The contamination of the soil, agricultural lands, and water bodies with petroleum wastes and other hydrocarbon pollutants has become a serious environmental concern as perceived by the impacts on the aquatic and marine ecosystem. Various investigations have provided novel insights into the significant roles of microbial activities in the cleanup of hydrocarbon contaminants.
- bioremediation
- eco-sustainable biotechnology
- environmental cleanup
1. Introduction
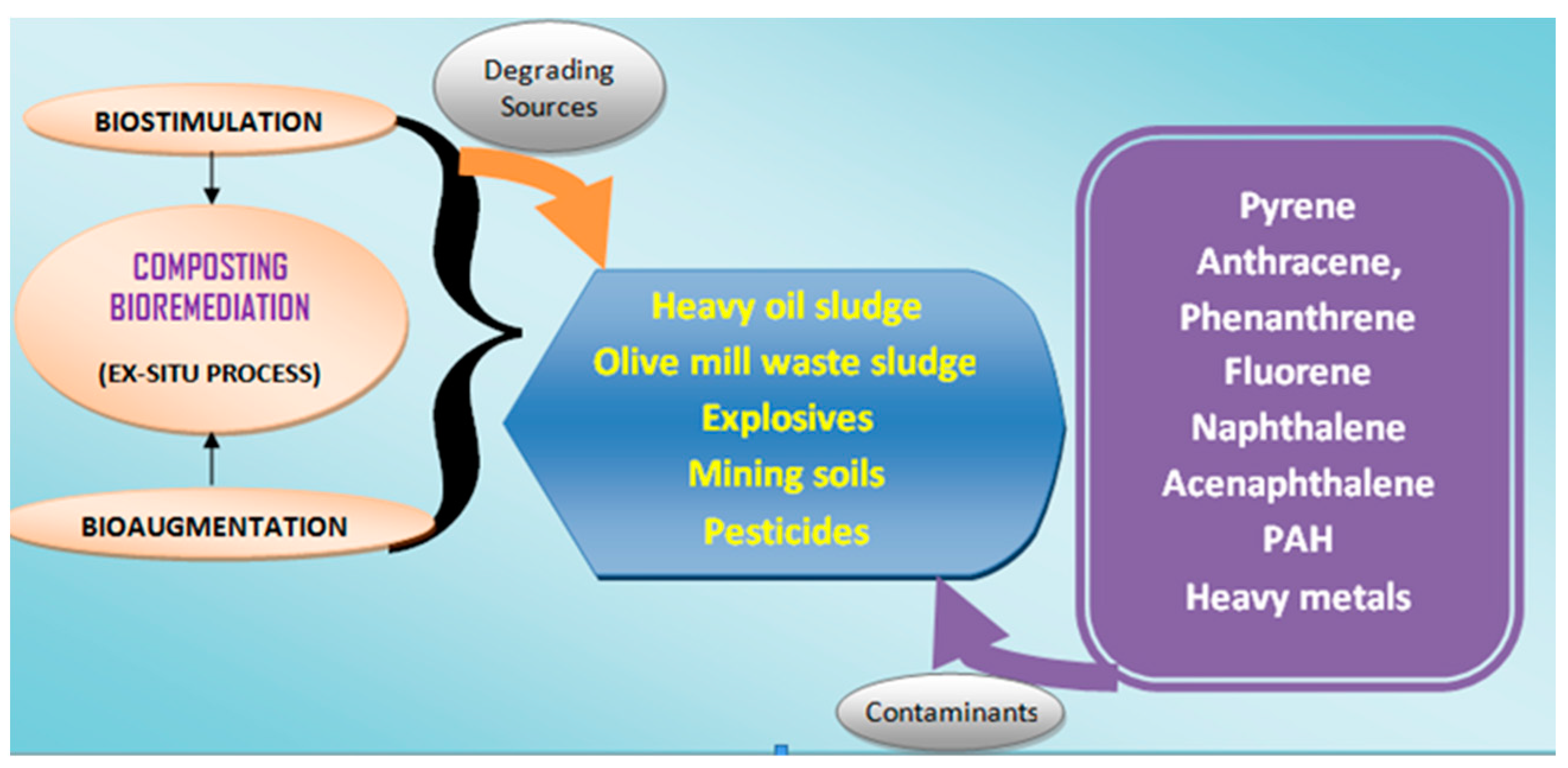
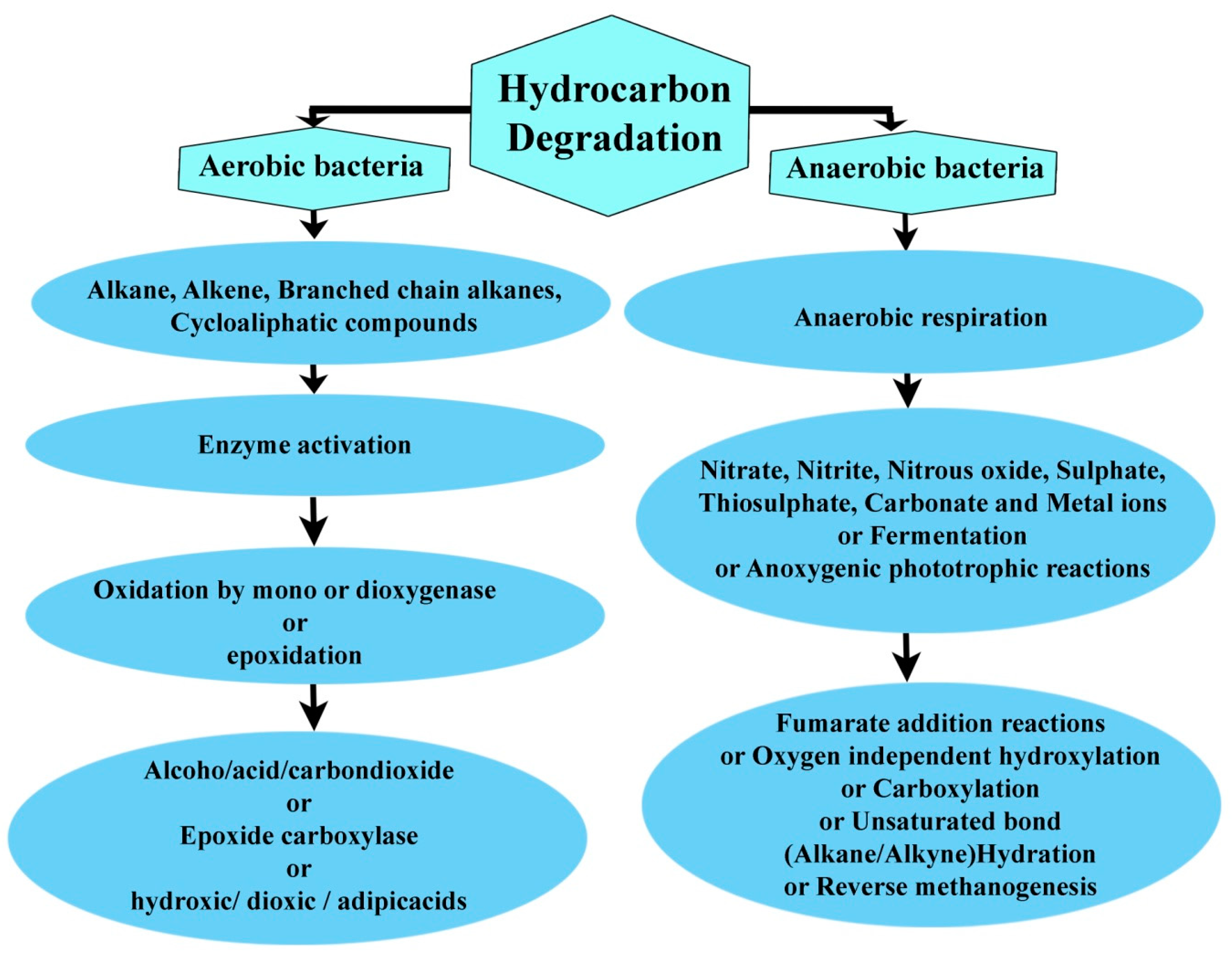
2. Role of Microorganisms in Hydrocarbon Biodegradation
|
Microorganisms Degrading Xenobiotics |
Isolation Sites |
|---|
|
Substrate |
Microbes |
References |
|||||
|---|---|---|---|---|---|---|---|
Duration |
Removal Efficiency (%) |
References |
|||||
|
Micrococcus and Pseudomonas |
Soil samples contaminated with spent engine oil; from a workshop in Ado-Ekiti |
||||||
|
Oily sludge |
Acinetobacter radioresistens KA2 |
Two stage (8 + 8 weeks) |
90 |
||||
|
Proteus vulgaris SR1 |
Freshly killed fish samples close to the point of oil spill in the Niger Delta, Nigeria |
[ | |||||
|
Petroleum waste sludge | ][21] |
||||||
Acinetobacter radioresistens KA5, Enterobacter hormaechei KA6 |
