Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Giulia Moltoni and Version 3 by Rita Xu.
Diffusion weighted imaging (DWI) is an imaging technique commonly used for the assessment of acute ischemia, inflammatory disorders, and CNS neoplasia. It has several benefits since it is a quick, easily replicable sequence that is widely used on many standard scanners. In addition to its normal clinical purpose, DWI offers crucial functional and physiological information regarding brain neoplasia and the surrounding milieu.
- diffusion-weighted imaging
- magnetic resonance imaging
- tumors
1. Introduction
Diffusion-weighted imaging (DWI) is a magnetic resonance imaging (MRI) sequence commonly used in neuroradiology for the assessment of acute ischemia, inflammatory diseases, and central nervous system (CNS) neoplasia [1][2][3][1,2,3].
It has several advantages, as it is a fast, easily reproducible, and extensively studied sequence in neuro-oncology, and it is widely available on many standard scanners, including those in non-academic centers [4][5][4,5].
In addition to being a simple instrument for everyday use, DWI can also offer functional and ultrastructural data, particularly when discussing tumor cellularity and the microenvironment through the measurement of water mobility. In fact, it yields an imaging biomarker for pathological tissue changes like cellularity increase or anomalies in the extracellular space by the assessment of water mobility [6][7][8][6,7,8].
Moreover, advanced diffusion-related sequences such as Diffusion Tensor Imaging (DTI) and Diffusion Kurtosis Imaging (DKI) are even playing a more important role than DWI in the neuro-oncology field.
Finally, multi-shell diffusion MRI allows for characterizing the water diffusion signal behavior by analyzing the data using multi-compartment diffusion models such as the Neurite Orientation Dispersion and Density Imaging (NODDI) model. Thus, it can provide a more specific characterization of brain tissue microstructures than conventional single-shell diffusion tensor imaging.
2. Gliomas and Cellularity
Brain tumors may show various degrees of diffusion changes related to tumor cellularity and the nucleus-cytoplasmic ratio [9]. Water diffusion restriction secondary to tumor high-cellularity results in low Apparent Diffusion Coefficient (ADC) values, which are useful for differentiating tumor type and grade [6][10][11][6,10,11]. The minimal ADC value has been proposed as one of the various parameters to use as a predictive tool in patients with malignant supratentorial astrocytomas [12][13][14][15][16][17][18][12,13,14,15,16,17,18]. For instance, Moon et al. [12], Yamasaki et al. [13], and Murakami et al. [14] showed an inverse correlation between the mean ADC value and tumor grade in astrocytic tumors. Generally, higher-grade tumors typically have lower ADC values (Figure 1A–H). Due to their variable cellularity and grade, astrocytomas exhibit heterogeneous diffusion signals, with most cellular areas generally exhibiting restricted diffusion [9]. For instance, the degree of ADC hypointensity will often be higher in lymphoma than in glioma or metastases, reflecting the high cellular density in this neoplasm. The drop in ADC values for non-necrotic high-grade gliomas and metastases will be higher than for low-grade malignancies. High-grade tumor-associated edema reduces ADC sensitivity by raising the average ADC intensity [6][7][6,7]. ADC values alone or in combination with other MRI parameters, such as relative cerebral blood volume (rCBV), derived by dynamic susceptibility contrast-enhanced MR-perfusion (DSC), can accurately grade gliomas [19]. Indeed, Wang et al. recently reported a high accuracy of DWI/ADC to distinguish low-grade gliomas (grades I and II) from high-grade ones (grades III and IV) with an area under the curve (AUC) of 0.91; therefore, with this value being very high it reflects an excellent diagnostic performance of DWI in this field [9][20][9,20].
