Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Giulia Moltoni.
DWI is an imaging technique commonly used for the assessment of acute ischemia, inflammatory disorders, and CNS neoplasia. It has several benefits since it is a quick, easily replicable sequence that is widely used on many standard scanners. In addition to its normal clinical purpose, DWI offers crucial functional and physiological information regarding brain neoplasia and the surrounding milieu.
- diffusion-weighted imaging
- magnetic resonance imaging
- tumors
1. Introduction
Diffusion-weighted imaging (DWI) is a magnetic resonance imaging (MRI) sequence commonly used in neuroradiology for the assessment of acute ischemia, inflammatory diseases, and central nervous system (CNS) neoplasia [1,2,3][1][2][3].
It has several advantages, as it is a fast, easily reproducible, and extensively studied sequence in neuro-oncology, and it is widely available on many standard scanners, including those in non-academic centers [4,5][4][5].
In addition to being a simple instrument for everyday use, DWI can also offer functional and ultrastructural data, particularly when discussing tumor cellularity and the microenvironment through the measurement of water mobility. In fact, it yields an imaging biomarker for pathological tissue changes like cellularity increase or anomalies in the extracellular space by the assessment of water mobility [6,7,8][6][7][8].
Moreover, advanced diffusion-related sequences such as Diffusion Tensor Imaging (DTI) and Diffusion Kurtosis Imaging (DKI) are even playing a more important role than DWI in the neuro-oncology field.
Finally, multi-shell diffusion MRI allows for characterizing the water diffusion signal behavior by analyzing the data using multi-compartment diffusion models such as the Neurite Orientation Dispersion and Density Imaging (NODDI) model. Thus, it can provide a more specific characterization of brain tissue microstructures than conventional single-shell diffusion tensor imaging.
2. Gliomas and Cellularity
Brain tumors may show various degrees of diffusion changes related to tumor cellularity and the nucleus-cytoplasmic ratio [9]. Water diffusion restriction secondary to tumor high-cellularity results in low Apparent Diffusion Coefficient (ADC) values, which are useful for differentiating tumor type and grade [6,10,11][6][10][11]. The minimal ADC value has been proposed as one of the various parameters to use as a predictive tool in patients with malignant supratentorial astrocytomas [12,13,14,15,16,17,18][12][13][14][15][16][17][18]. For instance, Moon et al. [12], Yamasaki et al. [13], and Murakami et al. [14] showed an inverse correlation between the mean ADC value and tumor grade in astrocytic tumors. Generally, higher-grade tumors typically have lower ADC values (Figure 1A–H). Due to their variable cellularity and grade, astrocytomas exhibit heterogeneous diffusion signals, with most cellular areas generally exhibiting restricted diffusion [9]. For instance, the degree of ADC hypointensity will often be higher in lymphoma than in glioma or metastases, reflecting the high cellular density in this neoplasm. The drop in ADC values for non-necrotic high-grade gliomas and metastases will be higher than for low-grade malignancies. High-grade tumor-associated edema reduces ADC sensitivity by raising the average ADC intensity [6,7][6][7]. ADC values alone or in combination with other MRI parameters, such as relative cerebral blood volume (rCBV), derived by dynamic susceptibility contrast-enhanced MR-perfusion (DSC), can accurately grade gliomas [19]. Indeed, Wang et al. recently reported a high accuracy of DWI/ADC to distinguish low-grade gliomas (grades I and II) from high-grade ones (grades III and IV) with an area under the curve (AUC) of 0.91; therefore, with this value being very high it reflects an excellent diagnostic performance of DWI in this field [9,20][9][20].
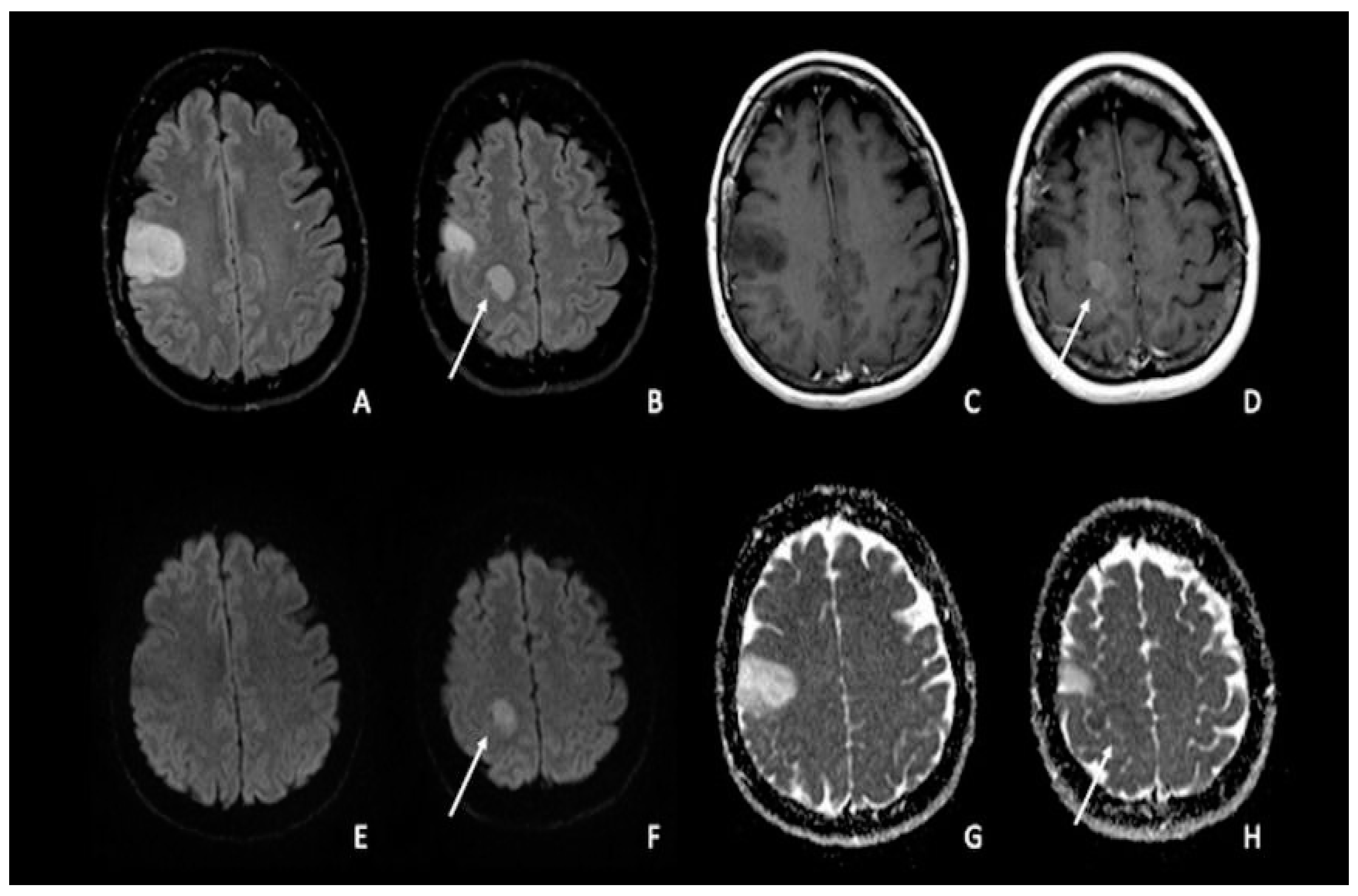
Figure 1. Double site of glioma infiltration in the right Rolandic frontal region: (A,B) Both lesions present similar hyperintensity on FLAIR images. The most cranial lesion (arrow) showed slight post-contrastographic enhancement (D) and restricted water diffusion on DWI (F), characterized by slightly low values on ADC map (H); this condition could be linked to an anaplastic aspect of the tumor, with restricted diffusion explained by an increase in tumor cellularity. The most caudal lesion did not show contrast enhancement, with a marked hypointense signal on post-contrastographic T1-weighted image (C); the same lesion presented no restricted diffusion with increased ADC values (E–G). FLAIR = fluid-attenuated inversion recovery; DWI = diffusion-weighted imaging; ADC: apparent diffusion coefficient.
