Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Diogo M.F. Santos and Version 2 by Catherine Yang.
Water electrolysis is a powerful technology for producing high-purity H2, with negligible emission of greenhouse gases and compatibility with renewable energy sources. Additionally, the electrolysis of organic compounds, such as lignin, is a promising method for localised H2 production, as it requires lower cell voltages than conventional water electrolysis. Industrial wastewater can be employed in those organic electrolysis systems due to their high organic content, decreasing industrial pollution through wastewater disposal. Electrocoagulation, indirect electrochemical oxidation, anodic oxidation, and electro-Fenton are effective electrochemical methods for treating industrial wastewater.
- water electrolysis
- CO2 reduction
- hydrogen production
- wastewater treatment
- industrial electrochemistry
1. Production of H2
Significant waste is produced in the agricultural, domestic, and industrial sectors [1][54]. Traditional wastewater treatment methods include physical, chemical, and anaerobic treatment, which are not efficient to produce harmless wastewater. On the other hand, wastewater contains large amounts of organic matter, making it highly valuable in resource utilisation [2][43]. This unused resource could be a potential source of energy content (17.8 kJ g−1 COD) if efficient and economically viable technologies are available to exploit it [3][56]. Biological H2 production has several advantages compared to photoelectrochemical or thermochemical processes, due to its low energy requirements and low investment costs [4][15]. Among the several types of wastewater bio-treatment technology, the bio-electrochemical method is a good way to achieve the goal of harmless wastewater production and resourceful utilisation. This approach is able to both treat wastewater to meet emission standards and produce high-value products. This includes electricity generation from microbial fuel cells (MFC) and H2 production from microbial electrolysis cells (MEC) [5][55].
The MEC has evolved with significant advancements in producing H2 and other value-added products, such as CH4. Biohydrogen production from MEC has been used with industrial effluents owing to the advantage of energy recovery from waste with simultaneous treatment [4][15]. MEC includes an anode chamber and a cathode chamber separated by a PEM. An exoelectrogenic microorganism oxidises the organic matter in the wastewater and releases protons and electrons that are transferred to the cathode side through the membrane and the external circuit, respectively. Electrons and protons then combine at the surface of the cathode catalyst to form H2 gas. However, as this process does not occur spontaneously due to thermodynamic limitations, a small amount of electrical energy must be supplied [4][15]. Still, the specific energy consumption is considerably inferior to the electricity consumed by the H2 production process in water electrolysis [5][55]. The MEC reactor can be divided into single (Figure 1) and two-chamber versions. The most critical drawback of the single chamber MEC design is that the produced H2 is consumed by microorganisms such as methanogens, electrogens, and homoacetogens, particularly during long-term operation.
The dual-chamber MEC includes a membrane to favour higher H2 recovery and higher H2 purity, and avoid H2 consumption by methanogenic bacteria [3][56]. In addition, MEC can use various carbon sources, such as domestic and industrial wastewater, to produce H2 and simultaneously reduce the chemical oxygen demand (COD) [4][15]. Jayabalan et al. [3][56] investigated the enhancement of biohydrogen production from sugar industry effluent using nanocomposite catalysts coated on a NF cathode in a dual-chamber MEC reactor. Each of the three MEC reactors fabricated was composed of an anodic and cathodic compartment separated by a Nafion®117 PEM from DuPont™, a plain graphite plate as the anode, a copper wire current collector, and a Ni foam cathode coated with a nanocomposite catalyst (NiO.rGO and Co3O4.rGO). The anode and cathode compartments (250 mL) were filled with industrial sugar wastewater and 50 mM phosphate buffer [3][56].
Furthermore, at an applied voltage of 1.0 V and ambient temperature, the MEC with NiO.rGO/NF attained the highest COD removal efficiency (56.6%), H2 production rate (4.83 ± 0.11 mmol L−1 D−1), and current density (1.16 A m−2). The organic matter conversion in wastewater is the primary mode of H2 generation in the form of splitting (anode side) and combining protons and electrons (cathode side). The transport of the protons and electrons influences the microcellular activity and impacts the COD removal efficiency. Jayabalan et al. [3][56] concluded that Ni-based catalysts exhibited high activity for HER, suggesting Ni should replace conventional precious metal catalysts.
