Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 4 by Jonaid Ahmad Malik and Version 3 by Queenie Zhao.
The death rate from breast cancer (BC) has dropped due to early detection and sophisticated therapeutic options, yet drug resistance and relapse remain barriers to effective, systematic treatment. Multiple mechanisms underlying miRNAs appear crucial in practically every aspect of cancer progression, including carcinogenesis, metastasis, and drug resistance, as evidenced by the elucidation of drug resistance.
- breast cancer
- miRNAs
- multiple drug resistance
1. Introduction
Breast cancer (BC) is a leading cause of morbidity, disability, and mortality in women worldwide and is a severe health risk [1]. For the first time in 2020, BC overtook lung cancer in terms of overall cancer diagnoses. It accounts for 15.5% of cancer-related fatalities worldwide and is the primary cause of cancer deaths in females in 110 countries. With more than 7.7 million women living 5 years after diagnosis, BC is the most common type of cancer [2]. Because BC is prone to recurrence and the spread of metastases to numerous important organs such as the lungs, brain, liver, and bone, it is imperative to comprehend the key players and the molecular mechanisms driving BC metastasis resulting in the patient’s death [3][4]. Despite significant improvements in the early detection and treatment of BC, some patient groups tend to have worse outcomes [5]. Cellular proteins and RNA interact in a complicated fashion for cellular survival, division, and adaptability. Only 5% of the human genome’s function is understood, while the remaining 95% is still under investigation [6].
Micro ribonucleic acids (miRNAs) are originally short non-coding RNAs made up of 22 nucleotides that allow gene expression by binding with target mRNAs’ 3′-untranslated regions [7]. They act as negative transcriptional regulators and control various biological processes such as cell survival, apoptosis, metastasis, and proliferation, and tumor miRNAs bind to complementary mRNA sequences and modify them post-translationally via repression, degradation, and silence. Transcription of RNA polymerase II, which folds back to generate another micro mRNA, has a distinctive hairpin form, and this structure is created using longer hairpin structures [8][9][10]. It is known that miRNAs are necessary for several biological activities, including animal development and reproduction, differentiation, maturation, and metabolism [11]. Many metabolic diseases are linked to modifications in miRNA expression [12]. Various diseases may also have altered expression, with significant changes in tumor tissues [13]. The biomolecular diagnosis of cancer has been connected to the miRNA profile [14]. Thus the use of miRNA in cancer diagnostics and treatment is increasing [15]. By interacting with target cell genes, miRNAs control tumor growth and metastasis. Exosomal shuttle small RNAs mediate cell-to-cell communication and cancer metastasis [16]. In altering these intracellular signaling processes, miRNAs can behave as tumor oncogenes or suppressors [17][18]. As shown in Figure 1, excessive expression of miRNAs is already known to impact tumor cell proliferation and development significantly by activating various oncogenic signaling pathways. The oncogenic activity of miRNAs is mediated by targeting numerous tumor suppressors, which causes tumor growth and metastasis in people with BC.
