Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Luiz Claudio Di Stasi and Version 2 by Beatrix Zheng.
Coumarin derivatives modulating the Nrf2 signaling pathway and displaying simultaneous intestinal anti-inflammatory activities, effects potentially useful in the management of intestinal inflammatory processes. Coumarin derivatives modulating the Nrf2 signaling pathway and displaying intestinal anti-inflammatory activity include simple coumarins, linear and angular furanocoumarins from plant origin, and coumarin derivatives produced by the fermentative process performed by gut microbiota on the plant-derived products commonly used in human feeding.
- intestinal inflammation
- natural coumarin
- Nrf2
- IBD
1. Simple Intestinal Anti-Inflammatory Coumarin Derivatives Targeting Nrf2 Signaling
The simple coumarin derivatives with simultaneous effects on the Nrf2 signaling pathway and intestinal inflammation include esculetin, 4-methylesculetin, esculin, daphnetin, umbelliferone, osthole, fraxetin, scopoletin, and scoparone. The previous in vitro and in vivo studies with these simple coumarin were analyzed to identify the most promising natural coumarins as lead compounds for the development of new drugs to control IBD.
2. Intestinal Anti-Inflammatory Furanocoumarin Derivatives Targeting Nrf2 Signaling
Imperatorim also known as ammidin, marmelosin, or marmelide, is a linear furanocoumarin isolated from several plants belonging Apiaceae family such as Angelica archangelica L., Angelica dahurica Fisch. Ex Hoffm.m and Glehnia littoralis F. Schimidt ex Miq. and other plant species from different genera and botanical families. Imperatorin (8-isopentenyloxypsoralen) is a psoralen derivative containing an oxy-isopentenyl group at C-8 (Figure 8). Psoralen, the basic structure of linear furanocoumarins, is found in Psoralea corylifolia L. (Babchi) and several food plants, including Apium graveolens L. (celery), Foeniculum vulgare Mill. (fennel), Daucus carota L. (carrot) belongs to the Apiaceae family, and several Rutaceae plant species, such as Ficus carica L. (Figure 8). Psoralen and imperatorin have been reported as active natural coumarin derivatives able to modulate the Nrf2 signaling pathway promoting beneficial effects useful to prevent and control several NCDs, including IBD [1][2][3][4][5][15,19,135,136,137].
Psoralen administered by gavage at a dose of 20 mg/Kg upregulated the Nrf2 signaling pathway protecting mice against radiation-induced bone injury [3][135]. Psoralen also ameliorated DSS-induced intestinal inflammation in C57BL/6 mice after intraperitoneal administration of 3 mg/Kg, inhibiting NLRP3 inflammasome, caspase-1, and IL-1β gene expression [5][137]. However, psoralen induces hepatotoxicity at a dose range of 20 to 800 mg/Kg in mice [6][7][8][138,139,140], limiting its use as a drug.
On the other hand, imperatorin effects on the Nrf2 have been also reported in several in vitro and in vivo studies. Treatment with imperatorin at concentrations ranging from 10 to 100 µg/mL in arsenic trioxide-induced cytotoxicity in H9c2 cells suppressed ROS generation and increased Nrf2, NQO1, and HO-1 expression and protein levels [9][141]. Imperatorin also suppressed allergic response in peritoneal rat mast cells through Nrf2/HO-1 activation and MAPK and NF-κB inhibition [10][142]. The upregulation of Nrf2 with reduction of oxidative stress and the inflammatory process was reported after oral administration of imperatorin at 15 and 30 mg/Kg in Sprague Dawley rats submitted to high-fat/high-fructose diet-induced cardiac remodeling and dysfunction [11][143]. In BALB/c mice asthma model using ovalbumin, imperatorin at 15, 30, and 60 mg/Kg regulated several signaling pathways, increasing the nuclear Nrf2 and HO-1 levels with a simultaneous reduction in cytosol Nrf2 and NF-κB, AKT, Erk, p-38 and JNK levels [12][144].