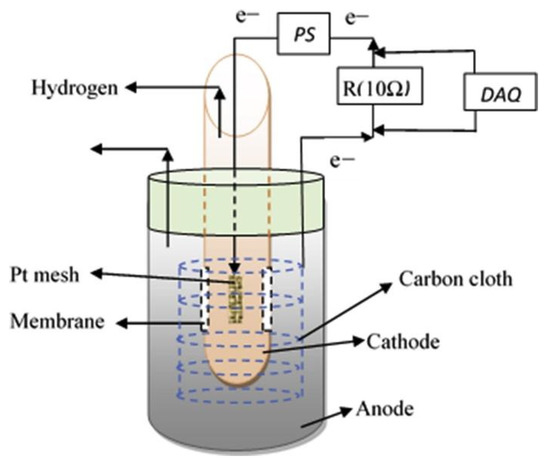
Spurgeon et al. [4][15] constructed a new compact design of MEC within an anaerobic digester. The cathode chamber is fitted in the anode chamber in a format similar to the typical dual-chamber MEC for wastewater treatment and H2 generation. Distillery wastewater was used in this study. The MEC was built using a glass bottle for the anodic chamber and a centrifuge tube for the cathodic chamber. A Nafion 117 PEM (Membrane Inc., USA) with an area of 5 cm2 separated the two chambers of the MEC. The anode was a 50 cm2 plain carbon cloth, and the cathode was a 2 cm2 Pt mesh. The anodic chamber contained 450 mL of artificial wastewater composed of sodium acetate, activated sludge, nutrients, minerals, and buffer solution. The cathodic chamber contained 25 mL of phosphate buffer with 50 mM K2HPO4 and 50 mM KH2PO4. The pH of the anolyte was adjusted to 7 using 2 M NaOH, and the pH of the catholyte was set to 7.5 using 0.5 M H2SO4. A titanium wire connected the anode and the cathode for the electronic transfer between the chambers. A variable voltage of 0.6 to 1.0 V was applied to study the effect of applied voltage on H2 generation. The tests were carried out for three days under batch mode operation at 1 atm and 35 °C [4][15].
A cumulative H2 production of 40.05 ± 0.5 and 30.12 ± 0.5 mL were obtained at current densities of 811.7 ± 20 and 908.3 ± 25 mA m−2, respectively, for the traditional design and the modified MEC system. The cathodic hydrogen recovery (CHR), corresponding to electron recovery as H2, presented a maximum of 46.5 ± 0.8 and 38.8 ± 0.5% in conventional and modified MEC, respectively. The Coulombic efficiency (CE), corresponding to the recovery of total electrons in acetate as current, was 17.25 ± 0.15 and 16.82 ± 0.1% for conventional and modified MEC, respectively. In addition, for traditional and modified MEC designs, the wastewater COD removal efficiency was observed to be 77.5 ± 1.0% and 75.6 ± 1.5 in 70 h. The modified compact design worked effectively to produce H2 under different COD concentrations (increased substrate concentration reduced the COD removal efficiency), anolyte and catholyte concentrations (H2 generation increased with catholyte concentration), and applied potentials (H2 production increased with the potential). In addition, the MEC performance in terms of CHR, CE, and COD removal efficiency for synthetic wastewater and distillery wastewater was compared. For the system operated with distillery wastewater, the results for these three parameters were 33.85 ± 0.5, 13.16 ± 0.2, and 72.5 ± 0.5%, respectively. The synthetic wastewater had higher performance than the real wastewater. According to Spurgeon et al., these results due to to the distillery wastewater containing complex substrates and degradation pathways of exoelectrogenic species. In summary, Spurgeon et al. concluded that the modified compact MEC, a retrofit to existing anaerobic digesters, can extend the use of anaerobic digesters and improve their economics in wastewater treatment [4][15].
Pathak et al. [6][53] studied an experimental method combining a solar PV-based electrolysis process and textile dyeing industry wastewater (TDIWW) for H2 production and simultaneous treatment of this wastewater. The typical wastewater from the textile industry is characterised by high ranges of pH, colour, total dissolved solids, total suspended solids, total solids, chloride, biological oxygen demand (BOD), COD, heavy metals, and sodium. The solid consists essentially of diverse organic and inorganic pollutants containing nitrogen, chloride, and phosphate. The ions’ attraction to the opposite charge electrode promotes solid and other pollutant removal. BOD is one of the primary pollutants in TDIWW, affecting aquatic life because of the present chemicals, metals, and other inorganic compounds. Lastly, COD can be removed through electrolysis, as this process promotes the decomposition of organic compounds. The used wastewater had a high BOD (402 mg L−1), which was removed up to 108 mg L−1 (73% removal) with a steel electrode at 12 V [6][53].
The study of Pathak et al. [6][53] involved three different electrodes: carbon, steel, and Pt. The carbon electrode had a surface area of 23 ± 1.5 cm2, the steel electrode area was 1.07 ± 0.23 cm2, and the Pt electrode had an area of 0.1 ± 0.02 cm2. The electrolysis process’s efficiency depends on the electrolyte’s ionic strength. As TDIWW has a high conductance and a high amount of total dissolved solids, its ionic concentration was considered sufficient to use it as an electrolyte in electrolysis processes. Furthermore, the wastewater produces more H2 than simple water because of the high content of organics and salts, which enhance the rate of ion formation and result in high H2 production with good pollutant removal efficiency [6][53].