In addition to standard DWI acquisition, more advanced models of quantitative DWI, such as DTI and DKI, have been created. These advanced sequences try to go beyond the theory that water diffusion occurs without boundaries via a uniform Gaussian distribution; indeed, it also depends on the configuration of intracellular organelles, cell membranes, and water compartments in cerebral tissues [6,21,22][6][21][22]. For instance, thanks to DKI, it is possible to quantify the deviation from a Gaussian distribution to produce a more accurate model [6,23,24][6][23][24]. The same result was reached by Abdalla et al. in a more recent expanded and updated meta-analysis, which found that DKI offers good diagnostic accuracy in differentiating high-grade from low-grade gliomas [6,25][6][25].
Finally, intravoxel incoherent motion (IVIM) and neurite orientation and dispersion imaging (NODDI), which make use of multiband imaging, are two promising advanced DTI approaches [6,26][6][26]. NODDI, in particular, measures the microstructure of dendrites and axons, revealing neuronal alterations, whereas IVIM may estimate tissue diffusivity and microcapillary perfusion [6].
3. Gliomas and Molecular Biology
When considering the glial line neoplasm, the 2016 World Health Organization (WHO) classification system has defined various groups of diffuse low-grade gliomas (LGGs) considering the isocitrate dehydrogenase (IDH) mutation and 1p/19q codeletion status [27,28][27][28]. The WHO classification of 2021 increased the importance of the molecular profile of brain tumors by categorizing adult-type diffuse glioma into three groups: IDH mutant with 1p/19q codeletion (Oligodendroglioma/IDH-mut codeleted LGGs), IDH mutant without codeletion (Astrocytoma/IDH-mut non-codeleted), and IDH wild type (Glioblastoma) [29]. The IDH gene plays a crucial role in metabolism, cellularity, and angiogenesis [30]. Recent research has shown that IDH mutant gliomas exhibit significantly greater survival and chemosensitivity than IDH wild-type glioblastomas [31,32][31][32]. When the IDH gene family is mutated, an oncometabolite called 2-hydroxyglutarate is produced, which inhibits tumor cell proliferation more than the wild type [33,34][33][34]. When compared to astrocytomas, oligodendrogliomas have a superior clinical outcome and treatment response [29,31,35,36,37][29][31][35][36][37]. As IDH mutant inhibitors become commercially available and might even be employed as neoadjuvant therapy, imaging biomarkers of IDH mutation would be a useful adjunct tool for clinical decision-making [38]. As a result, numerous research studies [33,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65][33][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65] investigated the imaging properties and/or high diagnostic performance of various MRI sequences for the prediction of IDH mutations in gliomas. According to several studies [41[41][48][56][60][62],48,56,60,62], IDH mutant glioma consistently displays higher mean ADC values on DWI than IDH wild-type glioblastoma [34]. It is still unclear how IDH-mutant and IDH wild-type gliomas differ from one another in terms of the ADC values; however, it could possibly be primarily related to tumor cellularity but also to the presence of cystic components, areas of necrosis, and interstitial water content [17,18,66][17][18][66]. While most IDH wild-type gliomas commonly present high-grade features such as necrosis and lower ADC mean values in solid sections, perhaps indicating more cellularity, most IDH-mutant gliomas show higher ADC mean values and MR imaging features consistent with a lower-grade nature [37] (Figure 2A–H). When considering oligodendrogliomas and the relationship between the 1p/19q codeletion status and ADC levels, the literature has shown contradictory findings [56,67,68,69][56][67][68][69]. According to Jenkinson et al., IDH-mut non-codeleted LGGs had much higher ADC values than IDH-mut-codeleted LGGs. Compared to IDH-mut non-codelated LGGs, the IDH-mut codeleted group may have fewer edematous areas and more cellulated areas, which could account for the lower ADC values [29,68][29][68]. Other diffusion-based sequences, such as DTI, DKI, and NODDI, have been successfully tested in predicting IDH status in gliomas, both with and without the use of artificial intelligence [70[70][71],71], with similar results between DTI and more advanced multi-shell methods [71,72][71][72]. Interestingly, mean diffusivity (MD) measures were increased in tumors not usually associated with high cellularity, probably reflecting changes in the extracellular volume that play a role in the diffusion signal [71]. Epidermal Growth Factor (EGFR) amplification is a molecular biomarker that could allow glioblastoma IDH wild-type designation even in tumors that appear histologically lower grade [29]. Hence, its prediction through MRI could be of therapeutic and prognostic use. A recent pilot study found that EGFR-amplified tumors showed lower mean ADC values compared to EGFR-non-amplified tumors [73]. Future research could focus on other important molecular information in gliomas, such as CDKN2A/B deletion or combined whole chromosome 7 gain and whole chromosome 10 loss (+7/−10) mutations.
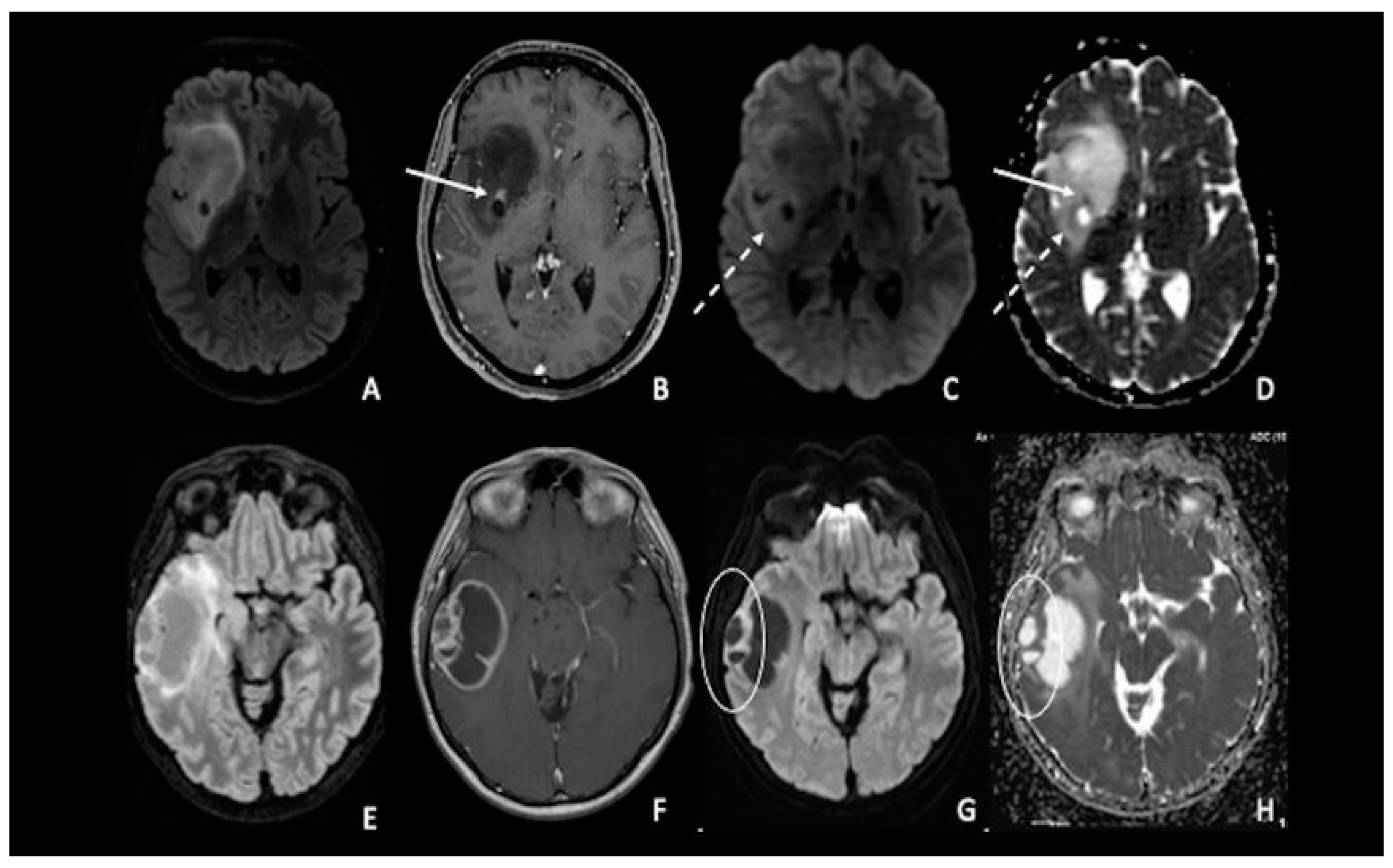
Figure 2. (A–D) IDH-1 mutated glioma in the right Sylvian region. The lesion is characterized by a heterogenous signal on FLAIR (A), with a small area of nodular enhancement on post-contrastographic T1 weighted images (arrow) (B); The site of pathological enhancement was in close proximity to an area of cystic-necrotic degeneration (dotted arrow) (C,D) and showed restricted diffusion on DWI confirmed by the ADC map (arrow); these findings were consistent with an anaplastic behavior of the tumor. (E–H) Right temporal lobe glioblastoma. The lesion was predominantly cystic and had a vivid peripherical post-contrastographic enhancement (F) with a solid component on the lateral side of the tumor; this region presented restricted diffusion on DWI confirmed by the ADC map (circle) (G,H), related to an increase in tumor cellularity. FLAIR = fluid-attenuated inversion recovery; DWI = diffusion-weighted imaging; ADC = apparent diffusion coefficient.