Two stage composting, 12 weeks |
84 |
Pseudomonas sp., Achromobacter sp., Bacillus sp. and Flavobacterium sp. |
Soil sample; obtained from a diesel spill region in north-central Alberta, British Columbia |
|||
|
Olive mill wastewater |
- |
Two stage composting |
84 |
Flavobacterium sp., and Acinetobacterium calcoaceticum |
Soil sample; collected from Amanzimtoti, South Africa | ||
|
Petroleum sludge |
Acinetobacter radioresistens KA2 |
||||||
In vessel reactor, two phase composting (8 + 8 weeks) |
88 |
Bacillus coagulans CR31, Klebsiella pneumonia CR23, Klebsiella aerogenes CR21 and Pseudomonas putrefacience CR33 |
Rhizosphere soil contaminated with spent engine oil in Sokoto, Nigeria |
||||
|
Oily sludge |
Enterobacter hormaechei KA6 | ||||||
In vessel experiment, 16 weeks |
81 |
Pseudomonas sp., Acinetobacter sp., Bacillus sp., Corynebacterium sp. and Flavobacterium sp. |
Soil samples from auto-mechanic workshops at Mgbukankpor, Nigeria |
[34 | |||
|
Contaminated soil |
- | ][25] |
|||||
Soil inoculated sewage sludge, wood chips and incubated for 19 months |
99 |
Pseudomonas putida, (Strain G1) and Pseudomonas aeruginosa (Strain K1) |
Soil samples from abandoned coal power plant (PHC) at Ijora-Olapa, Lagos |
||||
|
