Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Alberto Palazzuoli.
The moderator band (MB) is an intracavitary structure of the right ventricle composed of muscular fibers encompassing specialized Purkinje fibers, separated each other by collagen and adipose tissue. Premature ventricular complexes originating within the Purkinje network have been implicated in the genesis of life-threatening arrhythmias. However, right Purkinje network arrhythmias have been much less reported in the literature compared to the left counterpart. The MB has unique anatomical and electrophysiological properties, which may account for its arrhythmogenicity and may be responsible for a significant portion of idiopathic ventricular fibrillation.
- ventricular tachycardia
- electrical disorder
- moderator band
- Purkinje fibers
- ablation
- pharmacological treatment
1. Introduction
Moderator band (MB) related ventricular tachycardias (VTs) and premature ventricular contractions (PVC) are being increasingly recognized as a cause of idiopathic VTs. The MB is a muscular structure extending from the anterior septum to the anterior papillary muscle (APM) of the right ventricular (RV) free wall. This structure comprises a specialized Purkinje fibers network which allows for rapid activation of the RV-free wall [1]. The MB is composed of muscular fibers encompassing specialized Purkinje fibers, separated from the surrounding muscular cells by collagen and adipose tissue. Additionally, it is highly innervated by autonomic nervous system elements [2], with important implications in arrhythmogenesis. Idiopathic ventricular arrhythmias, defined by the absence of any identifiable structural heart disease or genetic arrhythmic disorder, have usually been considered “benign” in terms of prognosis [3]. Nonetheless, in the last decades, short coupling PVCs [4] e and PVCs originating within the Purkinje network have been described as a trigger of life-threatening arrhythmias and characterization and treatment of such idiopathic ventricular arrhythmias have become increasingly investigated [5,6,7][5][6][7]. After the development of sophisticated imaging tools such as intracardiac echocardiography (ICE) and intracardiac mapping, MB has been increasingly recognized as a source of short-coupling PVCs, VTs, and idiopathic ventricular fibrillation (IVF); [8,9][8][9]. The arrhythmogenic potential of MB involves multiple mechanisms due to the peculiar anatomical and electrical properties of this structure. Purkinje fibers may initiate arrhythmias by different mechanisms, mainly enhanced automaticity, trigger activity, and re-entry [10]. Sustained VTs are instead supported by a reentrant mechanism, favored by the action potential duration (APD) gradient established between MB and RV, and can be treated by catheter ablation [11].
2. Anatomy of the Moderator Band
The MB is an intracavitary structure of the RV which spans from the interventricular septum (IVS) to the RV-free wall at the base of the APM, and it is considered part of the septomarginal trabeculation. The MB has high interindividual variability in terms of origin and shape [12]. It might originate from the supraventricular crest to the ventricular apex, with single or double roots. Moreover, it can be classified into three major types according to its shape: cylindrical column (type 1), long and thin column (type 2), and wide and flat column (type 3) [13]. The most common configuration of MB is a single root origin from the middle IVS with a type 2 shape.
Originally described by Leonardo Da Vinci, the MB takes its name from what it was thought to be its function, which is to prevent RV excessive distension thanks to the anatomic link of the IVS and RV free wall. Later, it was found that part of the right half of the Purkinje system is contained in the MB, allowing for synchronous activation of the RV-free wall during systole. The right Purkinje system is composed of the right bundle branch (RBB), the free-running Purkinje fibers (PF), which branch from the RBB along its length, and the terminal PF, which connects with the free-running PF in the parietal RV wall. The terminal PF account for the largest part of the right Purkinje network, forming a more complex network than that in the left ventricle that cover the majority of the RV free wall and extends toward the tricuspid valve [12]. PF spreads across the IVS through the MB, becomes more superficial in the distal third of the MB, and finally joins the APM to the RV-free wall, forming the subendocardial ventricular plexus.
PF are a longitudinal assembly of Purkinje cells (PC) and are insulated from working myocytes by a connective tissue sheath that is lost before the PF form the terminal connections with the working myocytes through specialized junctions in the endocardium [14,15][14][15]. Recently, Walton et al. [11] demonstrated that the MB has a compartmentalized structure between the myocardium and PF, which have a coaxial configuration separated each other by a layer of collagen and lipid droplets that provide electrical insulation. Therefore, the MB is formed by these two excitable yet electrically uncoupled compartments.
The MB vasculature is perfused by the anterior interventricular coronary artery and the right coronary artery from the base of the APM, forming an important collateral circulation between the left and right coronary artery systems [1].