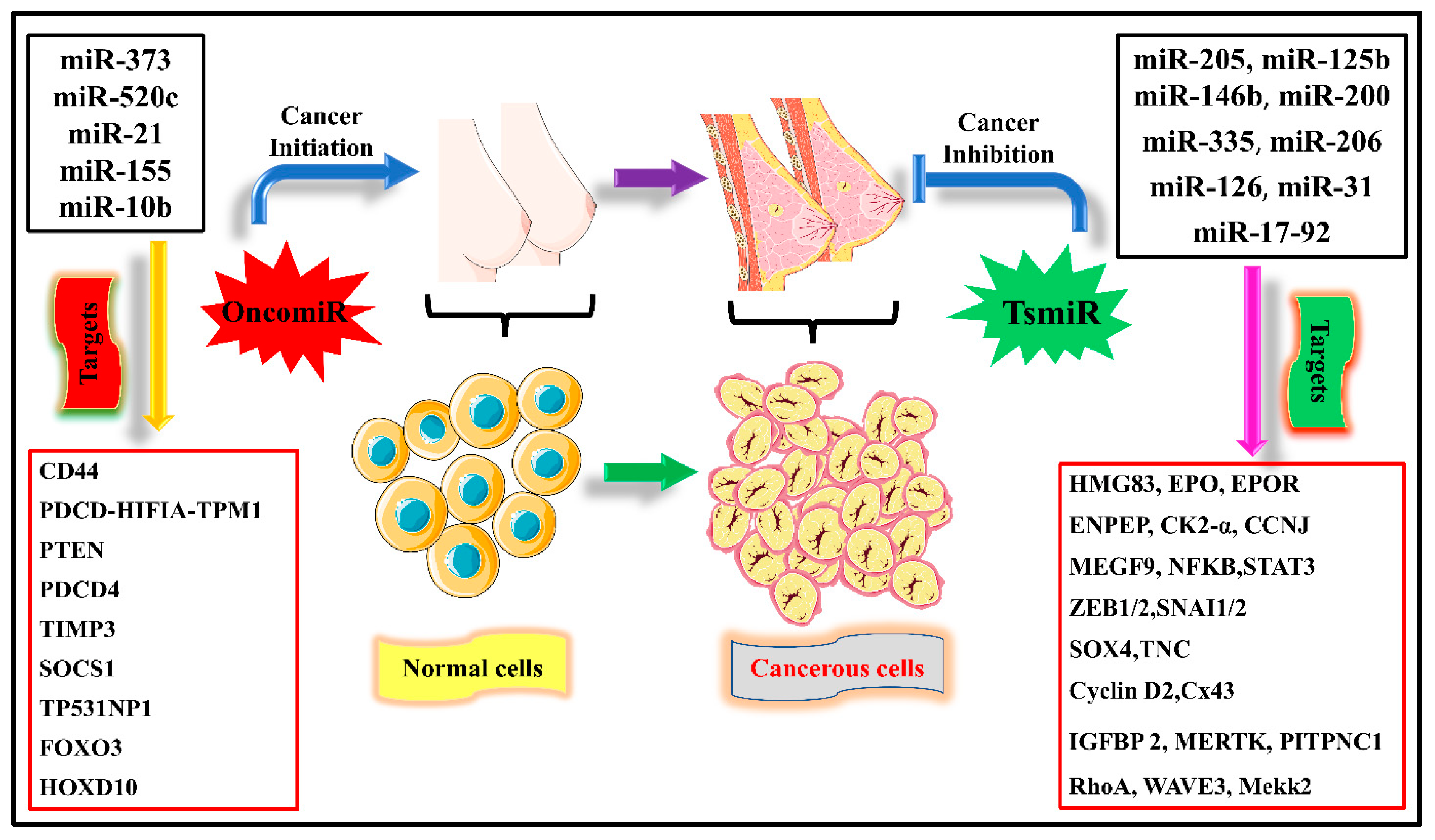
Figure 1. An overview of various miRNAs responsible for initiating cell proliferation and invasion in breast cancer, which are called oncogenic miRNAs (oncomiRs). While miRNAs responsible for tumor suppression are tumor suppressor miRNAs (TS miR), whose main function is the inhibition of migration, invasion, proliferation, and apoptosis. Different miRNAs have different targets for their activity in suppressing tumor growth.
2. Tumor Suppressor miRNAs in BC
The miRNA substitution is an innovative method for examining tumor suppressors’ therapeutic effects. Since they are much smaller than proteins, require merely the entrance into the cytoplasm of their target cells to become active, and may be administered systemically using siRNA delivery methods and technologies, miRNAs offer a fresh possibility [19]. Therefore, the transport barrier for miRNA duplicates appears lower than that for DNA that codes for proteins [20]. Cancer-associated fibroblasts (CAFs) in the TME have higher amounts of TS miRNA, substantially reducing their ability to increase, migrate, and expand tumors [21].
2.1. Mir-125 a, b
MiR-125a and miR-125b are downregulated in HER2-amplification, and HER2-overexpression breast cancers [22]. Overexpression of these miRNAs decreases HER3 and HER2 mRNA and protein levels in SKBR-3 cells (a HER2-dependent cancer cell line of human mammary tissues), resulting in cell migration, intrusiveness, and decreased anchorage-dependent growth. This effect is minor in the case of non-transformed HER2-independent BC [23].