Heavy oily sludge |
Staphylococcus equorum KA4, Enterobacter hormaechei KA3 | ||||||
Composting bioreactor (2 phase composting process 8 + 8 weeks) |
89 |
Bacillus sp. S6 and S35 |
Soil samples from storage centre of oil products in Tehran refinery and Siri Island |
||||
|
Hydrocarbon contaminated drill mud waste |
Brevibacterium casei, Bacillus sp. |
3. Optimization of Bioremediation Conditions
|
Substrate (s) |
Medium |
Conditions |
References |
|---|---|---|---|
|
Oil sludge |
Bushnell-Haas, 1% Kerosene |
150 rpm shaking, 1 week at 35 °C |
|
|
Heavy oil sludge |
Bushnell-Haas, 1% Crude Oil |
160 rpm shaking, 1 week at 30 °C |
|
|
Oily waste sludge |
Bushnell-Haas, 1% Crude Oil |
160 rpm shaking, 1 week at 30 °C |
|
|
Petroleum sludge |
Bushnell-Haas, 1% Crude Oil |
120 rpm shaking, 12 days at 30 °C |
|
|
Petroleum sludge |
Bushnell-Haas, 1% Crude Oil |
120 rpm shaking, 12 days at 30 °C |
|
|
Olive mill sludge |
Remazol brilliant blue R (RBBR) plate count agar-tannic acid or potato dextrose agar-tannic acid |
Incubation at 30 °C for 48 h (bacteria) and 96 h fungi |
References
- Sayara, T.; Sánchez, A. Bioremediation of PAH-contaminated soils: Process enhancement through composting/compost. Appl. Sci. 2020, 10, 3684.