4. Lymphomas
One of the uses of DWI on CNS neoplasia imaging is the differential diagnosis between glioblastoma and primary CNS lymphoma (PCNSL), two entities that may appear similar on conventional imaging when considering a patient presenting with an enhancing brain mass but with a completely different pathogenesis, origin, and treatment [74,75][74][75]. PCNSLs are highly cellular tumors with relatively little extracellular space, which limits the diffusivity of free water. As a result, compared to HGGs and metastases, PCNSLs have been found to have much lower ADC values (Figure 3A–H). Similar to ADC, PCNSLs have been found to have lower FA values than high-grade gliomas [4,76,77,78,79,80,81,82,83,84,85][4][76][77][78][79][80][81][82][83][84][85].
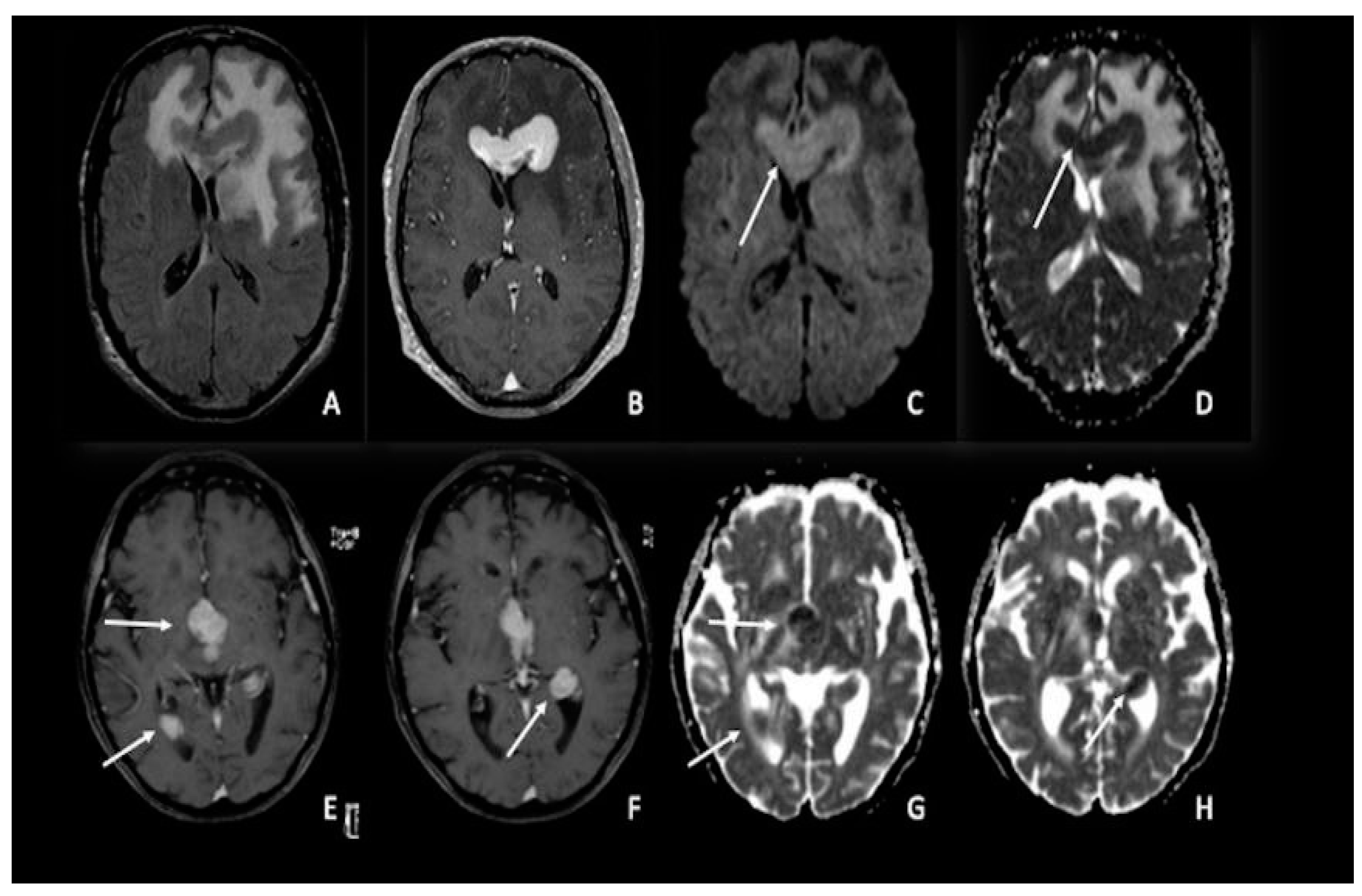
Figure 3. Two different cases of primitive cerebral lymphoma: the upper row showed a lymphoma in the pericallosal region (A–D) with a marked uniform enhancement on post-contrastographic T1 weighted image (B) and significant edema in the adjacent brain (A). The lower row showed a periventricular multicentric lymphoma (E–H) with multiple nodules of marked enhancement on post-contrast T1 weighted images (arrows) (E,F). Both cases presented restricted water diffusion with hyperintensity on DWI (arrow in C) and hypointense areas on ADC (arrows respectively in (D,G,H)), related to increasing tumor cellularity, as occurs typically in lymphomas. DWI = diffusion-weighted imaging; ADC = apparent diffusion coefficient.
5. Medulloblastomas
Medulloblastomas are highly cellular tumors; consequently, they present a substantial reduction in water molecule movement, resulting in a high diffusivity restriction on DWI and ADC images [86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103]. This finding helps in the differential diagnosis of other tumors typically located in the posterior fossa, such as ependymomas and pilocytic astrocytomas. Indeed, even if there is still overlap between these tumors [94,96[94][96][97][103][104][105][106],97,103,104,105,106], a high diffusion restriction is more suggestive of medulloblastomas than ependymomas or pilocytic astrocytomas [86,103,107][86][103][107] (Figure 4A–C). An ADC cut-off between 700 and 900 mm2/s has been proposed by the literature to distinguish medulloblastomas from pilocytic astrocytomas [106[106][108][109],108,109], whereas using a minimum ADC cut-off value of 660 mm2/s seems to allow for a good distinction with ependymomas [110]. Moreover, according to the literature, the ratio of ADC within the tumor compared to the grey matter ranges between 0.70 and 0.88 for the solid component [97,108,111][97][108][111] and 0.97 and 1.28 for the entire tumor [108,112,113,114][108][112][113][114]. Medulloblastomas, ependymomas, and pilocytic astrocytomas share the same fractional anisotropy [115,116,117][115][116][117]. As measured by lower ADC values, medulloblastomas frequently have lower rates of microscopic water diffusion than other common posterior fossa tumors in children [96]. This trait is most likely brought on by the frequent presence of cells with a high nuclear-to-cytoplasmic ratio and a high degree of cellularity in medulloblastomas, which results in additional membrane barriers impeding microscopic water diffusion [118]. Another much rarer tumor of the posterior fossa, typically affecting children, is the atypical teratoid/rhabdoid tumor (ATRT), which histologically resembles medulloblastoma [119] and exhibits similar DWI characteristics to medulloblastoma [86,111][86][111]. Finally, the distinction between medulloblastomas and glioblastomas cannot be made with DWI [86].