2.2. Let-7
Researchers looked at miRNA overexpression in distinguished and self-renewing cells from cell lines of cancerous breast cells. In a recent study, they discovered that expression of let-7 was much lower in BT-ICs (mammary tumor initiating cells) and increased with differentiation. Incorporating let-7 into BT-ICs stopped them from increasing, forming a mammosphere, or developing tumors and metastasis in vivo [24][25]. Overexpression of let-7 suppressed the expression of known cancerous targets, such as HMGA2 and H-RAS. By pulling down H-RAS, self-renewal was decreased but there was zero effect on differentiation in the BT-IC-enriched cell line.
In contrast, knocking down HMGA2 increased differentiation but did not affect self-renewal. Their findings showed that let-7 controls several BT-IC cell characteristics, and that let-7 offers a novel opportunity to target tumor stem cells with therapeutic RNA. Entry of the let-7 miRNA into tumors could decrease stem cells by stimulating proliferation and differentiation. RKIP (Raf kinase inhibitory protein) inhibits NF-κB signaling pathways and the MAPK-G protein-coupled receptor kinase-2. It inhibited bone metastasis and BC cell intravasation in a mouse model while growing expression of let-7 in BC cells. As a result, a chromatin-remodeling protein, such as HMGA-2, that activates pro-metastatic and pro-invasive genes like a snail, showed lower expression.
2.3. miR 31
MiR-31 has been shown to decrease the expression of pro-metastatic genes, preventing metastasis at many stages. The amount of miR-31 expressed by all normal breast cells is related to the tumor’s metastatic status. In non-malignant BC cells, it is observed in low amounts, reduced, and almost unnoticeable in human BC cell lines and metastatic mice. Moreover, introducing miR-31 in metastatic BC cells decreased in vivo and in vitro metastasis-related behaviors (resistance to anoikis, invasion, and motility) [26]. Even though miR-31 overexpressing mammary cancer cells produced more proliferative tumors, they were better enclosed and hence less invasive, demonstrating that miR-31 prevents early stages metastasis in case of disease. After being injected directly into the blood circulation, miR-31 overexpressing cells could not survive, demonstrating that miR-31 limits metastasis at various stages of the metastatic process. In the in vivo setting, inhibiting miR-31 activity increased metastasis and invasiveness. Frizzled3 (FZD3), myosin phosphatase rho interacting protein (M-RIP), matrix metallopeptidase 16 (MMP16) family member of ras homolog gene A (RhoA), integrin alpha-5 (ITGA5), and radixin (RDX) are six sigma aims of miR-31 in breast oncology. As miR-31 prevents metastasis by targeting several pro-metastatic human genes in a metastatic cascade, it undoubtedly has remarkable therapeutic promise for treating human BC.
2.4. miRNA-34a
The mechanism by which microRNA 34a (miR-34a) decreases cell growth is unknown, but miR-34a stops SIRT-1 from being expressed. A binding site of miR-34a exists in SIRT1’s 3 UTR. SIRT1 inhibition raises acetylated p53 levels, and PUMA and p21 are p53 transcriptional targets. These proteins participate in cell cycle processes as well as in apoptosis. In addition, inhibiting SIRT1 with miR-34 causes apoptosis in WT cells. However, p53 is missing from human colon cancer cells. Finally, the transcriptional target of p53 is miR-34a. A feedback mechanism could exist between miR-34a and p53. MiR-34a serves as an inhibitor in this way [27].
2.5. miR-200
One of mammals’ most well-known miRNA families is the miR-200 group, which comprises miR-429, miR-200b, and miR-200a. They play a vital role in EMT, drug resistance, cell proliferation, and other biological processes. The miR-200 family of microRNAs has been dysregulated in lung, ovarian, and stomach cancers, as well as BC.