Innervation of the MB is supplied by elements of the autonomic system, being the PF more sensible to the parasympathetic neurotransmitter acetylcholine (ACh) compared to ventricular muscle because of a higher expression of channels responsible for IKACh current [2,16][2][16].
3. Electrical Properties of the Moderator Band
PC are specialized myocardial cells that are responsible for the synchronous activation of the ventricles. Ion channel expression substantially differs from that of the working myocites and can explain the specialized electrical activity of the PC [12]. Differently from working myocardium, PC are poorly contractile and, therefore, have a lower expression of Ca2+-handling proteins such as RYR2 (Ryanodine receptor 2), RYR3 (Ryanodine receptor 3), SERCA2a (sarcoplasmatic/endoplasmic reticulum Ca2 ATPase 2a), and NCX (Na+-Ca2+ exchangers). In contrast, they are fast conducting because of high upstroke velocity during phase 0 of action potential and high conduction velocity. These properties are explained by a higher expression of Na+ channels, particularly Na1.1, and of the fast-conducting connexin Cx40. Regarding the K+ channel profile, PC show a lower expression of Kv1.4 and a higher expression of Kv4.2, the first being a slow recovering isoform while the second being a fast one, both responsible for Ito (transient outward current). On the other hand, the K+ conductance involved in phase 2 and phase 3, namely IKs, IKr, and IKur (for whom KvLQT1, ERG, and Kv1.5 are responsible, respectively), are predicted to be lower in PC as compared to working myocardium. In addition, the IK1 current generated by the Kir2 channels family (Kir2.1–2.4) responsible for the maintaining of resting potential is less represented in PC, and HCN 1 and HCN4, which underlie If current, are more expressed. All these differences account for the following differences in action potential: high upstroke velocity during phase 0, a prominent early rapid repolarization (phase 1), a negative potential plateau (phase 2), an increased APD, and a spontaneous diastolic depolarization (phase 4), which is normally inapparent because of overdrive suppression by the sinus node. Additionally, it has been shown that genetically determined upregulation of the DPP6 (dipeptidyl aminopeptidase-like protein 6) gene, which encodes for a beta subunit of Kv4 channels, enhances Ito in PF with consequent strong repolarization gradient between the PF and the adjacent ventricular myocardium, with possible phase 2 re-entry induced PVCs [17].
Moreover, the electrophysiological properties of MB may be influenced by its anatomic variability [11]. Particularly, variability of thickness and muscle/PFs ratio may be associated with a reduction of the effective refractory period, which is sensitive to tissue mass and coupling. Many factors may as well affect the dispersion of refractoriness within the myocardial syncytium represented by the MB, such as mechanical stretch (i.e., RV overload, bradycardia, conditions that induce a prolongation of APD; [18] and cardiac autonomic nervous system modulation [19]. These features may explain the ECG morphologies of PVC (Figure 1).
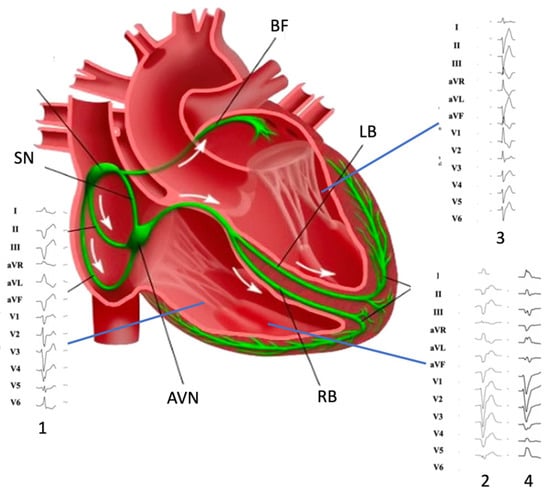
Figure 1. Morphology of premature ventricular complexes originating from (1) inferior tricuspid valve, (2) moderator band, (3) left posterior fascicle, and (4) moderator band (note DII/DIII discordance). SN: Sinus Node; AVN: atrio-ventricular node; RB right bundle; LB: left bundle; BF: Bachmann’s fibers.