- Mahiudddin, M.; Fakhruddin, A.N.M. Degradation of phenol via meta cleavage pathway by Pseudomonas fluorescens PU1. Int. Sch. Res. Not. 2012, 2012, 741820.
- Xi, B.; Dang, Q.; Wei, Y.; Li, X.; Zheng, Y.; Zhao, X. Biogas slurry as an activator for the remediation of petroleum contaminated soils through composting mediated by humic acid. Sci. Total Environ. 2020, 730, 139117.
- Gaur, V.K.; Gautam, K.; Sharma, P.; Gupta, P.; Dwivedi, S.; Srivastava, J.K.; Varjani, S.; Ngo, H.H.; Kim, S.-H.; Chang, J.-S. Sustainable strategies for combating hydrocarbon pollution: Special emphasis on mobil oil bioremediation. Sci. Total Environ. 2022, 832, 155083.
- Sharma, S.; Pandey, L.M. Biodegradation kinetics of binary mixture of Hexadecane and Phenanthrene by the bacterial microconsortium. Bioresour. Technol. 2022, 358, 127408.
- Haripriyan, U.; Gopinath, K.P.; Arun, J.; Govarthanan, M. Bioremediation of organic pollutants: A mini review on current and critical strategies for wastewater treatment. Arch. Microbiol. 2022, 204, 286.
- Martínez-Gallardo, M.R.; López, M.J.; Jurado, M.M.; Suárez-Estrella, F.; López-González, J.A.; Sáez, J.A.; Moral, R.; Moreno, J. Bioremediation of Olive Mill Wastewater sediments in evaporation ponds through in situ composting assisted by bioaugmentation. Sci. Total Environ. 2020, 703, 135537.
- Osei-Twumasi, D.; Fei-Baffoe, B.; Anning, A.K.; Danquah, K.O. Synergistic effects of compost, cow bile and bacterial culture on bioremediation of hydrocarbon-contaminated drill mud waste. Environ. Pollut. 2020, 266, 115202.
- Abtahi, H.; Parhamfar, M.; Saeedi, R.; Villasenor, J.; Sartaj, M.; Kumar, V.; Coulon, F.; Parhamfar, M.; Didehdar, M.; Koolivand, A. Effect of competition between petroleum-degrading bacteria and indigenous compost microorganisms on the efficiency of petroleum sludge bioremediation: Field application of mineral-based culture in the composting process. J. Environ. Manag. 2020, 258, 110013.
- Vasudevan, M.; Natarajan, N. Towards achieving sustainable bioplastics production and nutrient recovery from wastewater—A comprehensive overview on polyhydroxybutyrate. Biomass Convers. Biorefin. 2022, 1–20.
- Leech, C.; Tighe, M.K.; Pereg, L.; Winter, G.; McMillan, M.; Esmaeili, A.; Wilson, S.C. Bioaccessibility constrains the co-composting bioremediation of field aged PAH contaminated soils. Int. Biodeterior. Biodegrad. 2020, 149, 104922.
- Mahyudin, R.P.; Firmansyah, M.; Purwanti, M.A.; Najmina, D. Bioremediation of iron on diamond post mining soil using compost made from cow manure and traditional market organic waste. J. Ecol. Eng. 2020, 21, 221–228.
- Atagana, H.I. Compost bioremediation of hydrocarbon-contaminated soil inoculated with organic manure. Afr. J. Biotechnol. 2008, 7, 1516–1525.