Results demonstrated that the efficiency of pollutant removal, concerning conductivity, total dissolved solids, total suspended solids, BOD, COD, hardness, total nitrogen, and total phosphorus, ranged from 73 to 96% using steel electrodes at 12 V [6][53]. Maximum efficiencies of 49.6, 67.8, and 57.1% for carbon, steel, and Pt electrodes, respectively, were found for an input voltage of 3 V. However, for a higher voltage of 12 V, the H2 production rate was higher (0.27 mL min−1) with steel electrodes [6][53]. Pathak et al. explained that this might originate from an increase in the size of the bubbles, leading to floc formation and increasing the H2 production rate. As this study used a solar PV-based system (less carbon emission), Pathak et al. considered the higher voltage to achieve higher H2 production [6][53]. This study also analysed the hardness and nitrogen removal. Hardness is related to calcium (Ca2+) and magnesium (Mg2+) presence, and the maximum removal (90.7%) was attained at 12 V, owing to large amounts of flocs formation, as the charge-transfer rate is higher in the presence of hydroxide. Nitrogen removal showed a maximum efficiency of 93.6% with steel electrodes [6][53].
Consequently, TDIWW electrolysis using solar PV panels with storage systems for constant power supply has proven to be an efficient and energy-intensive approach for wastewater treatment coupled with H2 generation with clear advantages compared to traditional treatment methods [6][53].
2. Pollutant Removal
The treatment of industrial wastewater, especially if it is a mix, is challenging due to its high complexity [7][57]. With the ever-increasing standard of drinking water supply and the stringent environmental regulations regarding wastewater discharge, electrochemical technologies have regained their importance worldwide. Electrochemical methods are frequently used to treat wastewater containing organic oil, organic pollutants, heavy metals, and nitrate [8][14]. Some electrochemical techniques include electrochemical metal recovery, electrocoagulation, electroflotation, and electrooxidation [9][58]. These technologies are interesting because they lead to less sludge generation, are easy to operate, and do not require additional chemicals [9][58]. Namely, electrooxidation involves decomposing organic materials through oxidation into CO2, water, and other oxides [9][58]. Specifically, indirect electrochemical oxidation is an environmentally friendly technology that can completely mineralize recalcitrant organic matter, removing nitrogen species, and degrade pollutants through a powerful oxidant at the anodic surface [10][13].
Kliaugaite et al. [9][58] studied the electrochemical removal and recovery of humic-like substances from paper and food industry wastewater. The secondary effluent from these wastewaters still contains a high COD and colour intensity caused by complex degradable organic compounds that are primarily humic-like substances (HLS). Humic substances are heterogeneous and polyfunctional polymers formed by microbial decay from plant and animal residues and occur in soils, sediments, and natural waters. HLS consists of a complex, unresolved mixture of relatively small molecules. Streams containing HLS are usually produced in industrial operations involving leather, wood, and food processing.
Kliaugaite et al. [9][58] performed two continuous mode experiments: membrane electrolysis and electrocoagulation. The electrochemical cell consisted of 2 chambers: a plate electrode with a surface area of 22 cm2 and a flow channel. In the case of the membrane electrolysis experiment, these chambers were separated by a membrane (polyethersulfone microfiltration membrane, AEM, and CEM), and a titanium-coated electrode with 0.5 mg cm−2 Pt/Ir mixed metal oxide as the anode and a stainless-steel electrode as the cathode was used. In the electrocoagulation experiment, chambers were hydraulically connected, and both electrodes were low-cost iron plates [6][53]. The results indicated that membrane electrolysis and electrocoagulation could play an essential role in the HLS removal and recovery, and the decolourization of coloured wastewater. Compared to membrane electrolysis, electrocoagulation has lower operating costs and efficiently removes colour and COD, making it more suitable for highly polluted wastewater. Electrocoagulation showed removal effects similar to membrane electrolysis (65% of colour and 30% COD removal), spending 0.12 kWh m−3 energy consumption, which is 25 times cheaper than membrane electrolysis (3 kWh m−3). Kliaugaite et al. [9][58] showed that the type of membrane used in these applications does not largely impact the treatment efficiency; however, it was noticed that microfiltration membranes (MFMs) and AEMs are more suitable for wastewater electrolysis than CEMs. Using a CEM, 50% of colour and 10% of COD were removed, while 70% of colour and 20–30% of COD were removed using the other two membranes in the same conditions. So, it was demonstrated that the electrocoagulation treatment process efficiently removes COD and colour [9][58].