4. Moderator Band-Related Arrhythmias
4.1. Arrhythmogenic Mechanisms
All arrhythmogenic mechanisms may be involved in the genesis of MB-related arrhythmias, particularly triggered activity and re-entry. PF are often involved in the genesis of ventricular arrhythmias due to their susceptibility to the development of early afterdepolarizations (EADs). EADs seem to be ascribable mainly to inward calcium currents (L-type Ca2+) but also to late Na+ currents during late phase 2/early phase 3 of the action potential. Moreover, PVCs may originate from a phase 2 re-entry because of the strong repolarization gradient between the two compartments of the MB [20,21][20][21]. A critical number of cells exhibiting afterdepolarizations in a synchronous manner is essential to generate arrhythmias at the organ dimension. The susceptibility of the RV-free wall-MB junction to generate short-coupling PVCs may be related to the mechanical strain of the MB during proto-diastole, a “mechanoelectrical feedback” phenomenon that may induce ion channel activation [22].
Short-coupled PVCs originating in the proximal MB may determine a unidirectional conduction block within the myocardial compartment of the MB and consequently induce a sustained monomorphic VT through a macro re-entry circuit. The Purkinje network must overcome the large source-sink mismatch where it couples with the myocardium. The high density of Na+ channels is essential to provide sufficient safety factors for conduction. Moreover, a transitional layer of cells serves to amplify impulses and shield the Purkinje network from electrotonic loading. The wavefront propagates from the IVS to the RV-free wall and then activates it both in basal and apical directions. While the apical wavefront collides at the MB root with the repolarization wave tail, the basal propagation front encounters excitable tissues of the IVS, sufficiently delayed to allow the circuit completion. The demonstration of the involvement of these structures in the re-entry circuit has been provided by the experiment conducted by Walton et al. that showed the interruption of the macro reentry circuit after severing the MB in a sheep heart during sustained VT [11].
Additionally, polymorphic VT, like torsades de pointes, may result from a meandering or drifting spiral wave propagation. Both monomorphic and polymorphic VT, when an electrical or structural obstacle is encountered, may eventually degenerate into ventricular fibrillation (VF) because multiple spiral waves break up with the consequent genesis of daughter spirals. MB-related VF still represents a rare but not neglectable percentage of IVF [23]. Some case series described a VT and VF related to early coupled PVC originating from the MB [8]. Short coupled torsade de point (sc-TdP) should be classified as idiopathic VF from PF.
Furthermore, 3D-mapping and intracardiac echocardiography showed that sc-TdP predominantly originated from MB free wall and its PF network [24].
4.2. Right Purkinje Network Arrhythmias: In Addition to the Moderator Band
As mentioned, the right Purkinje system is composed of the RBB, free-running PF, and terminal PF, which form a complex network at the RV free wall extending up to the tricuspid valve (TV); [12]. The electrophysiological properties of the PC, which intrinsically justify a proarrhythmic behavior, may theoretically result in arrhythmias originating at each level of the complex right Purkinje network. However, right Purkinje network arrhythmias and their potential deleterious consequences have been less reported in the literature. Therefore, MB-related arrhythmias should be differentiated from other right-sided PF arrhythmias, such as those from APM and TV. VTs originating at the APM level seem to be provokable by isoproterenol or burst pacing, suggesting triggered activity as the underlying mechanism [25]. Moreover, the inability to entrain VTs from APM or terminate them by overdrive pacing supports automaticity instead of re-entry as a potential inducible mechanism.
The electrocardiographic (ECG) analysis may help clinicians in hypothesizing these specific arrhythmias. MB-related arrhythmias are typically classified among those with a superior axis configuration, such as those from inferior TV and left posterior fascicles [26,27,28][26][27][28] (Figure 1). Typically, MB-related arrhythmias have a left superior axis (positive D1 and negative DII/DIII) and late precordial transition (later than V4), similar to those originating from APM [29]. Sometimes, DII/DIII discordance may be observed, such as for lateral TV and para-Hissian origins. TV arrhythmias may display a variable precordial transition depending on the more septal or lateral origin (from V2 to V5) for those with a superior axis. On the contrary, left posterior fascicle arrhythmias usually mimic a typical RBB pattern with rsR’ and specifically have a QRS < 130 ms because of the rapid ventricular depolarization through the Purkinje system.
Sometimes monomorphic VTs may sustain through a re-entry circuit involving both the right and left bundle branches (called bundle branch re-entry), a single left fascicle (intrafascicular re-entry), or both left fascicles (interfascicular re-entry). However, the intrafascicular re-entry has not been demonstrated at the right Purkinje system level yet. Bundle branch re-entry VTs are often observed in patients with acquired heart disease, which might involve the His-Purkinje system, and, therefore, would lead to slow conduction within the Purkinje system necessary for the occurrence of re-entry. However, conduction disturbances in the His-Purkinje system of young patients with structurally normal hearts can also lead to susceptibility to bundle branch reentry VTs [30]. Finally, MB could be involved in atrioventricular reciprocating arrhythmias due to the insertion of an accessory pathway (Mahaim fascicles) into the PF of the MB.