- Chang, B.-V.; Chang, I.T.; Yuan, S.Y. Anaerobic degradation of phenanthrene and pyrene in mangrove sediment. Bull. Environ. Contam. Toxicol. 2008, 80, 145–149.
- Saha, J.K.; Selladurai, R.; Coumar, M.V.; Dotaniya, M.L.; Kundu, S.; Patra, A.K. Soil Pollution—An Emerging Threat to Agriculture; Springer: Singapore, 2017; ISBN 9811042748.
- Poorsoleiman, M.S.; Hosseini, S.A.; Etminan, A.; Abtahi, H.; Koolivand, A. Bioremediation of Petroleum Hydrocarbons by using a two-step inoculation composting process scaled-up from a mineral-based medium: Effect of biostimulation of an indigenous bacterial strain. Waste Biomass Valoriz. 2021, 12, 2089–2096.
- Poorsoleiman, M.S.; Hosseini, S.A.; Etminan, A.; Abtahi, H.; Koolivand, A. Effect of two-step bioaugmentation of an indigenous bacterial strain isolated from oily waste sludge on petroleum hydrocarbons biodegradation: Scaling-up from a liquid mineral medium to a two-stage composting process. Environ. Technol. Innov. 2020, 17, 100558.
- Parhamfar, M.; Abtahi, H.; Godini, K.; Saeedi, R.; Sartaj, M.; Villaseñor, J.; Coulon, F.; Kumar, V.; Soltanighias, T.; Ghaznavi-Rad, E. Biodegradation of heavy oily sludge by a two-step inoculation composting process using synergistic effect of indigenous isolated bacteria. Process Biochem. 2020, 91, 223–230.
- Blenis, N.; Hue, N.; Maaz, T.M.; Kantar, M. Biochar Production, Modification, and Its Uses in Soil Remediation: A Review. Sustainability 2023, 15, 3442.
- Vasudevan, M.; Nambi, I.M.; Kumar, G.S. Scenario-based modelling of mass transfer mechanisms at a petroleum contaminated field site-numerical implications. J. Environ. Manag. 2016, 175, 9–19.
- Vasudevan, M.; Suresh Kumar, G.; Nambi, I.M. Numerical modelling on rate-limited dissolution mass transfer of entrapped petroleum hydrocarbons in a saturated sub-surface system. ISH J. Hydraul. Eng. 2016, 22, 3–15.
- Koolivand, A.; Abtahi, H.; Villaseñor, J.; Saeedi, R.; Godini, K.; Parhamfar, M. Effective scale-up of oily sludge bioremediation from a culture-based medium to a two-phase composting system using an isolated hydrocarbon-degrading bacterium: Effect of two-step bioaugmentation. J. Mater. Cycles Waste Manag. 2020, 22, 1475–1483.
- Bhanse, P.; Kumar, M.; Singh, L.; Awasthi, M.K.; Qureshi, A. Role of plant growth-promoting rhizobacteria in boosting the phytoremediation of stressed soils: Opportunities, challenges, and prospects. Chemosphere 2022, 303, 134954.
- Liang, J.; Tang, S.; Gong, J.; Zeng, G.; Tang, W.; Song, B.; Zhang, P.; Yang, Z.; Luo, Y. Responses of enzymatic activity and microbial communities to biochar/compost amendment in sulfamethoxazole polluted wetland soil. J. Hazard. Mater. 2020, 385, 121533.
- Sáez, J.A.; Pérez-Murcia, M.D.; Vico, A.; Martínez-Gallardo, M.R.; Andreu-Rodríguez, F.J.; López, M.J.; Bustamante, M.A.; Sanchez-Hernandez, J.C.; Moreno, J.; Moral, R. Olive mill wastewater-evaporation ponds long term stored: Integrated assessment of in situ bioremediation strategies based on composting and vermicomposting. J. Hazard. Mater. 2021, 402, 123481.