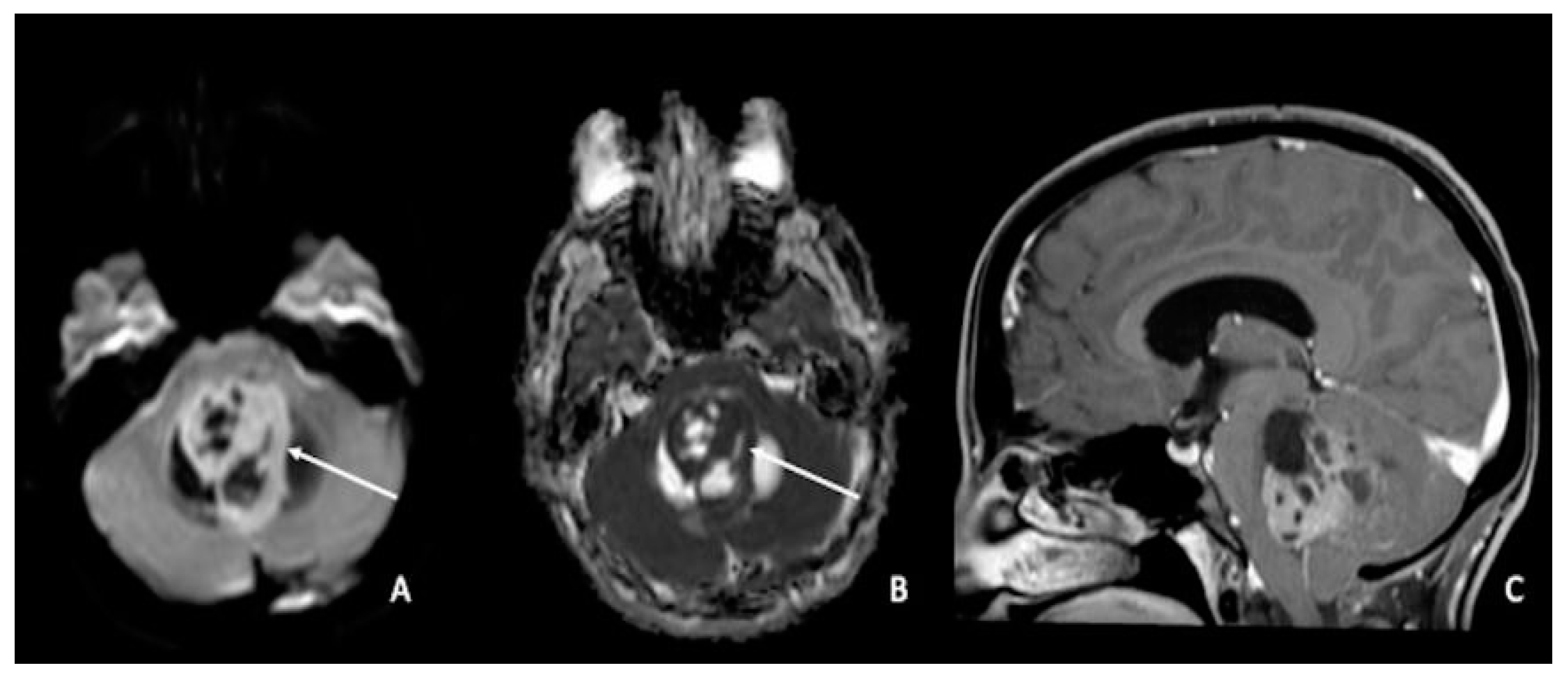
Figure 4. Medulloblastoma in a 30-year-old: (A,C) lesion of the posterior fossa with growth in the median line, close to the IV ventricle, characterized by inhomogeneous enhancement (arrow in (A)), mass effect, and (B) low ADC values (arrow). Using ADC values and a specific cut-off, it could be possible to distinguish medulloblastoma from ependymoma or pilocytic glioma. ADC = apparent diffusion coefficient.
According to Ahmed et al.’s study, the ADC ratio (ADC of the tumor divided by the ADC of the corresponding contralateral normal white matter) was unable to distinguish between medulloblastoma and ATRT. This could be explained by the fact that medulloblastoma and ATRT both have high grades in accordance with the WHO grading system, which indicates high tumoral cellularity and leads to a low ADC ratio [120].
References
- Martín-Noguerol, T.; Mohan, S.; Santos-Armentia, E.; Cabrera-Zubizarreta, A.; Luna, A. Advanced MRI assessment of non-enhancing peritumoral signal abnormality in brain lesions. Eur. J. Radiol. 2021, 143, 109900.
- Martín Noguerol, T.; Martínez Barbero, J.P. Advanced diffusion MRI and biomarkers in the central nervous system: A new approach. Radiologia 2017, 59, 273–285.
- Huisman, T.A. Diffusion-weighted imaging: Basic concepts and application in cerebral stroke and head trauma. Eur. Radiol. 2003, 13, 2283–2297.
- Svolos, P.; Kousi, E.; Kapsalaki, E.; Theodorou, K.; Fezoulidis, I.; Kappas, C.; Tsougos, I. The role of diffusion and perfusion weighted imaging in the differential diagnosis of cerebral tumors: A review and future perspectives. Cancer Imaging 2014, 14, 20.
- Romano, A.; Bozzao, A.; Bonamini, M.; Fasoli, F.; Ferrante, M.; Floris, R.; Colonnese, C.; Fantozzi, L.M. Diffusion-weighted MR Imaging: Clinical applications in neuroradiology. Radiol. Med. 2003, 106, 521–548.
- Overcast, W.B.; Davis, K.M.; Ho, C.Y.; Hutchins, G.D.; Green, M.A.; Graner, B.D.; Veronesi, M.C. Advanced imaging techniques for neuro-oncologic tumor diagnosis, with an emphasis on PET-MRI imaging of malignant brain tumors. Curr. Oncol. Rep. 2021, 23, 34.
- Dhermain, F.G.; Hau, P.; Lanfermann, H.; Jacobs, A.H.; van den Bent, M.J. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010, 9, 906–920.
- Falini, A.; Romano, A.; Bozzao, A. Tumours. Neurol. Sci. 2008, 29 (Suppl. S3), 327–332.
- Gaddamanugu, S.; Shafaat, O.; Sotoudeh, H.; Sarrami, A.H.; Rezaei, A.; Saadatpour, Z.; Singhal, A. Clinical applications of diffusion-weighted sequence in brain imaging: Beyond stroke. Neuroradiology 2022, 64, 15–30.
- Guo, A.C.; Cummings, T.J.; Dash, R.C.; Provenzale, J.M. Lymphomas and high-grade astrocytomas: Comparison of water diffusibility and histologic characteristics. Radiology 2002, 224, 177–183.
- Higano, S.; Yun, X.; Kumabe, T.; Watanabe, M.; Mugikura, S.; Umetsu, A.; Sato, A.; Yamada, T.; Takahashi, S. Malignant astrocytic tumors: Clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology 2006, 241, 839–846.
- Moon, W.J.; Choi, J.W.; Roh, H.G.; Lim, S.D.; Koh, Y.C. Imaging parameters of high grade gliomas in relation to the MGMT promoter methylation status: The CT, diffusion tensor imaging, and perfusion MR imaging. Neuroradiology 2012, 54, 555–563.
- Yamasaki, F.; Kurisu, K.; Satoh, K.; Arita, K.; Sugiyama, K.; Ohtaki, M.; Takaba, J.; Tominaga, A.; Hanaya, R.; Yoshioka, H.; et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology 2005, 235, 985–991.
- Murakami, R.; Sugahara, T.; Nakamura, H.; Hirai, T.; Kitajima, M.; Hayashida, Y.; Baba, Y.; Oya, N.; Kuratsu, J.; Yamashita, Y. Malignant supratentorial astrocytoma treated with postoperative radiation therapy: Prognostic value of pretreatment quantitative diffusion-weighted MR imaging. Radiology 2007, 243, 493–499.
- Romano, A.; Calabria, L.F.; Tavanti, F.; Minniti, G.; Rossi-Espagnet, M.C.; Coppola, V.; Pugliese, S.; Guida, D.; Francione, G.; Colonnese, C.; et al. Apparent diffusion coefficient obtained by magnetic resonance imaging as a prognostic marker in glioblastomas: Correlation with MGMT promoter methylation status. Eur. Radiol. 2013, 23, 513–520.
- Silber, J.R.; Blank, A.; Bobola, M.S.; Ghatan, S.; Kolstoe, D.D.; Berger, M.S. O6-methylguanine-DNA methyltransferase-deficient phenotype in human gliomas: Frequency and time to tumor progression after alkylating agent-based chemotherapy. Clin. Cancer Res. 1999, 5, 807–814.