References
- Loukas, M.; Klaassen, Z.; Tubbs, R.S.; Derderian, T.; Paling, D.; Chow, D.; Patel, S.; Anderson, R.H. Anatomical observations of the moderator band. Clin. Anat. 2010, 23, 443–450.
- Bojsen-Moller, F.; Tranum-Jensen, J. On nerves and nerve endings in the conducting sysetm of the moderator band (septomarginal trabecula). J. Anat. 1971, 108, 387–395.
- Belhassen, B.; Viskin, S. Idiopathic Ventricular Tachycardia and Fibrillation. J. Cardiovasc. Electrophysiol. 1993, 4, 356–368.
- Viskin, S.; Rosso, R.; Rogowski, O.; Belhassen, B. The “Short-Coupled” Variant of Right Ventricular Outflow Ventricular Tachycardia: A Not-So-Benign Form of Benign Ventricular Tachycardia? J. Cardiovasc. Electrophysiol. 2005, 16, 912–916.
- Boyden, P.A.; Dun, W.; Robinson, R.B. Cardiac Purkinje fibers and arrhythmias; The GK Moe Award Lecture 2015. Heart Rhythm. 2016, 13, 1172–1181.
- Haïssaguerre, M.; Shoda, M.; Jaïs, P.; Nogami, A.; Shah, D.C.; Kautzner, J.; Arentz, T.; Kalushe, D.; Lamaison, D.; Griffith, M.; et al. Mapping and Ablation of Idiopathic Ventricular Fibrillation. Circulation 2002, 106, 962–967.
- Haïssaguerre, M.; Shah, D.C.; Jaïs, P.; Shoda, M.; Kautzner, J.; Arentz, T.; Kalushe, D.; Kadish, A.; Griffith, M.; Gaita, F.; et al. Role of Purkinje conducting system in triggering of idiopathic ventricular fibrillation. Lancet 2002, 359, 677–678.
- Sadek, M.M.; Benhayon, D.; Sureddi, R.; Chik, W.; Santangeli, P.; Supple, G.E.; Hutchinson, M.D.; Bala, R.; Carballeira, L.; Zado, E.S.; et al. Idiopathic ventricular arrhythmias originating from the moderator band: Electrocardiographic characteristics and treatment by catheter ablation. Heart Rhythm. 2015, 12, 67–75.
- Anter, E.; Buxton, A.E.; Silverstein, J.R.; Josephson, M.E. Idiopathic Ventricular Fibrillation Originating from the Moderator Band. J. Cardiovasc. Electrophysiol. 2012, 24, 97–100.
- Nogami, A.; Sugiyasu, A.; Kubota, S.; Kato, K. Mapping and ablation of idiopathic ventricular fibrillation from the Purkinje system. Heart Rhythm. 2005, 2, 646–649.
- Walton, R.D.; Pashaei, A.; Martinez, M.E.; Constantin, M.; Duchateau, J.; Bear, L.; Cros, C.; Pascarel-Auclerc, C.; Guo, Y.; Benoist, D.; et al. Compartmentalized Structure of the Moderator Band Provides a Unique Substrate for Macroreentrant Ventricular Tachycardia. Circ. Arrhythmia Electrophysiol. 2018, 11, e005913.
- Atkinson, A.; Inada, S.; Li, J.; Tellez, J.O.; Yanni, J.; Sleiman, R.; Allah, E.A.; Anderson, R.H.; Zhang, H.; Boyett, M.R.; et al. Anatomical and molecular mapping of the left and right ventricular His–Purkinje conduction networks. J. Mol. Cell. Cardiol. 2011, 51, 689–701.
- Lee, J.Y.; Hur, M.S. Morphological classification of the moderator band and its relationship with the anterior papillary muscle. Anat. Cell Biol. 2019, 52, 38–42.
- Dobrzynski, H.; Anderson, R.H.; Atkinson, A.; Borbas, Z.; D’Souza, A.; Fraser, J.F.; Inada, S.; Logantha, S.J.; Monfredi, O.; Morris, G.M.; et al. Structure, function and clinical relevance of the cardiac conduction system, including the atrioventricular ring and outflow tract tissues. Pharmacol. Ther. 2013, 139, 260–288.
- Sommer, J.R.; Johnson, E.A. Cardiac muscle: A comparative study of Purkinje fibers and ventricular fibers. J. Cell Biol. 1968, 36, 497–526.