The Wnt/β-catenin pathway is important for BCSC stability and regulates mammary cell growth at various stages. The miRNAs such as miR-200c/141, miR-29b, and miR-600 regulate metastasis, progression, and therapy resistant Wnt/β-catenin-mediated self-renewal in BCSCs. As a result, the miRNA-catenin and Wnt axis can be evaluated as a great target in developing an effective BC treatment. Wnt/catenin was activated when the miR-200c/141 cluster was depleted, suggesting that miR-141/200c regulated BCSC proliferation and formation through modulating catenin/Wnt signaling [26][27].
2.6. miR-145
MicroRNAs are key gene regulators that can have an impact on cancer. MiR-145 is a tumor suppressor that prevents tumor cell proliferation both in vivo and in vitro. MiR-145 is a cellular microRNA with a specialized function. However, miR-145 prevents cell proliferation in HCT 116 and MCF-7 cells and does not affect metastatic mammary cancerous cell lines. However, miR-145 inhibits cell invasion in these cells, whereas miR-145 against the antisense oligo improves the cell invasion process. MiR-145 has been shown to reduce lung cell metastasis in an experimental metastatic animal model, and miR145 prevents step-by-step cell invasion by inhibiting the metastasis process of gene mucin 1 (MUC1). Researchers recognized MUC1 functions as a direct target for miR-145 by implementing luciferase reporters having the 3′-untranslated part of MUC1 along with immunofluorescence labeling and western blot. In addition, ectopic MUC1 expressions stimulate the cell invasion process, which miR-145 inhibits. MiR-145 inhibits MUC1 and causes a reduction in β-catenin and the oncogenic cadherin11. Finally, RNAi inhibition of MUC1 imitates miR-145’s invasion suppressive action, which is connected to β-catenin and cadherin11 downregulation. This information shows that the role of miR-145 is as a tumor suppressor, limiting tumor growth, cell invasion, and metastasis [28].
2.7. miR-335
MiR-335 prevents the metastatic cell invasion process and controls the expression of a collection of genes related to the danger of distal metastasis in various human tumors. By specifically targeting the extracellular matrix component tenascin C and progenitor cellular transcription factors, such as SOX4, miR-335 inhibits metastasis and migration. Most relapsed patients’ original breast tumors lose miR-335 expression, and loss of microRNA is connected to deficient distal metastasis-free existence. Thus, miR-335 has been reported as a metastatic suppressor micro-RNA in human mammary cancer cells [29].
MiRNAs can directly target the ER, resulting in an advanced phenotype with endocrine resistance. While miR-335-5p is usually considered a tumor suppressor, it represses ER in mammary cancer cells. It is increased by estrogen activation, implying that it could be engaged in estrogen signaling alterations. MiR-335 expression was greater in normal cells than in tumor cells, identifying miR-335 as a tumor cell suppressor. MiR-335 transcript-directed expression on an ER+ BC cell line indicated that two strands of miR-335 double helix conveyed across mammary cancer cell lines with no evident link to clinical tumor subtypes. The expression vector of a pre-miR-335 was firmly introduced into the parental MCF-7 cell line, and qPCR was used to show that the cell line of MCF-7-miR-335 had overexpression of miR-335-5p. MiR-335-5p and miR-335-3p from the cell line of MCF-7-miR-335 were overexpressed, with intact 5-p and 3-p miRNAs. Finally, miR-335-5p and miR-335-3p can modify the RAC1-activity, CDH1 stability, ER signaling, and PDGFR signaling network based on suppressed gene sets [30].
2.8. miR-203
MiR-203 is expressed more in triple positive carcinoma cells, suggesting that these miRNAs may limit tumor growth in ER-positive BC. Overexpression of miR-203 decreased BT474 cell growth considerably, but antisense-mediated gene silencing repression of miR-203 massively increases cell proliferation. In addition, miR-203 prevents the growth of mammary cancer cells. Overexpression of miR-203 reduced BT474 cells’ migratory potential and invasiveness, but antisense-mediated reduction of miR-203 enhanced migration invasiveness and intensity, showing that miR-203 suppresses mammary cancer cell invasion and migration. Overexpression of miR-203 boosted cell cycle inhibitors p27 and p21, reduced cell-cycle activators CDK6 and cyclin D2, and increased apoptosis-associated protein Bcl 2 levels. Inhibition of miR-203 reduced the number of cell cycle inhibitors p27 and p21, elevated cell-cycle activators CDK6 and cyclinD2, and inhibited the apoptosis-related protein Bcl-2, corroborating previous findings. These studies examined the molecular mechanism of miR-203-induced mammary cell growth arrest. The amounts of MMP mRNA in miR-203-modified human mammary cancer cells were studied. Researchers discovered that miR-203 suppressed matrix metalloproteinase MMP7, 2 (MMP2), and MMP9 in BC cells but not other MMPs. Thus, the primary target is to study the inhibition of miR-203-mediated mammary cell invasion [31].