- Sugahara, T.; Korogi, Y.; Kochi, M.; Ikushima, I.; Shigematu, Y.; Hirai, T.; Okuda, T.; Liang, L.; Ge, Y.; Komohara, Y.; et al. Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J. Magn. Reson. Imaging 1999, 9, 53–60.
- Gupta, R.K.; Cloughesy, T.F.; Sinha, U.; Garakian, J.; Lazareff, J.; Rubino, G.; Rubino, L.; Becker, D.P.; Vinters, H.V.; Alger, J.R. Relationships between choline magnetic resonance spectroscopy, apparent diffusion coefficient and quantitative histopathology in human glioma. J. Neurooncol. 2000, 50, 215–226.
- Qin, J.B.; Zhang, H.; Wang, X.C.; Tan, Y.; Wu, X.F. Combination value of diffusion-weighted imaging and dynamic susceptibility contrast-enhanced MRI in astrocytoma grading and correlation with GFAP, Topoisomerase IIα and MGMT. Oncol. Lett. 2019, 18, 2763–2770.
- Wang, Q.P.; Lei, D.Q.; Yuan, Y.; Xiong, N.X. Accuracy of ADC derived from DWI for differentiating high-grade from low-grade gliomas: Systematic review and meta-analysis. Medicine 2020, 99, e19254.
- Van Cauter, S.; Veraart, J.; Sijbers, J.; Peeters, R.R.; Himmelreich, U.; De Keyzer, F.; Van Gool, S.W.; Van Calenbergh, F.; De Vleeschouwer, S.; Van Hecke, W.; et al. Gliomas: Diffusion kurtosis MR imaging in grading. Radiology 2012, 263, 492–501.
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion tensor imaging of the brain. Neurotherapeutics 2007, 4, 316–329.
- Jensen, J.H.; Helpern, J.A. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010, 23, 698–710.
- Raab, P.; Hattingen, E.; Franz, K.; Zanella, F.E.; Lanfermann, H. Cerebral gliomas: Diffusional kurtosis imaging analysis of microstructural differences. Radiology 2010, 254, 876–881.
- Abdalla, G.; Dixon, L.; Sanverdi, E.; Machado, P.M.; Kwong, J.S.W.; Panovska-Griffiths, J.; Rojas-Garcia, A.; Yoneoka, D.; Veraart, J.; Van Cauter, S.; et al. The diagnostic role of diffusional kurtosis imaging in glioma grading and differentiation of gliomas from other intra-axial brain tumours: A systematic review with critical appraisal and meta-analysis. Neuroradiology 2020, 62, 791–802.
- Wen, Q.; Kelley, D.A.; Banerjee, S.; Lupo, J.M.; Chang, S.M.; Xu, D.; Hess, C.P.; Nelson, S.J. Clinically feasible NODDI characterization of glioma using multiband EPI at 7 T. Neuroimage Clin. 2015, 9, 291–299.
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820.
- Appin, C.L.; Brat, D.J. Molecular genetics of gliomas. Cancer J. 2014, 20, 66–72.
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251.
- Kickingereder, P.; Sahm, F.; Radbruch, A.; Wick, W.; Heiland, S.; Deimling, A.V.; Bendszus, M.; Wiestler, B. IDH mutation status is associated with a distinct hypoxia/angiogenesis transcriptome signature which is non-invasively predictable with rCBV imaging in human glioma. Sci. Rep. 2015, 5, 16238.
- Brat, D.J.; Verhaak, R.G.; Aldape, K.D.; Yung, W.K.; Salama, S.R.; Cooper, L.A.; Rheinbay, E.; Miller, C.R.; Vitucci, M.; Morozova, O.; et al. Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas. N. Engl. J. Med. 2015, 372, 2481–2498.
- Miller, J.J.; Shih, H.A.; Andronesi, O.C.; Cahill, D.P. Isocitrate dehydrogenase-mutant glioma: Evolving clinical and therapeutic implications. Cancer 2017, 123, 4535–4546.
- Yu, J.; Shi, Z.; Lian, Y.; Li, Z.; Liu, T.; Gao, Y.; Wang, Y.; Chen, L.; Mao, Y. Noninvasive IDH1 mutation estimation based on a quantitative radiomics approach for grade II glioma. Eur. Radiol. 2017, 27, 3509–3522.
- Suh, C.H.; Kim, H.S.; Jung, S.C.; Choi, C.G.; Kim, S.J. Imaging prediction of isocitrate dehydrogenase (IDH) mutation in patients with glioma: A systemic review and meta-analysis. Eur. Radiol. 2019, 29, 745–758.
- van den Bent, M.J.; Brandes, A.A.; Taphoorn, M.J.; Kros, J.M.; Kouwenhoven, M.C.; Delattre, J.Y.; Bernsen, H.J.; Frenay, M.; Tijssen, C.C.; Grisold, W.; et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: Long-term follow-up of EORTC brain tumor group study 26951. J. Clin. Oncol. 2013, 31, 344–350.
- Chamberlain, M.C.; Born, D. Prognostic significance of relative 1p/19q codeletion in oligodendroglial tumors. J. Neurooncol. 2015, 125, 249–251.
- Wu, C.C.; Jain, R.; Radmanesh, A.; Poisson, L.M.; Guo, W.Y.; Zagzag, D.; Snuderl, M.; Placantonakis, D.G.; Golfinos, J.; Chi, A.S. Predicting Genotype and Survival in Glioma Using Standard Clinical MR Imaging Apparent Diffusion Coefficient Images: A Pilot Study from the Cancer Genome Atlas. AJNR Am. J. Neuroradiol. 2018, 39, 1814–1820.
- Dang, L.; Yen, K.; Attar, E.C. IDH mutations in cancer and progress toward development of targeted therapeutics. Ann. Oncol. 2016, 27, 599–608.
- Zhou, H.; Vallières, M.; Bai, H.X.; Su, C.; Tang, H.; Oldridge, D.; Zhang, Z.; Xiao, B.; Liao, W.; Tao, Y.; et al. MRI features predict survival and molecular markers in diffuse lower-grade gliomas. Neuro-Oncology 2017, 19, 862–870.
- Zhang, B.; Chang, K.; Ramkissoon, S.; Tanguturi, S.; Bi, W.L.; Reardon, D.A.; Ligon, K.L.; Alexander, B.M.; Wen, P.Y.; Huang, R.Y. Multimodal MRI features predict isocitrate dehydrogenase genotype in high-grade gliomas. Neuro-Oncology 2017, 19, 109–117.
- Xing, Z.; Yang, X.; She, D.; Lin, Y.; Zhang, Y.; Cao, D. Noninvasive Assessment of IDH Mutational Status in World Health Organization Grade II and III Astrocytomas Using DWI and DSC-PWI Combined with Conventional MR Imaging. AJNR Am. J. Neuroradiol. 2017, 38, 1138–1144.
- Tietze, A.; Choi, C.; Mickey, B.; Maher, E.A.; Parm Ulhøi, B.; Sangill, R.; Lassen-Ramshad, Y.; Lukacova, S.; Østergaard, L.; von Oettingen, G. Noninvasive assessment of isocitrate dehydrogenase mutation status in cerebral gliomas by magnetic resonance spectroscopy in a clinical setting. J. Neurosurg. 2018, 128, 391–398.
- Tan, W.; Xiong, J.; Huang, W.; Wu, J.; Zhan, S.; Geng, D. Noninvasively detecting Isocitrate dehydrogenase 1 gene status in astrocytoma by dynamic susceptibility contrast MRI. J. Magn. Reson. Imaging 2017, 45, 492–499.
- Stadlbauer, A.; Zimmermann, M.; Kitzwögerer, M.; Oberndorfer, S.; Rössler, K.; Dörfler, A.; Buchfelder, M.; Heinz, G. MR Imaging-derived Oxygen Metabolism and Neovascularization Characterization for Grading and IDH Gene Mutation Detection of Gliomas. Radiology 2017, 283, 799–809.
- Price, S.J.; Allinson, K.; Liu, H.; Boonzaier, N.R.; Yan, J.L.; Lupson, V.C.; Larkin, T.J. Less Invasive Phenotype Found in Isocitrate Dehydrogenase-mutated Glioblastomas than in Isocitrate Dehydrogenase Wild-Type Glioblastomas: A Diffusion-Tensor Imaging Study. Radiology 2017, 283, 215–221.