- Schmid, P.G.; Greif, B.J.; Lund, D.D.; Roskoski, R., Jr. Regional choline acetyltransferase activity in the guinea pig heart. Circ. Res. 1978, 42, 657–660.
- Xiao, L.; Koopmann, T.T.; Ördög, B.; Postema, P.G.; Verkerk, A.O.; Iyer, V.; Sampson, K.J.; Boink, G.J.; Mamarbachi, M.A.; Varro, A.; et al. Unique cardiac Purkinje fiber transient outward current β-subunit composition: A potential molecular link to idiopathic ventricular fibrillation. Circ. Res. 2013, 112, 1310–1322.
- Myerburg, R.J.; Stewart, J.W.; Hoffman, B.F. Electrophysiological Properties of the Canine Peripheral A-V Conducting System. Circ. Res. 1970, 26, 361–378.
- He, B.; Lu, Z.; He, W.; Huang, B.; Yu, L.; Wu, L.; Cui, B.; Hu, X.; Jiang, H. The effects of atrial ganglionated plexi stimulation on ventricular electro-physiology in a normal canine heart. J. Interv. Card. Electrophysiol. 2013, 37, 1–8.
- Antzelevitch, C.; Sicouri, S. Clinical relevance of cardiac arrhythmias generated by afterdepolarizations: Role of M cells in the generation of U waves, triggered activity and torsade de pointes. J. Am. Coll. Cardiol. 1994, 23, 259–277.
- Li, P.; Rudy, Y. A model of canine purkinje cell electrophysiology and Ca(2+) cycling: Rate dependence, triggered activity, and comparison to ventricular myocytes. Circ. Res. 2011, 109, 71–79.
- Quinn, T.A.; Jin, H.; Lee, P.; Kohl, P. Mechanically Induced Ectopy via Stretch-Activated Cation-Nonselective Channels Is Caused by Local Tissue Deformation and Results in Ventricular Fibrillation if Triggered on the Repolarization Wave Edge (Commotio Cordis). Circ. Arrhythmia Electrophysiol. 2017, 10, e004777.
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126.
- Steinfurt, J.; Nazer, B.; Aguilar, M.; Moss, J.; Higuchi, S.; Zarse, M.; Trolese, L.; Gressler, A.; Faber, T.S.; Odening, K.E.; et al. Catheter ablation of short-coupled variant of torsade de pointes. Clin. Res. Cardiol. 2021, 111, 502–510.
- Crawford, T.; Mueller, G.; Good, E.; Jongnarangsin, K.; Chugh, A.; Pelosi, F.; Ebinger, M.; Oral, H.; Morady, F.; Bogun, F. Ventricular arrhythmias originating from papillary muscles in the right ventricle. Heart Rhythm. 2010, 7, 725–730.
- Enriquez, A.; Baranchuk, A.; Briceno, D.; Saenz, L.; Garcia, F. How to use the 12-lead ECG to predict the site of origin of idiopathic ventricular arrhythmias. Heart Rhythm. 2019, 16, 1538–1544.
- Efremidis, M.; Vlachos, K.; Kyriakopoulou, M.; Mililis, P.; Martin, C.A.; Bazoukis, G.; Dragasis, S.; Megarisiotou, A.; Unger, P.; Frontera, A.; et al. The RV1-V3 transition ratio: A novel electrocardiographic criterion for the differentiation of right versus left outflow tract premature ventricular complexes. Heart Rhythm. O2 2021, 2, 521–528.
- Fries, B.; Johnson, V.; Rutsatz, W.; Schmitt, J.; Bogossian, H. Lokalisation ventrikulärer Extrasystolen im 12-Kanal-EKG . Herzschrittmachertherapie Elektrophysiologie 2021, 32, 21–26.
- Santoro, F.; Di Biase, L.; Hranitzky, P.; Sanchez, J.E.; Santangeli, P.; Perini, A.P.; Burkhardt, J.D.; Natale, A. Ventricular Tachycardia Originating from the Septal Papillary Muscle of the Right Ventricle: Electrocardiographic and Electrophysiological Characteristics. J. Cardiovasc. Electrophysiol. 2014, 26, 145–150.
- Chen, H.; Shi, L.; Yang, B.; Ju, W.; Zhang, F.; Yang, G.; Gu, K.; Li, M.; Cao, K.; Ouyang, F.; et al. Electrophysiological Characteristics of Bundle Branch Reentry Ventricular Tachycardia in Patients Without Structural Heart Disease. Circ. Arrhythmia Electrophysiol. 2018, 11, e006049.
More