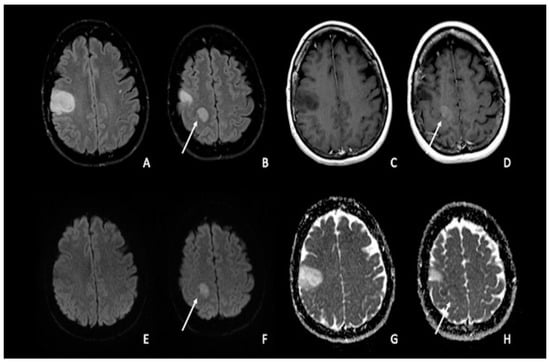
Figure 1. Double site of glioma infiltration in the right Rolandic frontal region: (A,B) Both lesions present similar hyperintensity on FLAIR images. The most cranial lesion (arrow) showed slight post-contrastographic enhancement (D) and restricted water diffusion on DWI (F), characterized by slightly low values on ADC map (H); this condition could be linked to an anaplastic aspect of the tumor, with restricted diffusion explained by an increase in tumor cellularity. The most caudal lesion did not show contrast enhancement, with a marked hypointense signal on post-contrastographic T1-weighted image (C); the same lesion presented no restricted diffusion with increased ADC values (E–G). FLAIR = fluid-attenuated inversion recovery; DWI = diffusion-weighted imaging; ADC: apparent diffusion coefficient.
In addition to standard DWI acquisition, more advanced models of quantitative DWI, such as DTI and DKI, have been created. These advanced sequences try to go beyond the theory that water diffusion occurs without boundaries via a uniform Gaussian distribution; indeed, it also depends on the configuration of intracellular organelles, cell membranes, and water compartments in cerebral tissues [6][21][22][6,21,22]. For instance, thanks to DKI, it is possible to quantify the deviation from a Gaussian distribution to produce a more accurate model [6][23][24][6,23,24]. The same result was reached by Abdalla et al. in a more recent expanded and updated meta-analysis, which found that DKI offers good diagnostic accuracy in differentiating high-grade from low-grade gliomas [6][25][6,25].
Finally, intravoxel incoherent motion (IVIM) and neurite orientation and dispersion imaging (NODDI), which make use of multiband imaging, are two promising advanced DTI approaches [6][26][6,26]. NODDI, in particular, measures the microstructure of dendrites and axons, revealing neuronal alterations, whereas IVIM may estimate tissue diffusivity and microcapillary perfusion [6].
3. Gliomas and Molecular Biology
When considering the glial line neoplasm, the 2016 World Health Organization (WHO) classification system has defined various groups of diffuse low-grade gliomas (LGGs) considering the isocitrate dehydrogenase (IDH) mutation and 1p/19q codeletion status [27][28][27,28]. The WHO classification of 2021 increased the importance of the molecular profile of brain tumors by categorizing adult-type diffuse glioma into three groups: IDH mutant with 1p/19q codeletion (Oligodendroglioma/IDH-mut codeleted LGGs), IDH mutant without codeletion (Astrocytoma/IDH-mut non-codeleted), and IDH wild type (Glioblastoma) [29]. The IDH gene plays a crucial role in metabolism, cellularity, and angiogenesis [30]. Recent research has shown that IDH mutant gliomas exhibit significantly greater survival and chemosensitivity than IDH wild-type glioblastomas [31][32][31,32]. When the IDH gene family is mutated, an oncometabolite called 2-hydroxyglutarate is produced, which inhibits tumor cell proliferation more than the wild type [33][34][33,34]. When compared to astrocytomas, oligodendrogliomas have a superior clinical outcome and treatment response [29][31][35][36][37][29,31,35,36,37]. As IDH mutant inhibitors become commercially available and might even be employed as neoadjuvant therapy, imaging biomarkers of IDH mutation would be a useful adjunct tool for clinical decision-making [38]. As a result, numerous research studies [33][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][33,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65] investigated the imaging properties and/or high diagnostic performance of various MRI sequences for the prediction of IDH mutations in gliomas. According to several studies [41][48][56][60][62][41,48,56,60,62], IDH mutant glioma consistently displays higher mean ADC values on DWI than IDH wild-type glioblastoma [34]. It is still