- Patel, S.H.; Poisson, L.M.; Brat, D.J.; Zhou, Y.; Cooper, L.; Snuderl, M.; Thomas, C.; Franceschi, A.M.; Griffith, B.; Flanders, A.E.; et al. T2-FLAIR Mismatch, an Imaging Biomarker for IDH and 1p/19q Status in Lower-grade Gliomas: A TCGA/TCIA Project. Clin. Cancer Res. 2017, 23, 6078–6085.
- Nakae, S.; Murayama, K.; Sasaki, H.; Kumon, M.; Nishiyama, Y.; Ohba, S.; Adachi, K.; Nagahisa, S.; Hayashi, T.; Inamasu, J.; et al. Prediction of genetic subgroups in adult supra tentorial gliomas by pre- and intraoperative parameters. J. Neurooncol. 2017, 131, 403–412.
- Leu, K.; Ott, G.A.; Lai, A.; Nghiemphu, P.L.; Pope, W.B.; Yong, W.H.; Liau, L.M.; Cloughesy, T.F.; Ellingson, B.M. Perfusion and diffusion MRI signatures in histologic and genetic subtypes of WHO grade II-III diffuse gliomas. J. Neurooncol. 2017, 134, 177–188.
- Lasocki, A.; Tsui, A.; Gaillard, F.; Tacey, M.; Drummond, K.; Stuckey, S. Reliability of noncontrast-enhancing tumor as a biomarker of IDH1 mutation status in glioblastoma. J. Clin. Neurosci. 2017, 39, 170–175.
- Jiang, S.; Zou, T.; Eberhart, C.G.; Villalobos, M.A.V.; Heo, H.Y.; Zhang, Y.; Wang, Y.; Wang, X.; Yu, H.; Du, Y.; et al. Predicting IDH mutation status in grade II gliomas using amide proton transfer-weighted (APTw) MRI. Magn. Reson. Med. 2017, 78, 1100–1109.
- Hsieh, K.L.; Chen, C.Y.; Lo, C.M. Radiomic model for predicting mutations in the isocitrate dehydrogenase gene in glioblastomas. Oncotarget 2017, 8, 45888–45897.
- Hempel, J.M.; Schittenhelm, J.; Brendle, C.; Bender, B.; Bier, G.; Skardelly, M.; Tabatabai, G.; Castaneda Vega, S.; Ernemann, U.; Klose, U. Histogram analysis of diffusion kurtosis imaging estimates for in vivo assessment of 2016 WHO glioma grades: A cross-sectional observational study. Eur. J. Radiol. 2017, 95, 202–211.
- Grabner, G.; Kiesel, B.; Wöhrer, A.; Millesi, M.; Wurzer, A.; Göd, S.; Mallouhi, A.; Knosp, E.; Marosi, C.; Trattnig, S.; et al. Local image variance of 7 Tesla SWI is a new technique for preoperative characterization of diffusely infiltrating gliomas: Correlation with tumour grade and IDH1 mutational status. Eur. Radiol. 2017, 27, 1556–1567.
- Delfanti, R.L.; Piccioni, D.E.; Handwerker, J.; Bahrami, N.; Krishnan, A.; Karunamuni, R.; Hattangadi-Gluth, J.A.; Seibert, T.M.; Srikant, A.; Jones, K.A.; et al. Imaging correlates for the 2016 update on WHO classification of grade II/III gliomas: Implications for IDH, 1p/19q and ATRX status. J. Neurooncol. 2017, 135, 601–609.
- Yamashita, K.; Hiwatashi, A.; Togao, O.; Kikuchi, K.; Hatae, R.; Yoshimoto, K.; Mizoguchi, M.; Suzuki, S.O.; Yoshiura, T.; Honda, H. MR Imaging-Based Analysis of Glioblastoma Multiforme: Estimation of IDH1 Mutation Status. AJNR Am. J. Neuroradiol. 2016, 37, 58–65.
- Xiong, J.; Tan, W.; Wen, J.; Pan, J.; Wang, Y.; Zhang, J.; Geng, D. Combination of diffusion tensor imaging and conventional MRI correlates with isocitrate dehydrogenase 1/2 mutations but not 1p/19q genotyping in oligodendroglial tumours. Eur. Radiol. 2016, 26, 1705–1715.
- Wang, K.; Wang, Y.; Fan, X.; Wang, J.; Li, G.; Ma, J.; Ma, J.; Jiang, T.; Dai, J. Radiological features combined with IDH1 status for predicting the survival outcome of glioblastoma patients. Neuro-Oncology 2016, 18, 589–597.
- Choi, C.; Raisanen, J.M.; Ganji, S.K.; Zhang, S.; McNeil, S.S.; An, Z.; Madan, A.; Hatanpaa, K.J.; Vemireddy, V.; Sheppard, C.A.; et al. Prospective Longitudinal Analysis of 2-Hydroxyglutarate Magnetic Resonance Spectroscopy Identifies Broad Clinical Utility for the Management of Patients with IDH-Mutant Glioma. J. Clin. Oncol. 2016, 34, 4030–4039.
- Biller, A.; Badde, S.; Nagel, A.; Neumann, J.O.; Wick, W.; Hertenstein, A.; Bendszus, M.; Sahm, F.; Benkhedah, N.; Kleesiek, J. Improved Brain Tumor Classification by Sodium MR Imaging: Prediction of IDH Mutation Status and Tumor Progression. AJNR Am. J. Neuroradiol. 2016, 37, 66–73.
- Wasserman, J.K.; Nicholas, G.; Yaworski, R.; Wasserman, A.M.; Woulfe, J.M.; Jansen, G.H.; Chakraborty, S.; Nguyen, T.B. Radiological and pathological features associated with IDH1-R132H mutation status and early mortality in newly diagnosed anaplastic astrocytic tumours. PLoS ONE 2015, 10, e0123890.
- Sonoda, Y.; Shibahara, I.; Kawaguchi, T.; Saito, R.; Kanamori, M.; Watanabe, M.; Suzuki, H.; Kumabe, T.; Tominaga, T. Association between molecular alterations and tumor location and MRI characteristics in anaplastic gliomas. Brain Tumor Pathol. 2015, 32, 99–104.
- Lee, S.; Choi, S.H.; Ryoo, I.; Yoon, T.J.; Kim, T.M.; Lee, S.H.; Park, C.K.; Kim, J.H.; Sohn, C.H.; Park, S.H.; et al. Evaluation of the microenvironmental heterogeneity in high-grade gliomas with IDH1/2 gene mutation using histogram analysis of diffusion-weighted imaging and dynamic-susceptibility contrast perfusion imaging. J. Neurooncol. 2015, 121, 141–150.
- Reyes-Botero, G.; Dehais, C.; Idbaih, A.; Martin-Duverneuil, N.; Lahutte, M.; Carpentier, C.; Letouzé, E.; Chinot, O.; Loiseau, H.; Honnorat, J.; et al. Contrast enhancement in 1p/19q-codeleted anaplastic oligodendrogliomas is associated with 9p loss, genomic instability, and angiogenic gene expression. Neuro-Oncology 2014, 16, 662–670.
- Qi, S.; Yu, L.; Li, H.; Ou, Y.; Qiu, X.; Ding, Y.; Han, H.; Zhang, X. Isocitrate dehydrogenase mutation is associated with tumor location and magnetic resonance imaging characteristics in astrocytic neoplasms. Oncol Lett. 2014, 7, 1895–1902.
- Carrillo, J.A.; Lai, A.; Nghiemphu, P.L.; Kim, H.J.; Phillips, H.S.; Kharbanda, S.; Moftakhar, P.; Lalaezari, S.; Yong, W.; Ellingson, B.M.; et al. Relationship between tumor enhancement, edema, IDH1 mutational status, MGMT promoter methylation, and survival in glioblastoma. AJNR Am. J. Neuroradiol. 2012, 33, 1349–1355.
- Lee, E.J.; Lee, S.K.; Agid, R.; Bae, J.M.; Keller, A.; Terbrugge, K. Preoperative grading of presumptive low-grade astrocytomas on MR imaging: Diagnostic value of minimum apparent diffusion coefficient. AJNR Am. J. Neuroradiol. 2008, 29, 1872–1877.