2.9. miRNA-339-5p
The transcription factor called the p53 tumor suppressor, which offers a framework for several stress-sensing pathways, is crucial for the cellular reply to oxidative stress. MiRNAs are 20–24 nucleotide short non-coding RNAs that affect several biological processes, including the regulation of p53. MicroRNAs (miRNAs) can either increase or decrease tumor growth. They control numerous important cancer-related pathways [32]. Thus, it has been discovered that miRNA dysregulation is a common trait in several human cancers. The tumor suppressor gene p53, which slows cell development in response to stress, is the one that is deleted and changed most frequently in human cancers. The oncoprotein MDM2 prevents p53 from working properly. The researcher, using a high throughput screening approach, found that, miR-339-5p acts as a p53 pathway regulator. By targeting the 3′-untranslated area of the MDM2 mRNA, miR-339-5p enhances p53 activity by lowering MDM2 levels [33]. Therefore, miR-339-5p overexpression encourages p53-regulated cellular actions such as stoppage of proliferation and cell death, whereas miR-339-5p inhibition inhibits the response of p53 in cancer cells, enabling enhanced proliferation [34]. Additionally, miR-339-5p levels are lower in tumors with wild-type TP53, indicating that lowering miR-339-5p levels decreases the p53 response in p53-competent tumor cells.
2.10. miRNA-433
As per the research done in 2020 by Jinhui Xue et al., the existence speed of the high-expression group was much higher than that of the low-expression group. This result raised the possibility that miRNA-433 could be a marker for the malignancy of mammary cancer [35]. Shiqin Liu et al. initially argued that miRNA-433 could decrease tumor growth in ER+ BC by preventing M2 macrophage polarisation. The results of these investigations have shown that miRNA-433 deserves attention, has significant research value, and could open up a new way to treat breast cancer targeting miRNA-433 [36].
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Ginsburg, O.; Bray, F.; Coleman, M.P.; Vanderpuye, V.; Eniu, A.; Kotha, S.R.; Sarker, M.; Huong, T.T.; Allemani, C.; Dvaladze, A.; et al. The Global Burden of Women’s Cancers: A Grand Challenge in Global Health. Lancet 2017, 389, 847–860.
- Lv, Y.; Wang, X.; Li, X.; Xu, G.; Bai, Y.; Wu, J.; Piao, Y.; Shi, Y.; Xiang, R.; Wang, L. Nucleotide de Novo Synthesis Increases Breast Cancer Stemness and Metastasis via CGMP-PKG-MAPK Signaling Pathway. PLoS Biol. 2020, 18, e3000872.
- Mohankumar, K.M.; Currle, D.S.; White, E.; Boulos, N.; Dapper, J.; Eden, C.; Nimmervoll, B.; Thiruvenkatam, R.; Connelly, M.; Kranenburg, T.A.; et al. An in Vivo Screen Identifies Ependymoma Oncogenes and Tumor-Suppressor Genes. Nat. Genet. 2015, 47, 878–887.
- Garreffa, E.; Arora, D. Breast Cancer in the Elderly, in Men and during Pregnancy. Available online: https://agegap.shef.ac.uk (accessed on 12 January 2023).
- Ying, S.Y.; Chang, D.C.; Lin, S.L. The MicroRNA (MiRNA): Overview of the RNA Genes That Modulate Gene Function. Mol. Biotechnol. 2008, 38, 257–268.