unclear how IDH-mutant and IDH wild-type gliomas differ from one another in terms of the ADC values; however, it could possibly be primarily related to tumor cellularity but also to the presence of cystic components, areas of necrosis, and interstitial water content [17][18][66][17,18,66]. While most IDH wild-type gliomas commonly present high-grade features such as necrosis and lower ADC mean values in solid sections, perhaps indicating more cellularity, most IDH-mutant gliomas show higher ADC mean values and MR imaging features consistent with a lower-grade nature [37] (Figure 2A–H). When considering oligodendrogliomas and the relationship between the 1p/19q codeletion status and ADC levels, the literature has shown contradictory findings [56][67][68][69][56,67,68,69]. According to Jenkinson et al., IDH-mut non-codeleted LGGs had much higher ADC values than IDH-mut-codeleted LGGs. Compared to IDH-mut non-codelated LGGs, the IDH-mut codeleted group may have fewer edematous areas and more cellulated areas, which could account for the lower ADC values [29][68][29,68]. Other diffusion-based sequences, such as DTI, DKI, and NODDI, have been successfully tested in predicting IDH status in gliomas, both with and without the use of artificial intelligence [70][71][70,71], with similar results between DTI and more advanced multi-shell methods [71][72][71,72]. Interestingly, mean diffusivity (MD) measures were increased in tumors not usually associated with high cellularity, probably reflecting changes in the extracellular volume that play a role in the diffusion signal [71]. Epidermal Growth Factor (EGFR) amplification is a molecular biomarker that could allow glioblastoma IDH wild-type designation even in tumors that appear histologically lower grade [29]. Hence, its prediction through MRI could be of therapeutic and prognostic use. A recent pilot study found that EGFR-amplified tumors showed lower mean ADC values compared to EGFR-non-amplified tumors [73]. Future research could focus on other important molecular information in gliomas, such as CDKN2A/B deletion or combined whole chromosome 7 gain and whole chromosome 10 loss (+7/−10) mutations.
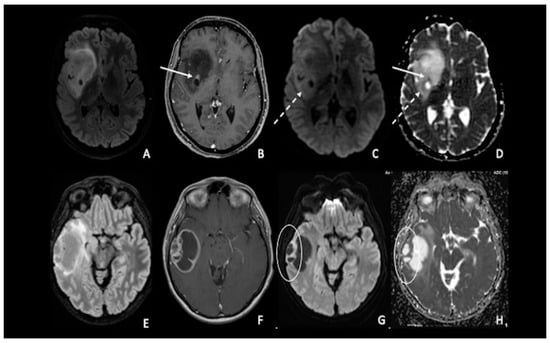
Figure 2. (A–D) IDH-1 mutated glioma in the right Sylvian region. The lesion is characterized by a heterogenous signal on FLAIR (A), with a small area of nodular enhancement on post-contrastographic T1 weighted images (arrow) (B); The site of pathological enhancement was in close proximity to an area of cystic-necrotic degeneration (dotted arrow) (C,D) and showed restricted diffusion on DWI confirmed by the ADC map (arrow); these findings were consistent with an anaplastic behavior of the tumor. (E–H) Right temporal lobe glioblastoma. The lesion was predominantly cystic and had a vivid peripherical post-contrastographic enhancement (F) with a solid component on the lateral side of the tumor; this region presented restricted diffusion on DWI confirmed by the ADC map (circle) (G,H), related to an increase in tumor cellularity. FLAIR = fluid-attenuated inversion recovery; DWI = diffusion-weighted imaging; ADC = apparent diffusion coefficient.
4. Lymphomas
One of the uses of DWI on CNS neoplasia imaging is the differential diagnosis between glioblastoma and primary CNS lymphoma (PCNSL), two entities that may appear similar on conventional imaging when considering a patient presenting with an enhancing brain mass but with a completely different pathogenesis, origin, and treatment [74][75][74,75]. PCNSLs are highly cellular tumors with relatively little extracellular space, which limits the diffusivity of free water. As a result, compared to HGGs and metastases, PCNSLs have been found to have much lower ADC values (Figure 3A–H). Similar to ADC, PCNSLs have been found to have lower FA values than high-grade gliomas [4][76][77][78][79][80][81][82][83][84][85][4,76,77,78,79,80,81,82,83,84,85].