- Fellah, S.; Caudal, D.; De Paula, A.M.; Dory-Lautrec, P.; Figarella-Branger, D.; Chinot, O.; Metellus, P.; Cozzone, P.J.; Confort-Gouny, S.; Ghattas, B.; et al. Multimodal MR imaging (diffusion, perfusion, and spectroscopy): Is it possible to distinguish oligodendroglial tumor grade and 1p/19q codeletion in the pretherapeutic diagnosis? AJNR Am. J. Neuroradiol. 2013, 34, 1326–1333.
- Jenkinson, M.D.; Smith, T.S.; Brodbelt, A.R.; Joyce, K.A.; Warnke, P.C.; Walker, C. Apparent diffusion coefficients in oligodendroglial tumors characterized by genotype. J. Magn. Reson. Imaging 2007, 26, 1405–1412.
- Park, Y.W.; Han, K.; Ahn, S.S.; Bae, S.; Choi, Y.S.; Chang, J.H.; Kim, S.H.; Kang, S.G.; Lee, S.K. Prediction of IDH1-Mutation and 1p/19q-Codeletion Status Using Preoperative MR Imaging Phenotypes in Lower Grade Gliomas. AJNR Am. J. Neuroradiol. 2018, 39, 37–42.
- Eichinger, P.; Alberts, E.; Delbridge, C.; Trebeschi, S.; Valentinitsch, A.; Bette, S.; Huber, T.; Gempt, J.; Meyer, B.; Schlegel, J.; et al. Diffusion tensor image features predict IDH genotype in newly diagnosed WHO grade II/III gliomas. Sci. Rep. 2017, 7, 13396.
- Figini, M.; Riva, M.; Graham, M.; Castelli, G.M.; Fernandes, B.; Grimaldi, M.; Baselli, G.; Pessina, F.; Bello, L.; Zhang, H.; et al. Prediction of Isocitrate Dehydrogenase Genotype in Brain Gliomas with MRI: Single-Shell versus Multishell Diffusion Models. Radiology 2018, 289, 788–796.
- Gao, A.; Zhang, H.; Yan, X.; Wang, S.; Chen, Q.; Gao, E.; Qi, J.; Bai, J.; Zhang, Y.; Cheng, J. Whole-Tumor Histogram Analysis of Multiple Diffusion Metrics for Glioma Genotyping. Radiology 2022, 302, 652–661.
- Park, Y.W.; Ahn, S.S.; Park, C.J.; Han, K.; Kim, E.H.; Kang, S.G.; Chang, J.H.; Kim, S.H.; Lee, S.K. Diffusion and perfusion MRI may predict EGFR amplification and the TERT promoter mutation status of IDH-wildtype lower-grade gliomas. Eur. Radiol. 2020, 30, 6475–6484.
- Drake-Pérez, M.; Boto, J.; Fitsiori, A.; Lovblad, K.; Vargas, M.I. Clinical applications of diffusion weighted imaging in neuroradiology. Insights Imaging 2018, 9, 535–547.
- Lin, X.; Lee, M.; Buck, O.; Woo, K.M.; Zhang, Z.; Hatzoglou, V.; Omuro, A.; Arevalo-Perez, J.; Thomas, A.A.; Huse, J.; et al. Diagnostic Accuracy of T1-Weighted Dynamic Contrast-Enhanced-MRI and DWI-ADC for Differentiation of Glioblastoma and Primary CNS Lymphoma. AJNR Am. J. Neuroradiol. 2017, 38, 485–491.
- Wang, S.; Kim, S.; Chawla, S.; Wolf, R.L.; Knipp, D.E.; Vossough, A.; O’Rourke, D.M.; Judy, K.D.; Poptani, H.; Melhem, E.R. Differentiation between glioblastomas, solitary brain metastases, and primary cerebral lymphomas using diffusion tensor and dynamic susceptibility contrast-enhanced MR imaging. AJNR Am. J. Neuroradiol. 2011, 32, 507–514.
- Toh, C.H.; Castillo, M.; Wong, A.M.; Wei, K.C.; Wong, H.F.; Ng, S.H.; Wan, Y.L. Primary cerebral lymphoma and glioblastoma multiforme: Differences in diffusion characteristics evaluated with diffusion tensor imaging. AJNR Am. J. Neuroradiol. 2008, 29, 471–475.
- Lu, X.; Xu, W.; Wei, Y.; Li, T.; Gao, L.; Fu, X.; Yao, Y.; Wang, L. Diagnostic performance of DWI for differentiating primary central nervous system lymphoma from glioblastoma: A systematic review and meta-analysis. Neurol. Sci. 2019, 40, 947–956.
- Ahn, S.J.; Shin, H.J.; Chang, J.H.; Lee, S.K. Differentiation between primary cerebral lymphoma and glioblastoma using the apparent diffusion coefficient: Comparison of three different ROI methods. PLoS ONE 2014, 9, e112948.
- Horger, M.; Fenchel, M.; Nägele, T.; Moehle, R.; Claussen, C.D.; Beschorner, R.; Ernemann, U. Water diffusivity: Comparison of primary CNS lymphoma and astrocytic tumor infiltrating the corpus callosum. AJR Am. J. Roentgenol. 2009, 193, 1384–1387.
- Calli, C.; Kitis, O.; Yunten, N.; Yurtseven, T.; Islekel, S.; Akalin, T. Perfusion and diffusion MR imaging in enhancing malignant cerebral tumors. Eur. J. Radiol. 2006, 58, 394–403.
- Server, A.; Kulle, B.; Maehlen, J.; Josefsen, R.; Schellhorn, T.; Kumar, T.; Langberg, C.W.; Nakstad, P.H. Quantitative apparent diffusion coefficients in the characterization of brain tumors and associated peritumoral edema. Acta Radiol. 2009, 50, 682–689.
- Rizzo, L.; Crasto, S.G.; Moruno, P.G.; Cassoni, P.; Rudà, R.; Boccaletti, R.; Brosio, M.; De Lucchi, R.; Fava, C. Role of diffusion- and perfusion-weighted MR imaging for brain tumour characterisation. Radiol. Med. 2009, 114, 645–659.
- Kinoshita, M.; Hashimoto, N.; Goto, T.; Kagawa, N.; Kishima, H.; Izumoto, S.; Tanaka, H.; Fujita, N.; Yoshimine, T. Fractional anisotropy and tumor cell density of the tumor core show positive correlation in diffusion tensor magnetic resonance imaging of malignant brain tumors. Neuroimage 2008, 43, 29–35.
- Rollin, N.; Guyotat, J.; Streichenberger, N.; Honnorat, J.; Tran Minh, V.A.; Cotton, F. Clinical relevance of diffusion and perfusion magnetic resonance imaging in assessing intra-axial brain tumors. Neuroradiology 2006, 48, 150–159.
- Yamashita, Y.; Kumabe, T.; Higano, S.; Watanabe, M.; Tominaga, T. Minimum apparent diffusion coefficient is significantly correlated with cellularity in medulloblastomas. Neurol. Res. 2009, 31, 940–946.
- Poussaint, T.Y.; Panigrahy, A.; Huisman, T.A. Pediatric brain tumors. Pediatr. Radiol. 2015, 45 (Suppl. S3), S443–S453.
- Rasalkar, D.D.; Chu, W.C.; Paunipagar, B.K.; Cheng, F.W.; Li, C.K. Paediatric intra-axial posterior fossa tumours: Pictorial review. Postgrad. Med. J. 2013, 89, 39–46.
- Fruehwald-Pallamar, J.; Puchner, S.B.; Rossi, A.; Garre, M.L.; Cama, A.; Koelblinger, C.; Osborn, A.G.; Thurnher, M.M. Magnetic resonance imaging spectrum of medulloblastoma. Neuroradiology 2011, 53, 387–396.
- Plaza, M.J.; Borja, M.J.; Altman, N.; Saigal, G. Conventional and advanced MRI features of pediatric intracranial tumors: Posterior fossa and suprasellar tumors. AJR Am. J. Roentgenol. 2013, 200, 1115–1124.
- Eran, A.; Ozturk, A.; Aygun, N.; Izbudak, I. Medulloblastoma: Atypical CT and MRI findings in children. Pediatr. Radiol. 2010, 40, 1254–1262.
- Sarrazin, J.L. Tumeurs de la fosse postérieure . J. Radiol. 2006, 87 Pt 2, 748–763.
