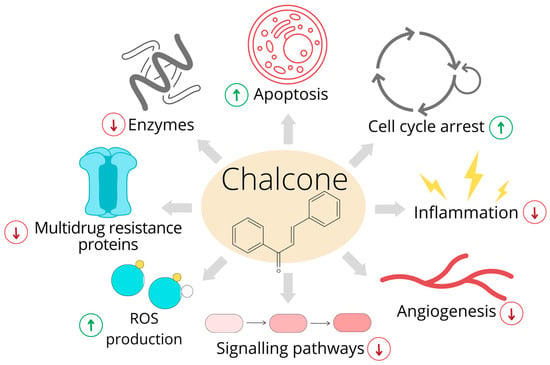
2.3. Enzyme Inhibition
Topoisomerases play essential roles in DNA replication by preventing deleterious excessive supercoiling. Topoisomerase inhibitors are commonly used in anticancer therapy, including irinotecan, topotecan (topoisomerase I inhibitors; TOPO I), etoposide and teniposide (topoisomerase II inhibitors; TOPO II)
[60][101].
Recently, Mohammed et al.
[61][102] described the antiproliferative effects of novel urea-ciprofloxacin–chalcone hybrids on HCT116 colon cancer cells. These effects were associated with G2/M cell cycle arrest and apoptosis induction. Further analyses showed that the most potent hybrids inhibited TOPO I and TOPO II, which was comparable to clinically used drugs. Later, they also reported significant inhibitory activity against TOPO I and TOPO II in triazole linked ciprofloxacin chalcone-treated colon cancer cells. This anti-topoisomerase effect has also been associated with DNA damage, G2/M arrest and tubulin dysregulation
[62][103].
2.4. Antiangiogenic Effect of Chalcones
One of the key factors in cancer development and metastasis is angiogenesis
[63][125]. The process of neovascularization is gently orchestrated by numerous positive and negative regulators of angiogenesis. Among them, the vascular endothelial growth factor (VEGF) and its receptor (VEGFR-2) are important targets in the treatment of various cancer types, including gastrointestinal tract cancers
[64][65][66][126,127,128]. In addition, numerous studies have shown the potential of natural compounds, including chalcones, in blocking different steps during angiogenesis
[67][68][69][129,130,131].
Hypoxia-inducible factor (HIF)-1α plays an important role in cancer adaptation to hypoxic microenvironments
[70][134]. Among other activities, HIF-1α also regulates angiogenesis via the up-regulation of several pro-angiogenic factors, such as VEGF, transforming growth factor-β, plasminogen activator-1 and erythropoietin. Due to its wide spectrum of regulatory activities, HIF-1α signaling is a promising target for anticancer therapy. In addition to synthetic compounds, several phytochemicals have also been reported to modulate HIF-1α activity
[71][135].
Furthermore, human umbilical vein endothelial cells (HUVECs) have also often been used as models to study the effects of agents targeting angiogenesis. Tube formation and the migration and invasion of endothelial cells are crucial features of angiogenesis. Recently, Wang et al.
[72][142] synthesized new chalcones based on 2-methoxyestradiol. Among other effects, they found that the most active compound was able to block the migration of HUVECs. This effect was associated with the reduced expression of the VEGF receptor, as well as the reduced phosphorylation of focal adhesion kinase and SHC, which are two proteins involved in endothelial cell migration. In addition, these compounds significantly suppressed angiogenesis in a CAM assay. Furthermore, newly synthesized α-substituted hetero-aromatic chalcone hybrids have been found to inhibit several steps of angiogenesis. In a wound healing model, chalcone 7m significantly inhibited HUVEC migration and also suppressed HUVEC invasion in a dose-dependent manner, as detected by a transwell assay. Additionally, HUVEC tube formation and length were dramatically inhibited in the chalcone-treated group. Furthermore, the in vitro results were then confirmed in vivo using a zebrafish embryo model
[73][143]. Later, they also documented a similar antiangiogenic effect also for new α-fluorinated chalcone
[74][144].