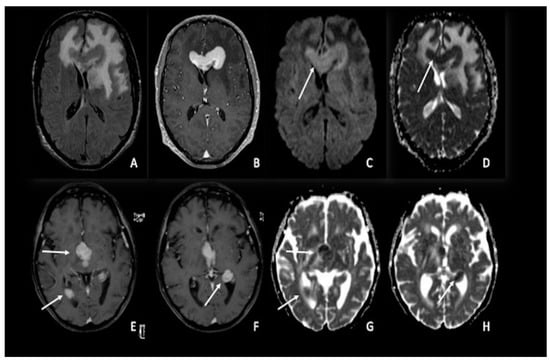
Figure 3. Two different cases of primitive cerebral lymphoma: the upper row showed a lymphoma in the pericallosal region (A–D) with a marked uniform enhancement on post-contrastographic T1 weighted image (B) and significant edema in the adjacent brain (A). The lower row showed a periventricular multicentric lymphoma (E–H) with multiple nodules of marked enhancement on post-contrast T1 weighted images (arrows) (E,F). Both cases presented restricted water diffusion with hyperintensity on DWI (arrow in C) and hypointense areas on ADC (arrows respectively in (D,G,H)), related to increasing tumor cellularity, as occurs typically in lymphomas. DWI = diffusion-weighted imaging; ADC = apparent diffusion coefficient.
5. Medulloblastomas
Medulloblastomas are highly cellular tumors; consequently, they present a substantial reduction in water molecule movement, resulting in a high diffusivity restriction on DWI and ADC images [86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103]. This finding helps in the differential diagnosis of other tumors typically located in the posterior fossa, such as ependymomas and pilocytic astrocytomas. Indeed, even if there is still overlap between these tumors [94][96][97][103][104][105][106][94,96,97,103,104,105,106], a high diffusion restriction is more suggestive of medulloblastomas than ependymomas or pilocytic astrocytomas [86][103][107][86,103,107] (Figure 4A–C). An ADC cut-off between 700 and 900 mm2/s has been proposed by the literature to distinguish medulloblastomas from pilocytic astrocytomas [106][108][109][106,108,109], whereas using a minimum ADC cut-off value of 660 mm2/s seems to allow for a good distinction with ependymomas [110]. Moreover, according to the literature, the ratio of ADC within the tumor compared to the grey matter ranges between 0.70 and 0.88 for the solid component [97][108][111][97,108,111] and 0.97 and 1.28 for the entire tumor [108][112][113][114][108,112,113,114]. Medulloblastomas, ependymomas, and pilocytic astrocytomas share the same fractional anisotropy [115][116][117][115,116,117]. As measured by lower ADC values, medulloblastomas frequently have lower rates of microscopic water diffusion than other common posterior fossa tumors in children [96]. This trait is most likely brought on by the frequent presence of cells with a high nuclear-to-cytoplasmic ratio and a high degree of cellularity in medulloblastomas, which results in additional membrane barriers impeding microscopic water diffusion [118]. Another much rarer tumor of the posterior fossa, typically affecting children, is the atypical teratoid/rhabdoid tumor (ATRT), which histologically resembles medulloblastoma [119] and exhibits similar DWI characteristics to medulloblastoma [86][111][86,111]. Finally, the distinction between medulloblastomas and glioblastomas cannot be made with DWI [86].
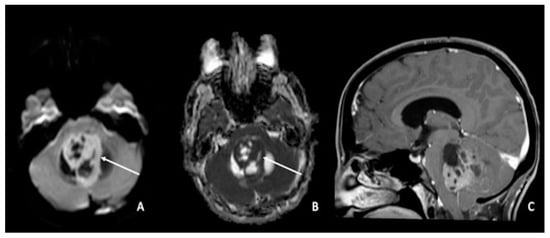
Figure 4. Medulloblastoma in a 30-year-old: (A,C) lesion of the posterior fossa with growth in the median line, close to the IV ventricle, characterized by inhomogeneous enhancement (arrow in (A)), mass effect, and (B) low ADC values (arrow). Using ADC values and a specific cut-off, it could be possible to distinguish medulloblastoma from ependymoma or pilocytic glioma. ADC = apparent diffusion coefficient.
According to Ahmed et al.’s study, the ADC ratio (ADC of the tumor divided by the ADC of the corresponding contralateral normal white matter) was unable to distinguish between medulloblastoma and ATRT. This could be explained by the fact that medulloblastoma and ATRT both have high grades in accordance with the WHO grading system, which indicates high tumoral cellularity and leads to a low ADC ratio [120].