- Waigi, M.G.; Kang, F.; Goikavi, C.; Ling, W.; Gao, Y. Phenanthrene biodegradation by sphingomonads and its application in the contaminated soils and sediments: A review. Int. Biodeterior. Biodegrad. 2015, 104, 333–349.
- Grover, R.; Burse, S.A.; Shankrit, S.; Aggarwal, A.; Kirty, K.; Narta, K.; Srivastav, R.; Ray, A.K.; Malik, G.; Vats, A. Myg1 exonuclease couples the nuclear and mitochondrial translational programs through RNA processing. Nucleic Acids Res. 2019, 47, 5852–5866.
- Hussain, F.; Hussain, I.; Khan, A.H.A.; Muhammad, Y.S.; Iqbal, M.; Soja, G.; Reichenauer, T.G.; Yousaf, S. Combined application of biochar, compost, and bacterial consortia with Italian ryegrass enhanced phytoremediation of petroleum hydrocarbon contaminated soil. Environ. Exp. Bot. 2018, 153, 80–88.
- Nzila, A. Current status of the degradation of aliphatic and aromatic petroleum hydrocarbons by thermophilic microbes and future perspectives. Int. J. Environ. Res. Public Health 2018, 15, 2782.
- Ostrem Loss, E.M.; Lee, M.-K.; Wu, M.-Y.; Martien, J.; Chen, W.; Amador-Noguez, D.; Jefcoate, C.; Remucal, C.; Jung, S.; Kim, S.-C. Cytochrome P450 monooxygenase-mediated metabolic utilization of benzo pyrene by Aspergillus species. MBio 2019, 10, e00558-19.
- Li, J.; Yang, H. Polycyclic Aromatic Hydrocarbon Improves the Anaerobic Biodegradation of Benz Anthracene in Sludge Via Boosting the Microbial Activity and Bioavailability. Pak. J. Zool. 2021, 53, 2445–2450.
- Soleimani, M.; Farhoudi, M.; Christensen, J.H. Chemometric assessment of enhanced bioremediation of oil contaminated soils. J. Hazard. Mater. 2013, 254, 372–381.
- Pandya, D.K.; Kumar, M.A. Chemo-metric engineering designs for deciphering the biodegradation of polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2021, 411, 125154.
- Dudhagara, D.R.; Rajpara, R.K.; Bhatt, J.K.; Gosai, H.B.; Dave, B.P. Bioengineering for polycyclic aromatic hydrocarbon degradation by Mycobacterium litorale: Statistical and artificial neural network (ANN) approach. Chemom. Intell. Lab. Syst. 2016, 159, 155–163.
- Oliveira, L.G.; Araújo, K.C.; Barreto, M.C.; Bastos, M.E.P.; Lemos, S.G.; Fragoso, W.D. Applications of chemometrics in oil spill studies. Microchem. J. 2021, 166, 106216.
- Tavares, T.S.; da Rocha, E.P.; Esteves Nogueira, F.G.; Torres, J.A.; Silva, M.C.; Kuca, K.; Ramalho, T.C. Δ-FeOOH as support for immobilization peroxidase: Optimization via a chemometric approach. Molecules 2020, 25, 259.
- de Castro, A.A.; Soares, F.V.; Pereira, A.F.; Silva, T.C.; Silva, D.R.; Mancini, D.T.; Caetano, M.S.; da Cunha, E.F.F.; Ramalho, T.C. Asymmetric biodegradation of the nerve agents Sarin and VX by human dUTPase: Chemometrics, molecular docking and hybrid QM/MM calculations. J. Biomol. Struct. Dyn. 2019, 37, 2154–2164.
- Lange, I.; Kotiukov, P.; Lebedeva, Y. Analyzing Physical-Mechanical and Hydrophysical Properties of Sandy Soils Exposed to Long-Term Hydrocarbon Contamination. Sustainability 2023, 15, 3599.