Several studies using TNBS and DSS experimental models of intestinal inflammation demonstrated the intestinal anti-inflammatory properties of imperatorin, suggesting its potential to control and prevent IBD. In the DSS model in mice, 25, 50, and 100/Kg of imperatorin attenuated macroscopic and microscopic intestinal damage, avoiding body weight loss and bloody diarrhea and reducing macroscopic and microscopic scores of the lesion [13][145]. The effects of imperatorin at concentrations of 6.25, 12.5, and 25 µM were also evaluated on the human intestinal epithelial HCT116, LS174T, human leukemia THP-1, and HEK293T cell lines as an attempt to elucidate the main actions of imperatorin, which produced a range of effects, mainly acting as an agonist of pregnane X receptor and inhibiting the NF-κB-mediated TNF-α, IL-1β, and IL-6 pro-inflammatory cytokines production [13][145]. The intestinal anti-inflammatory activity of imperatorin was also demonstrated in the TNBS model and associated with several pharmacological mechanisms. Imperatorin administered by an intraperitoneal route at concentrations of 15, 30, and 60 mg/Kg in Sprague Dawley rats ameliorated the macroscopic and microscopic intestinal damage induced by TNBS as well as reduced the colon levels of TNF-α and IL-6, upregulating the Nrf2, ARE, and HO-1 expression [4][136]. Moreover, imperatorin was reported as the main active component of Angelica dahurica and Angelica albicans plant extracts, which were able to reduce TNBS-induced intestinal inflammation [14][15][146,147].
3. Intestinal Anti-Inflammatory Gut Microbial Coumarins Targeting Nrf2 Signaling
A remarkable group of natural coumarins derived from gut microbiota enzymatic action and named urolithins has been highlighted in recent years due to their pharmacological actions, particularly antioxidant properties. Urolithins are benzocoumarins derived from diphenylpyran-6-one and classified as a combination of coumarin and isocoumarin chemical structure [16][17][148,149]. Urolithins include several penta-, tetra-, tri-, di-, and monohydroxylated compounds dependent on the level of hydroxylation on the ellagitannins from the diet promoted by gut bacteria, mainly Gordonibacter urolithinfaciens and Gordobacter pamelaceae, on the ellagitannins from the diet [17][149]. Other gut bacteria that can participate in the production of urolithins are the Ellagibacteris isourilithinifaciens and strains of Bifidobacteria, mainly Bifidobacterium pseudocatenulatum [18][150]. The main dietary sources of ellagitannins include pomegranate (Punica granatum L., Lythraceae botanical family) fruits, several nuts, mainly walnuts (Juglans regia L., Junglandaceae botanical family), and several species of raspberries, mainly red raspberries (Rubus idaeus L., Rosaceae family) and black raspberries (Rubus occidentalis L.), and black tea (Camelia sinensis (L.) Kuntze, Theaceae botanical family). Ellagitannins are firstly converted to ellagic acid by tannases for further production of intermediate luteic acid, which generated urolithin M5, the key precursor of several bioactive urolithins, mainly the bioactive urolithins A and urolithin B (Figure 19) [18][19][150,151]. Urolithin A and urolithin B display several pharmacological properties, including anti-cancer, neuroprotective, hepatoprotective, nephroprotective, anti-metabolic, anti-inflammatory, and against autoimmune, cardiovascular, genetic, and aging-associated diseases acting by different signaling pathway modulation [16][18][148,150].
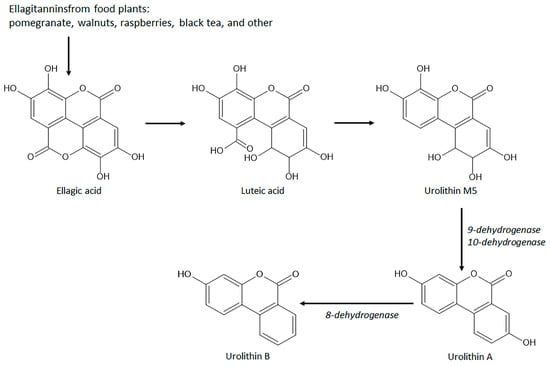
Figure 19. Chemical structures and biosynthetic route of gut microbiota-derived coumarins with antioxidant and intestinal anti-inflammatory properties.
Urolithin A and urolithin B modulate oxidative stress and are recognized as emerging antioxidant coumarin derivatives, reducing ROS generation, free radical scavenging, and activating the Nrf2 signaling pathway through Nrf2 nuclear translocation with further upregulation of HO-1, SOD, glutathione-related antioxidant system, and NQO1 [16][148]. The antioxidant properties of urolithin A and urolithin B were related to the control of several diseases such as diabetes, skin aging, sclerosis, kidney and liver toxicity, inflammation, and osteoclastogenesis [20][21][22][23][24][25][26][27][152,153,154,155,156,157,158,159].