- Rahmani, F.; Tadayyon Tabrizi, A.; Hashemian, P.; Alijannejad, S.; Rahdar, H.A.; Ferns, G.A.; Hassanian, S.M.; Shahidsales, S.; Avan, A. Role of Regulatory MiRNAs of the Wnt/β-Catenin Signaling Pathway in Tumorigenesis of Breast Cancer. Gene 2020, 754, 144892.
- Chi, Y.; Zhou, D. MicroRNAs in Colorectal Carcinoma—From Pathogenesis to Therapy. J. Exp. Clin. Cancer Res. 2016, 35, 1–11.
- Bartel, D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell 2009, 136, 215–233.
- De Rie, D.; Abugessaisa, I.; Alam, T.; Arner, E.; Arner, P.; Ashoor, H.; Åström, G.; Babina, M.; Bertin, N.; Burroughs, A.M.; et al. An Integrated Expression Atlas of MiRNAs and Their Promoters in Human and Mouse. Nat. Biotechnol. 2017, 35, 872–878.
- Wang, J.; Samuels, D.C.; Zhao, S.; Xiang, Y.; Zhao, Y.Y.; Guo, Y. Current Research on Non-Coding Ribonucleic Acid (RNA). Genes 2017, 8, 366.
- Blenkiron, C.; Miska, E.A. MiRNAs in Cancer: Approaches, Aetiology, Diagnostics and Therapy. Hum. Mol. Genet. 2007, 16, R106–R113.
- He, Y.; Lin, J.; Kong, D.; Huang, M.; Xu, C.; Kim, T.K.; Etheridge, A.; Luo, Y.; Ding, Y.; Wang, K. Current State of Circulating MicroRNAs as Cancer Biomarkers. Clin. Chem. 2015, 61, 1138–1155.
- Liu, Q.; Peng, F.; Chen, J. The Role of Exosomal Micrornas in the Tumor Microenvironment of Breast Cancer. Int. J. Mol. Sci. 2019, 20, 3884.
- Yang, Z.; Liu, Z. The Emerging Role of MicroRNAs in Breast Cancer. J. Oncol. 2020, 2020, 9160905.
- Nurzadeh, M.; Naemi, M.; Sheikh Hasani, S. A Comprehensive Review on Oncogenic MiRNAs in Breast Cancer. J. Genet. 2021, 100, 1–21.
- Grimaldi, A.M.; Salvatore, M.; Incoronato, M. MiRNA-Based Therapeutics in Breast Cancer: A Systematic Review. Front. Oncol. 2021, 11, 668464.
- Musumeci, M.; Coppola, V.; Addario, A.; Patrizii, M.; Maugeri-Saccá, M.; Memeo, L.; Colarossi, C.; Francescangeli, F.; Biffoni, M.; Collura, D.; et al. Control of Tumor and Microenvironment Cross-Talk by MiR-15a and MiR-16 in Prostate Cancer. Oncogene 2011, 30, 4231–4242.
- Baselga, J.; Swain, S.M. Novel Anticancer Targets: Revisiting ERBB2 and Discovering ERBB3. Nat. Rev. Cancer 2009, 9, 463–475.
- Mattie, M.D.; Benz, C.C.; Bowers, J.; Sensinger, K.; Wong, L.; Scott, G.K.; Fedele, V.; Ginzinger, D.; Getts, R.; Haqq, C. Optimized High-Throughput MicroRNA Expression Profiling Provides Novel Biomarker Assessment of Clinical Prostate and Breast Cancer Biopsies. Mol. Cancer 2006, 5, 1–14.
- Scott, G.K.; Goga, A.; Bhaumik, D.; Berger, C.E.; Sullivan, C.S.; Benz, C.C. Coordinate Suppression of ERBB2 and ERBB3 by Enforced Expression of Micro-RNA MiR-125a or MiR-125b. J. Biol. Chem. 2007, 282, 1479–1486.
- Yu, F.; Yao, H.; Zhu, P.; Zhang, X.; Pan, Q.; Gong, C.; Huang, Y.; Hu, X.; Su, F.; Lieberman, J.; et al. Let-7 Regulates Self Renewal and Tumorigenicity of Breast Cancer Cells. Cell 2007, 131, 1109–1123.