2.5. Chalcones and Inflammation
It is well known that tumors originate in areas that are infiltrated by cells from the immune system, i.e., areas that are repeatedly exposed to inflammation
[75][146]. It is now believed that chronic inflammation is responsible for changing sensitive cells via neoplastic transformation. In general, the elderly are at the greatest risk of developing cancer as they have experienced multiple inflammatory responses induced by infectious agents and other stressors during their lifetimes. Long-term exposure to carcinogenic factors, including inflammation, is a cause of cancer.
The presence of immune cells in tumors demonstrates that cells with innate immunity actively model tumor processes. The processes of chronic inflammation (i.e., leukocyte infiltration and angiogenesis) are thought to play important roles in tumor progression. Tumor microenvironments (TMEs) modulate tumor growth and progression through the production of reactive oxygen species (ROS), epigenetic changes and the simultaneous promotion of tumorigenesis through the production of growth factors and pro-inflammatory cytokines
[76][147]. Growing tumors influence TMEs via feedback pathways through the production of cytokines and chemokines. An increased risk of cancer has also been shown to be associated with chronic intestinal diseases, which can be linked to microbial infections or inappropriate diets.
Many scientific studies have shown that chalcones and their flavonoid derivatives are effective anti-inflammatory substances that protect against the development of cancer. Their antimicrobial, antibacterial and antiparasitic activities have been proven in relation to CRC. In general, anti-cancer chalcones could simultaneously be considered as anti-inflammatory agents due to the fact that they increase the synthesis of anti-inflammatory cytokines
[77][78][149,150].
The Toll-like receptor 4 (TLR4) is abundantly expressed in intestinal epithelial cells and is thought to play a key role in intestinal innate immunity
[79][151]. It is also involved in the pathogenesis of inflammatory bowel disease, with TLR4 activation resulting in the nuclear translocation of transcriptional NF-κB and/or the activation of MAPKs, leading to the production of pro-inflammatory cytokines and chemokines
[80][152]. Many studies have shown that NF-κB plays a major role in the development and progression of cancer because it regulates more than 400 genes that are involved in inflammation and carcinogenesis
[81][153]. Therefore, it is desirable to down-regulate TLR4 expression, block the activation of the NF-κB and MAPK signaling pathways, and inhibit the pro-inflammatory gene expression of COX, prostaglandin E2, inducible nitric oxide synthase (iNOS) and numerous cytokines (e.g., IL-1β, IL-6, IL-8 and TNF-α).
Chalcones, such as LiA, are important inhibitors of TLR4-mediated NF-κB activation, with higher accumulation in Caco-2 cells
[82][154]. Licochalcone A exudes anti-UC activity, in part by blocking the MAPK pathway
[83][155]. Another natural chalcone, ISL, reduces the incidence of colitis that is associated with colorectal cancer through the modulation of gut microbiota and pro-inflammatory cytokines
[84][156]. Furthermore, xanthoangelol D (25 mg/kg) reduces the disease activity index (DAI) of colitis and the dextran sulfate sodium-induced increases in colonic MCP-1, IL-1β and TNF-α levels
[85][157]. The inhibition of NF-κB activity, p38-regulated/activated kinase (PRAK) and MAPK-activated protein kinase (MAPKAP-K)-3 suppression have been reported effects of flavokawain A, which is isolated from
Piper methysticum [86][158].
The anti-inflammatory properties of chalcones due to the inhibition of NF-κB activity are related to the downregulation of iNOS, COX-2, TNF-α and IL-6. Scientific studies on chalcones have demonstrated their anti-inflammatory properties via the observation of multiple pro-inflammatory markers. Xn
[87][159] and cardamonin
[88][160] have been found to be significant COX-2 inhibitors that also simultaneously inhibit iNOS. Kim et al. demonstrated that ISL reduced iNOS, COX-2, TNF-α and IL-6
[89][161]. Similarly, LiA inhibited the expression of iNOS and COX-2 and suppressed the production of NO and PGE
2 [90][162]. Chalcone 4,2′,5′-trihydroxy-4′-methoxychalcone is isolated from the heartwood of
D. odorifera and has been found to inhibit the production of NO, the expression of COX-2 and nitric oxide synthase and the release of TNF-α and IL-1β
[91][163]. Chalcones isolated from
Humulus lupulus, such as xanthohumol B, xanthohumol D and dihydroxanthohumol, have shown the potential to inhibit LPS-induced NO production without any cytotoxicity, as well as inhibiting iNOS expression
[92][164].
