Nonalcoholic fatty liver disease (NAFLD), a chronic condition associated with metabolic dysfunction and obesity, has reached epidemic proportions worldwide. Although early NAFLD can be treated with lifestyle changes, the treatment of advanced liver pathology, such as nonalcoholic steatohepatitis (NASH), remains a challenge. There are currently no FDA-approved drugs for NAFLD. Fibroblast growth factors (FGFs) play essential roles in lipid and carbohydrate metabolism and have recently emerged as promising therapeutic agents for metabolic diseases. Among them, endocrine members (FGF19 and FGF21) and classical members (FGF1 and FGF4) are key regulators of energy metabolism. FGF-based therapies have shown therapeutic benefits in patients with NAFLD, and substantial progress has recently been made in clinical trials. These FGF analogs are effective in alleviating steatosis, liver inflammation, and fibrosis.
1. Introduction
Due to a sedentary lifestyle and excess nutrition, overweight and obesity, as well as a series of metabolic-related diseases, are developing among modern people
[1]. Nonalcoholic fatty liver disease (NAFLD) is closely related to metabolic syndrome and has become the most prevalent chronic liver disease in recent decades
[2]. The disease burden of NAFLD varies by geographic region and ethnicity
[3]. According to a meta-analysis in 2022, the global prevalence of NAFLD is approximately 30% and increasing
[4]. NAFLD refers to a group of diseases, ranging from mild steatosis to severe nonalcoholic steatohepatitis (NASH), that dramatically increase overall mortality. Obesity is a significant risk factor for the progression of NAFLD, in which excess lipids are stored as triglycerides in hepatocytes, resulting in steatosis
[5][6][5,6]. There is also as a known genetic predisposition to hepatic fat accumulation (e.g., PNPLA3 gene polymorphisms and TM6SF2 gene variations)
[7][8][7,8]. The overall mortality of NAFLD patients is increased compared to non-NAFLD patients
[9]. Cardiovascular disease (CVD) and cancer are the main causes of death in people with NAFLD
[10][11][10,11]. NASH is a kind of aggressive fatty liver disease distinguished by the presence of hepatocyte ballooning and lobular inflammation with or without fibrosis
[12][13][12,13]. The severity of fibrosis is a crucial indicator of the long-term prognosis in NASH patients for it progresses in about 40% of NASH patients
[14][15][16][14,15,16]. The progressive form of NAFLD and NASH is a leading indication for a liver transplant
[17]. In 2020, several expert panels advocated for metabolic-associated fatty liver disease (MAFLD) as a better term to reflect the heterogeneity of NAFLD.
The exact mechanism of NAFLD is yet to be elucidated. Excess lipids are the primary damage factors, and the subsequent effects of pathogenic drivers, including insulin resistance, lipotoxicity, and immune system activation, all contribute to the pathogenesis of NAFLD
[18]. NAFLD is thought to develop with early steatosis, which is insufficient in triggering inflammation and fibrosis. As the condition advances, the second hit (via oxidative stress and other factors) aggravates the liver damage
[19]. Furthermore, it was found that multiple factors, including hepatic inflammation and the synergistic effect of multiple mechanisms, including oxidative stress, lipid peroxidation, the endotoxin-induced activation of hepatic stellate cells (HSCs), and mitochondrial dysfunction, promoted the progression of NASH to fibrosis, and this hypothesis was dubbed “multi-hit”
[20][21][20,21]. Although the development of NAFLD varies from person to person, it is generally categorized into four stages
[22][23][22,23]. The first stage is fat accumulation in the liver, which is believed to be innocuous
[24]. The second stage, early NASH (F0: no fibrosis and F1: negligible fibrosis), is characterized by fatty infiltration and liver inflammation. The diagnosis of NASH requires the detection of steatosis, ballooning, and lobular inflammation on the liver biopsy. Pathological alterations in NASH include portal inflammation, polymorphonuclear infiltration, Mallory–Denk bodies, microvacuolar steatosis, and giant mitochondria. The third stage entails chronic liver inflammation and damage, which both promote persistent inflammation and hepatic fibrosis. The fourth stage is cirrhosis (F4), a severe stage of NAFLD/NASH. Patients with advanced fibrosis and cirrhosis are at an increased risk for liver-related complications (i.e., liver decompensation and HCC) and liver-related mortality
[25]. In the early stages, liver fibrosis can both progress and regress. The reversal of fibrosis is often observed with weight loss in obese patients with NAFLD
[26]. However, with the progressive inflammation and fibrosis of the liver parenchyma with the disruption of the hepatic architecture, aberrant regeneration eventually led to the irreversible loss of liver function
[27]. Thus, a timely intervention for the diseases is needed to improve quality of life and reduce liver-related mortality
[28].
In recent years, despite the fact that many valuable breakthroughs have been made in the pathogenesis and treatment of NAFLD, the condition still remains challenging to manage. Currently, the treatment for NAFLD is mainly based on lifestyle changes (such as increased exercise; weight loss; and a diet low in calories, cholesterol, saturated fat, and fructose). With regard to the amelioration of weight loss due to NAFLD, a 5% change can improve steatosis, a 7–9% change is able to improve most histopathological changes, while a weight loss of 10% or more can improve fibrosis
[29]. However, lifestyle adjustments and weight loss are uneasy for the patients, let alone long-term maintenance
[30]. With most advanced diseases and severe fibrosis being rarely curable with lifestyle interventions, appropriate pharmacological interventions remain in high demand. Despite tremendous scientific efforts being made to develop therapeutics, no specific drug for NAFLD/NASH was approved by the Food and Drug Administration (FDA). There are very few available drugs to treat NAFLD, most of which have been withdrawn from the market due to their side effects
[31]. Currently, only vitamin E and the proliferator-activated receptor gamma (PPAR-y) ligand pioglitazone are recommended for selected patients by the European and American Association for the Study of the Liver
[32]. Several drugs have been investigated in clinical trials for the histopathology of NASH or aggravation of fibrosis, while the translation to clinical applications still requires additional investigations
[33][34][33,34]. In addition, individual variation among patients makes it difficult for a single treatment to achieve the desired effect in NAFLD/NASH. In consideration of the above, combination therapies and individualized treatment models of drug intervention appear to be better options.