Urolithin A at a concentration of 10 µM on high glucose-induced human retinal endothelial (HRE) cells counteracted the oxidative stress, increasing SOD activity and GSH levels and reducing MDA, IL-6, IL-1β, and TNF-α levels and gene expression [20][152]. The antioxidant properties were related to the activation of Nrf2 and HO-1 levels and Nrf2 activity in HRE cells as well as in streptozotocin-induced diabetic Sprague Dawley rats treated with intraperitoneal administration of 2.5 mg/Kg/day for 12 weeks with urolithin A [20][152]. In human dermal fibroblasts exposed to ultraviolet A (UVA) radiation, pretreatment with urolithin A at a concentration of 0.2 µM ameliorated UVA-induced proliferative fibroblast dysfunction, protected fibroblast from DNA damage, promoted ROs scavenging activity and Nrf2 activation subsequently driving the activation of antioxidant enzymes, corroborating the antiaging properties of urolithin A previously reported on senescent human skin fibroblasts [20][28][152,160]. In vivo studies also demonstrated urolithin A producing several pharmacological activities via Nrf2 activation. The anti-atherosclerotic activity was reported after oral administration of urolithin A at a dose of 3mg/Kg/day for three weeks in rats with a diet rich in cholesterol and subjected to balloon injury of the aorta [24][156]. Oral administration of urolithin A (20, 50, and 100 mg/Kg/day for 7 days) in C57BL/6 mice subjected to renal/ischemia surgery model alleviated kidney injury via the Keap1-Nrf2 pathway [23][155]. The hepatoprotective effect was also reported in an acetaminophen hepatotoxicity model in mice, in which urolithin A at intraperitoneal doses of 50, 100, and 150 mg/Kg inhibited oxidative stress accumulation and activated the Nrf2 pathway [22][154]. Moreover, urolithin A (25 mg/Kg, oral administration) in a model of LPS-induced osteoporosis in C57BL/6 mice attenuated osteoclastogenesis through simultaneous regulating of p28 MAPK and Nrf2 signaling pathways [25][157].
Urolithin B displayed cardioprotective effects and anti-inflammatory and anticlastogenesis activities modulating the Nrf2 signaling pathway as described by in vitro and in vivo studies [26][27][29][158,159,161]. Pretreatment of LPS-stimulated BV2 microglia cells with 30, 50, and 100 µM of urolithin B promoted anti-inflammatory effects by modulating pro-inflammatory markers, reducing nitric oxide, TNF-α, IL-6, ROS generation levels, and increasing HO-1 levels and gene expression [26][158]. These effects were also related to the suppression of NF-κB activity via inhibition of IκBα phosphorylation and AP-1 activity [26][158]. Urolithin B (0.7 mg/Kg, intraperitoneal route) in the ischemia/reperfusion damage model in Sprague Dawley rats reduced myocardial apoptosis and alleviated cardiac impairment through ROS reduction [29][161]. In H9c2 cells, urolithin B (5, 10, 20, and 40 µM) reduced ROS production through p62-Keap1 interaction and Nrf2 nuclear translocation with subsequent increase of HO-1, NQO1, and GSTP1 protein expression [29][161]. Moreover, urolithin B (10, 30, 50, and 100 µM) suppressed osteoclastogenesis through the reduction in ROS production in RANKL-stimulated osteoclast formation and activation in RAW264.7 cells with simultaneous attenuation of NF-κB, MAPK, and Akt signaling pathways and upregulation of Nrf2 and antioxidant enzymes [27][159].
Urolithins display several actions and a key role in the maintenance of intestinal health, acting by different mechanisms such as the regulation of intestinal microbiota, increasing beneficial bacteria such as Lactobacillus, Akkermansia, Gordonibacter, Bifidobacterium and Clostridium, and reducing bacterial infection with an improvement of intestinal barrier function [17][149]. In the DSS-induced intestinal inflammation in male Fischer rats, urolithin A at a dose of 15 mg/Kg/day for 25 days before damage induction ameliorated macroscopic and microscopic intestinal inflammation parameters as well as promoted an increase of bifidobacteria and lactobacilli and antioxidant properties as evidenced by ROS scavenging activity, downregulation of COX-1 and iNOS expression and NO production [30][162]. In a recent review was reported that several compounds, including urolithin A, can act on the aryl hydrocarbon receptor to protect and control intestinal inflammatory processes [31][163]. Urolithin A (40 µM) and urolithin B (5 µM) were also evaluated in human acute monocytic THP-1 cells and colon fibroblasts and promoted anti-inflammatory effects with a significant reduction of IL-1β, TNF-α, a downregulation of PGE2 and IL-8 and other regulators of cell migration and adhesion [32][164].