2.6. Chalcone-Induced Oxidative Stress
Cellular physiological processes are inherently associated with the production of reactive oxygen species (ROS), which are involved in several signaling pathways
[93][172]. When ROS formation is uncontrolled, oxidative stress occurs, which disrupts redox signaling and causes damage to biomolecules, at the expense of cellular antioxidant defense mechanisms
[94][173]. In cells, oxidation–reduction homeostasis is ensured through the glutathione
[95][174] and thioredoxin systems
[96][175]. Moreover, several antioxidant enzymes are present in cells, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione S-transferase (GST), which maintain redox status and protect the cells against oxidative and nitrosative stress. It is well known that several types of ROS are produced continuously by mitochondrial bioenergetics and oxidative metabolism, including superoxide anions (O
2−), hydroxyl radicals (·HO), perhydroxyl radicals (·HOO), non-radical hydrogen peroxide (H
2O
2) and nitrogen (RNS) species, such as nitric oxide (·NO) and peroxynitrite (ONOO
−)
[97][176].
In cancer research and therapy, radiotherapy and chemotherapy are the possible approaches to the treatment of tumor cells, which kill tumor cells through the production of ROS. Additionally, the anti-cancer effects of several drugs have been connected with ROS generation and/or decreases in ROS attenuators and antagonists. As previously described, chalcones are promising antitumor substances of natural origin, which also possess pro-oxidant activity and pro-apoptotic effects that are associated with oxidative stress and the modulation of ROS-dependent signaling pathways and DNA damage
[23][36]. It is well known that the antioxidant properties of chalcones are based on structural variations and diversity in their molecules. Stabilized radicals are formed during reactions between chalcones and ROS.
2.7. Chalcones and Multidrug Resistance
Multidrug resistance (MDR) caused by efflux membrane proteins from the ATP-binding cassette transporter superfamily (ABC transporters) is considered to be one of the potential mechanisms that leads to insufficient therapeutic outcomes
[98][99][100][195,196,197].
Previous research has indicated that MDR is mainly the result of the action of ATP-binding cassette subfamily B member 1 protein (ABCB1), also known as P-glycoprotein (P-gp) or multidrug resistance protein 1 (MDR1). The literature has also shown the elevated expression of this transporter in both colorectal cancer
[101][198] and gastric cancer
[102][199]. Additionally, several studies have demonstrated the association between ABCB1 overexpression and resistant phenotypes in such tumors
[103][104][200,201]. Conversely, the downregulation of ABCB1 in cancer cells leads to increased chemosensitivity to 5-FU, epirubicin, irinotecan and oxaliplatin
[105][106][107][108][109][202,203,204,205,206], drugs that are still commonly used in the treatment of gastrointestinal carcinomas
[110][111][207,208].
Further research has revealed that chalcones may inhibit the ABCB1 function by disrupting the integration or function of cell membranes. For example, IBC has been shown to exhibit interaction potential with membranes
[112][220]. It has also been found to elevate the intracellular accumulation of doxorubicin in doxorubicin-resistant human adenocarcinoma colon cancer cells (HT29/Dx), although the effect was milder than that of verapamil. Those authors assumed that the substance acts more as a competitive inhibitor than a displacer of substrates with lower affinity to the binding sites. However, the compound has not demonstrated significant intrinsic cytotoxic effects on any of the tested human colon cancer cells and has not been found to sensitize cells to doxorubicin, indicating that efflux by ABCB1 is not the only mechanism of resistance in these cells.