- Chawla, A.; Emmanuel, J.V.; Seow, W.T.; Lou, J.; Teo, H.E.; Lim, C.C. Paediatric PNET: Pre-surgical MRI features. Clin. Radiol. 2007, 62, 43–52.
- Schneider, J.F.; Confort-Gouny, S.; Viola, A.; Le Fur, Y.; Viout, P.; Bennathan, M.; Chapon, F.; Figarella-Branger, D.; Cozzone, P.; Girard, N. Multiparametric differentiation of posterior fossa tumors in children using diffusion-weighted imaging and short echo-time 1H-MR spectroscopy. J. Magn. Reson. Imaging 2007, 26, 1390–1398.
- Wu, G.; Pang, H.; Ghimire, P.; Liu, G. (1)H magnetic resonance spectroscopy and diffusion weighted imaging findings of medulloblastoma in 3.0T MRI: A retrospective analysis of 17 cases. Neural. Regen. Res. 2012, 7, 2554–2559.
- Bull, J.G.; Saunders, D.E.; Clark, C.A. Discrimination of paediatric brain tumours using apparent diffusion coefficient histograms. Eur. Radiol. 2012, 22, 447–457.
- Gimi, B.; Cederberg, K.; Derinkuyu, B.; Gargan, L.; Koral, K.M.; Bowers, D.C.; Koral, K. Utility of apparent diffusion coefficient ratios in distinguishing common pediatric cerebellar tumors. Acad. Radiol. 2012, 19, 794–800.
- Poussaint, T.Y.; Rodriguez, D. Advanced neuroimaging of pediatric brain tumors: MR diffusion, MR perfusion, and MR spectroscopy. Neuroimaging Clin. N. Am. 2006, 16, 169–192, ix.
- Rodallec, M.; Colombat, M.; Krainik, A.; Kalamaridès, M.; Redondo, A.; Feydy, A. Diffusion-weighted MR imaging and pathologic findings in adult cerebellar medulloblastoma. J. Neuroradiol. 2004, 31, 234–237.
- Quadery, F.A.; Okamoto, K. Diffusion-weighted MRI of haemangioblastomas and other cerebellar tumours. Neuroradiology 2003, 45, 212–219.
- Wilke, M.; Eidenschink, A.; Müller-Weihrich, S.; Auer, D.P. MR diffusion imaging and 1H spectroscopy in a child with medulloblastoma. A case report. Acta Radiol. 2001, 42, 39–42.
- Kotsenas, A.L.; Roth, T.C.; Manness, W.K.; Faerber, E.N. Abnormal diffusion-weighted MRI in medulloblastoma: Does it reflect small cell histology? Pediatr. Radiol. 1999, 29, 524–526.
- Zitouni, S.; Koc, G.; Doganay, S.; Saracoglu, S.; Gumus, K.Z.; Ciraci, S.; Coskun, A.; Unal, E.; Per, H.; Kurtsoy, A.; et al. Apparent diffusion coefficient in differentiation of pediatric posterior fossa tumors. Jpn. J. Radiol. 2017, 35, 448–453.
- Pillai, S.; Singhal, A.; Byrne, A.T.; Dunham, C.; Cochrane, D.D.; Steinbok, P. Diffusion-weighted imaging and pathological correlation in pediatric medulloblastomas-“They are not always restricted!”. Childs Nerv. Syst. 2011, 27, 1407–1411.
- Douglas-Akinwande, A.C.; Payner, T.D.; Hattab, E.M. Medulloblastoma mimicking Lhermitte-Duclos disease on MRI and CT. Clin. Neurol. Neurosurg. 2009, 111, 536–539.
- Jaremko, J.L.; Jans, L.B.; Coleman, L.T.; Ditchfield, M.R. Value and limitations of diffusion-weighted imaging in grading and diagnosis of pediatric posterior fossa tumors. AJNR Am. J. Neuroradiol. 2010, 31, 1613–1616.
- Forbes, J.A.; Reig, A.S.; Smith, J.G.; Jermakowicz, W.; Tomycz, L.; Shay, S.D.; Sun, D.A.; Wushensky, C.A.; Pearson, M.M. Findings on preoperative brain MRI predict histopathology in children with cerebellar neoplasms. Pediatr. Neurosurg. 2011, 47, 51–59.
- Orman, G.; Bosemani, T.; Higgins, L.; Carson, K.A.; Huisman, T.A.; Poretti, A. Pediatric Cerebellar Tumors: Does ADC Analysis of Solid, Contrast-Enhancing Tumor Components Correlate Better with Tumor Grade than ADC Analysis of the Entire Tumor? J. Neuroimaging 2015, 25, 785–791.
- Porto, L.; Jurcoane, A.; Schwabe, D.; Kieslich, M.; Hattingen, E. Differentiation between high and low grade tumours in paediatric patients by using apparent diffusion coefficients. Eur. J. Paediatr. Neurol. 2013, 17, 302–307.
- Pierce, T.; Kranz, P.G.; Roth, C.; Leong, D.; Wei, P.; Provenzale, J.M. Use of apparent diffusion coefficient values for diagnosis of pediatric posterior fossa tumors. Neuroradiol. J. 2014, 27, 233–244.
- Pierce, T.T.; Provenzale, J.M. Evaluation of apparent diffusion coefficient thresholds for diagnosis of medulloblastoma using diffusion-weighted imaging. Neuroradiol. J. 2014, 27, 63–74.
- Koral, K.; Mathis, D.; Gimi, B.; Gargan, L.; Weprin, B.; Bowers, D.C.; Margraf, L. Common pediatric cerebellar tumors: Correlation between cell densities and apparent diffusion coefficient metrics. Radiology 2013, 268, 532–537.
- Koral, K.; Alford, R.; Choudhury, N.; Mossa-Basha, M.; Gargan, L.; Gimi, B.; Gao, A.; Zhang, S.; Bowers, D.C.; Koral, K.M.; et al. Applicability of apparent diffusion coefficient ratios in preoperative diagnosis of common pediatric cerebellar tumors across two institutions. Neuroradiology 2014, 56, 781–788.
- Domínguez-Pinilla, N.; Martínez de Aragón, A.; Diéguez Tapias, S.; Toldos, O.; Hinojosa Bernal, J.; Rigal Andrés, M.; González-Granado, L.I. Evaluating the apparent diffusion coefficient in MRI studies as a means of determining paediatric brain tumour stages. Neurologia 2016, 31, 459–465.
- Wagner, M.W.; Narayan, A.K.; Bosemani, T.; Huisman, T.A.; Poretti, A. Histogram Analysis of Diffusion Tensor Imaging Parameters in Pediatric Cerebellar Tumors. J. Neuroimaging 2016, 26, 360–365.
- Assis, Z.A.; Saini, J.; Ranjan, M.; Gupta, A.K.; Sabharwal, P.; Naidu, P.R. Diffusion tensor imaging in evaluation of posterior fossa tumors in children on a 3T MRI scanner. Indian J. Radiol. Imaging 2015, 25, 445–452.
- Dangouloff-Ros, V.; Varlet, P.; Levy, R.; Beccaria, K.; Puget, S.; Dufour, C.; Boddaert, N. Imaging features of medulloblastoma: Conventional imaging, diffusion-weighted imaging, perfusion-weighted imaging, and spectroscopy: From general features to subtypes and characteristics. Neurochirurgie 2021, 67, 6–13.
- Gauvain, K.M.; McKinstry, R.C.; Mukherjee, P.; Perry, A.; Neil, J.J.; Kaufman, B.A.; Hayashi, R.J. Evaluating pediatric brain tumor cellularity with diffusion-tensor imaging. AJR Am. J. Roentgenol. 2001, 177, 449–454.
- Burger, P.C.; Yu, I.T.; Tihan, T.; Friedman, H.S.; Strother, D.R.; Kepner, J.L.; Duffner, P.K.; Kun, L.E.; Perlman, E.J. Atypical teratoid/rhabdoid tumor of the central nervous system: A highly malignant tumor of infancy and childhood frequently mistaken for medulloblastoma: A Pediatric Oncology Group study. Am. J. Surg. Pathol. 1998, 22, 1083–1092.
- Phuttharak, W.; Wannasarnmetha, M.; Wara-Asawapati, S.; Yuthawong, S. Diffusion MRI in Evaluation of Pediatric Posterior Fossa Tumors. Asian Pac. J. Cancer Prev. 2021, 22, 1129–1136.
More