Popat et al. [10][13] studied a treatment for mixed industrial wastewater (from pharmaceuticals, dyes, textiles, etc.) that consisted of an electrochemical advanced oxidation process (EAOP) followed by a biological process. The EAOP applied in this study was the electro-Fenton process. A total of two anodes (Ti/Pt mesh) and two cathodes (graphite felt) were used for the experiments. The initial value of COD in raw wastewater was 1152 mg L−1, which was possible to reduce to 691 mg L−1 by EAOPs process at optimum conditions: 10 V, interelectrode distance of 1 cm, area of 25 cm2, catalyst loading of 10 mg L−1 and a persulphate dosage of 200 mg L−1. Due to the COD reduction, the BOD/COD ratio increased from 0.34 to 0.52, which proves the enhancement in the biodegradability of the wastewater after EAOPs treatment. However, the COD of any effluent disposed of in any surface water body should not exceed 250 mg L−1. As a result, the electro-Fenton process was followed by a biological process that further reduced the COD to 60.8 mg L−1. The efficiency of this last wastewater treatment process was increased because non-biodegradable organic compounds were converted into biodegradable forms by the electro-Fenton process. As the combined process had a COD removal efficiency of 94%, Popat et al. concluded that combining EAOPs and biological treatment seems a feasible and economical option for degrading mixed industrial wastewater [10][13].
Nidheesh et al. [8][14] studied the electrochemical treatment of mixed industrial wastewater, specifically electrocoagulation and indirect electrochemical oxidation processes, through COD and colour removal studies. The experiments for electrocoagulation performance evaluation were conducted in a rectangular tank with a working volume of 1 L. Commercially accessible aluminium plates with a 25 cm2 surface area were used as both anodes and cathodes (separated by 1 cm). The experiments were carried out at room temperature. A similar experimental setup was used for the indirect electrochemical oxidation experiments, using commercially available graphite plate electrodes with a surface area of 25 cm2 [8][14].
Additionally, both processes are more efficient at the wastewater pH, i.e., 7.7. The efficiency of electrocoagulation was assessed at different cell configurations, specifically monopolar and bipolar designs in parallel connection. The results found that the monopolar connection was more effective for COD and colour removal from the wastewater than the bipolar design. The monopolar connection exhibited COD and colour removals of 55 and 56%, respectively, whereas the bipolar connection led to a lower removal of 43 and 48% at the wastewater pH and an applied voltage of 1.5 V for 1 h. In the case of the indirect electrochemical oxidation process using graphite electrodes, the COD and colour removal efficiencies of the indirect electrooxidation process were found to be 55 and 99.8%, respectively. These results were obtained for 1 h of electrolysis at a pH of 7.7, an applied voltage of 4 V, and a NaCl concentration of 1 g L−1, as it was proven that adding external chloride to the system boosts COD removal efficiency and nearly complete colour removal. Hence, Nidheesh et al. [8][14] concluded that electrocoagulation with aluminium electrodes and indirect electrochemical oxidation processes are potential tools for mixed industrial wastewater treatment. Table 1 summarises the conditions used in different studies on COD/BOD removal from industrial wastewater using electrochemical treatment.
Table 1.
Summary of the operation conditions, catalyst, H
2
production, and pollutant removal using electrochemical treatment of industrial wastewater.
| Operating Conditions | Electrolyte | Electrodes, Catalyst | H | 2 | Production |
COD/BOD and Colour Removal | Source | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nafion 117 membrane 1 V |
Sugar industrial wastewater with 50 mM phosphate buffer | Graphite anode Nickel cathode Copper wire current collector nanocomposite cathode catalyst (NiO.rGO and Co | 3 | O | 4 | .rGO) | 4.83 mmol L | −1 | D | −1 | - | [3] | [56] | |
| Nafion 117 membrane | Wastewater anolyte 50 mM K | 2 | HPO | 4 | and 50 mM of KH | 2 | PO | 4 | catholyte | Carbon anode Pt cathode |
30.12 mL | 75.6% | [4] | [15] |
| 12 V | textile dyeing industry wastewater | Steel electrodes | 16.4 mL h | −1 | 73–96% | [6] | [53] | |||||||
| Membrane (MFM or AEM) | paper and food industry wastewater | Titanium anode Stainless steel cathode 0.5 mg.cm | −2 | Pt/Ir mixed metal oxide anode catalyst | - | Membrane electrolysis: 20–30% COD 70% colour Electrocoagulation: 30% COD 65% colour |
[9] | [58] | ||||||
| 10 V | Mixed wastewater | Ti/Pt anodes Graphite cathodes Fe | 2+ | catalyst | - | 40% | [10] | [13] | ||||||
| T | room | Electrocoagulation: 1.5V Indirect electrochemical oxidation: 4 V |
- | Electrocoagulation: Aluminium Electrodes Indirect electrochemical oxidation: Graphite electrodes |
- | Electrocoagulation 55% COD 56% colour Indirect electrochemical oxidation: 55% COD 99.8% colour |
[8] | [14] |