2. The Rationale for FGFs in the Treatment of NAFLD
Fibroblast growth factors (FGFs) are a family of 22 signaling proteins that regulate reproduction, development, repairment, and metabolism
[34][35][36][34,35,36]. The human FGF family can be classified into seven subfamilies based on sequence homology and phylogenetic divergence. The majority of FGFs are members of the classical FGF subfamilies (FGF1-10, FGF16-18, FGF20, and FGF22), which act as autocrine and paracrine factors that bind to and activate FGF receptors. Moreover, three endocrine members in the FGF subfamily (FGF19, FGF21, and FGF23) are released from the extracellular matrix into the blood and are involved in the metabolism of lipids, carbohydrates, bile acids, as well as phosphates in distant organs
[37]. Endocrine FGFs activate their cognate fibroblast growth factor receptors (FGFRs), which operate as coreceptors with the corresponding glycoproteins. Specifically, FGF19 and FGF21 act as crucial bile acid and glycolipid metabolism regulators by binding to the FGFRs/β-Klotho complex. Meanwhile, FGF23 plays a critical role in regulating phosphate and vitamin D in homeostasis by stimulating the FGFR/α-Klotho complex in the kidney and parathyroid gland. Several other FGF members have also been found in preclinical studies to maintain energy homeostasis and regulate glucose/lipid metabolism
[38][39][38,39]. FGF analogs have been shown to effectively alleviate the pathological states of hepatic steatosis, steatohepatitis, and hepatic fibrosis (
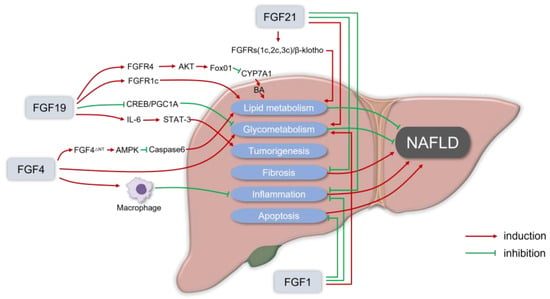
Figure 1).
Figure 1. The rationale for fibroblast growth factors (FGFs) in the treatment of nonalcoholic fatty liver disease (NAFLD). FGF19 usually binds to the FGFR1c or FGFR4/β-Klotho complex to enhance lipid metabolism that can alleviate NAFLD. FGF19 inhibits the orphan nuclear receptor small heterodimeric partner (SHP)-dependent cholesterol 7α hydroxylase (CYP7A1) to prevent BA-induced liver injury in NASH. For glycometabolism, FGF19 inhibits gluconeogenesis by suppressing the cAMP response element-binding protein (CREB)/peroxisome proliferator-activated receptor γ coactivator 1-α (PGC1α) signaling cascade. Meanwhile, in FGF19 transgenic mice, it was observed that FGF19 could transform normal hepatocytes into malignant cells via IL-6 by activating the STAT-3 pathway. FGF21 usually recruits β-Klotho and FGFRs (FGFR1c, FGFR2c, or FGFR3c) as coreceptors for activation, to reduce lipid deposition in hepatocytes in a non-insulin way, and the FGF21 receptor agonists can inhibit liver inflammation, fat content, and liver fibrosis in the in vitro and in vivo models of liver fibrosis and NASH. Physiological doses of FGF21 can reduce the body weight, and fat content can also alleviate insulin resistance, hyperglycemia, and dyslipidemia. In the NASH mice model, the administration of recombinant FGF1 (rFGF1) ameliorated liver inflammation and hepatocyte injury, and FGF1 normalized the blood glucose levels of both gene- and diet-induced obese mice. FGF4 is also essential in regulating glucose and lipid metabolism, as well as maintaining systemic metabolic homeostasis, which can ameliorate insulin resistance and inhibit the infiltration and the development of inflammation. FGF4 acts directly on macrophages to block inflammatory responses in the liver and adipose tissue. rFGF4ΔNT, a non-mitotic rFGF4 analog, can have a significant protective effect on NASH through an AMPK-dependent signaling pathway.
Considering the potent beneficial roles of endocrine FGFs in NAFLD and metabolic diseases, more and more researchers are paying extra attention to the usage of FGFs in various promising therapeutic approaches. The characteristics of several FGFs with potential therapeutic effects in NAFLD are summarized in
Table 1. FGFs and their analogs have pleiotropic metabolic effects. A growing number of FGF analogs and/or receptor agonists (particularly for FGF19 and FGF21) are being explored as potential agents for NAFLD and other metabolic-related diseases
[40][41][40,41]. To date, a variety of FGF-based drugs have shown therapeutic potential in preclinical studies and clinical trials for NAFLD/NASH
[42][43][44][45][42,43,44,45]. FGF19 and FGF21 may be the most promising potent drugs for NAFLD, especially in the relief of liver steatosis, steatohepatitis, and liver fibrosis
[46].