- Dangi-Garimella, S.; Yun, J.; Eves, E.M.; Newman, M.; Erkeland, S.J.; Hammond, S.M.; Minn, A.J.; Rosner, M.R. Raf Kinase Inhibitory Protein Suppresses a Metastasis Signalling Cascade Involving LIN28 and Let-7. EMBO J. 2009, 28, 347–358.
- Sarkadi, B.; Homolya, L.; Szakács, G.; Váradi, A. Human Multidrug Resistance ABCB and ABCG Transporters: Participation in a Chemoimmunity Defense System. Physiol. Rev. 2006, 86, 1179–1236.
- Barrette-Lee, P. A Pleiotropically Acting MicroRNA, MiR-31, Inhibits Breast Cancer Metastasis. Adv. Breast Cancer 2009, 6, 24–25.
- Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J. MiR-34a Repression of SIRT1 Regulates Apoptosis. 2008. Available online: www.pnas.org/cgi/content/full/ (accessed on 1 January 2023).
- Asghari, F.; Haghnavaz, N.; Baradaran, B.; Hemmatzadeh, M.; Kazemi, T. Tumor Suppressor MicroRNAs: Targeted Molecules and Signaling Pathways in Breast Cancer. Biomed. Pharmacother. 2016, 81, 305–317.
- Das, P.K.; Siddika, M.A.; Asha, S.Y.; Aktar, S.; Rakib, M.A.; Khanam, J.A.; Pillai, S.; Islam, F. MicroRNAs, a Promising Target for Breast Cancer Stem Cells. Mol. Diagn. Ther. 2020, 24, 69–83.
- Sachdeva, M.; Mo, Y.Y. MicroRNA-145 Suppresses Cell Invasion and Metastasis by Directly Targeting Mucin 1. Cancer Res. 2010, 70, 378–387.
- Tavazoie, S.F.; Alarcón, C.; Oskarsson, T.; Padua, D.; Wang, Q.; Bos, P.D.; Gerald, W.L.; Massagué, J. Endogenous Human MicroRNAs That Suppress Breast Cancer Metastasis. Nature 2008, 451, 147–152.
- Heyn, H.; Engelmann, M.; Schreek, S.; Ahrens, P.; Lehmann, U.; Kreipe, H.; Schlegelberger, B.; Beger, C. MicroRNA MiR-335 Is Crucial for the BRCA1 Regulatory Cascade in Breast Cancer Development. Int. J. Cancer 2011, 129, 2797–2806.
- Palareti, G.; Legnani, C.; Cosmi, B.; Antonucci, E.; Erba, N.; Poli, D.; Testa, S.; Tosetto, A. Comparison between Different D-Dimer Cutoff Values to Assess the Individual Risk of Recurrent Venous Thromboembolism: Analysis of Results Obtained in the DULCIS Study. Int. J. Lab. Hematol. 2016, 38, 42–49.
- Yu, X.; Zhang, X.; Dhakal, I.B.; Beggs, M.; Kadlubar, S.; Luo, D. Induction of Cell Proliferation and Survival Genes by Estradiol-Repressed MicroRNAs in Breast Cancer Cells. BMC Cancer 2012, 12, 29.
- Wu, Z.-S.; Wu, Q.; Wang, C.-Q.; Wang, X.-N.; Wang, Y.; Zhao, J.-J.; Mao, S.-S.; Zhang, G.-H.; Zhang, N.; Xu, X.-C. MiR-339-5p Inhibits Breast Cancer Cell Migration and Invasion In Vitro and May Be a Potential Biomarker for Breast Cancer Prognosis. 2010. Available online: http://www.biomedcentral.com/1471-2407/10/542 (accessed on 10 January 2023).
- Tang, J.; Chen, J.; Wang, Y.; Zhou, S. The Role of MiRNA-433 in Malignant Tumors of Digestive Tract as Tumor Suppressor. Cancer Rep. 2022, 5, e1694.
- Lou, W.; Liu, J.; Ding, B.; Xu, L.; Fan, W. Identification of Chemoresistance-Associated MiRNAs in Breast Cancer. Cancer Manag. Res. 2018, 10, 4747–4757.
More
