Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Fréderic Dumur and Version 4 by Rita Xu.
Photopolymerization offers a unique opportunity to convert liquid monomers to polymers using light as the activation source. Major efforts have been devoted to developing visible light photo-initiating systems, and the search for new dyes that can be incorporated into photocurable resins and polymerize a resin within a few seconds is still ongoing.
- quinoxaline
- photopolymerization
- UV light
- visible light
1. Introduction
During the past decades, photopolymerization has been an active research field, mainly supported by the development of 3D printing but also by the necessity to replace in the near future the historical UV photo-initiating systems with visible light photo-initiating systems in the industry [1][2][3][4][5][6][7][8][9][10][11][1,2,3,4,5,6,7,8,9,10,11]. Indeed, UV photopolymerization is more and more the focus of safety concerns, originating from the numerous drawbacks of UV light. Notably, UV light can cause eye damage and skin cancers [12][13][14][15][12,13,14,15]. Parallel to this, molecular oxygen can be converted to ozone during the polymerization process, constituting an additional drawback of this approach [15]. In addition, photopolymerization constitutes an appealing polymerization technique, which exhibits numerous specificities and advantages compared to traditional thermal polymerization. In order to illustrate this, the possibility of polymerizing without solvents to obtain efficient spatial and temporal control during the polymerization process can be cited as relevant examples [16][17][18][19][20][21][22][23][24][25][26][27][28][29][16,17,18,19,20,21,22,23,24,25,26,27,28,29]. Development of photo-initiating systems is not new since the first report mentioning a photoinduced electron transfer between triethanolamine and electron-accepting dyes (xanthenes, acridines, thiazines) was reported as soon as 1954 by Oster and coworkers [30]. Since 1954, photopolymerization has greatly evolved, enabling now polymerization in safer and energy-saving conditions. As a breakthrough, visible light photopolymerization has emerged as a promising alternative to the historical UV photopolymerization. As the main advantage of visible light photopolymerization, a higher light penetration within the photocurable resins can be obtained than in the UV range (See Figure 1). Indeed, if the light penetration is limited to a few hundred micrometers in the UV range, this value can increase up to 4 mm at 450 nm and even reach 5 cm at 800 nm [31]. In these conditions, photopolymerization becomes capable of polymerizing thick samples and is not limited anymore to the polymerization of thin samples, as in the past when UV light was used [32]. However, light penetration within the photocurable resins is an important issue of photopolymerization, and some clarifications should be given. For instance, the light penetration is strongly related to the molar extinction coefficient of the photoinitiator at the wavelength used for irradiation and on the photoinitiator concentration in the system. Only in the case of photo-bleachable initiators can the light penetrate deeply. This concerns the light of any wavelengths, with the exception that short-wavelength UV may also be absorbed by monomers. Figure 1 depicts the light penetration only in the light-scattering system, which cannot be generalized to all photocurable systems because most photocurable resins are transparent and not light-scattering. Consequently, the statement that the main advantage of visible light photopolymerization is the higher light penetration within the photocurable resins than in the case of UV light is not applicable to all resins. In complement to this first point, one of the main drawbacks of visible light photopolymerization remains the difficulty of obtaining colorless coatings [33]. Indeed, UV photoinitiators are colorless compounds, enabling the ability to obtain easily colorless polymers. Conversely, visible light photopolymerization makes use of dyes absorbing in the visible range, and these dyes are often responsible for the final color of the polymers. In order to address this issue, major efforts have been devoted to developing photo-bleachable photo-initiating systems, with more or less success [33].
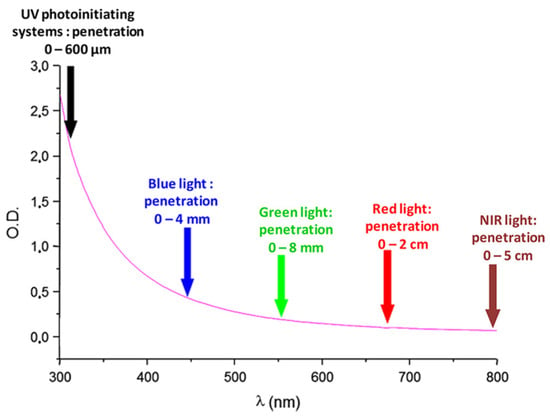
Figure 1. Light penetration in a polystyrene latex with an average particle diameter of 112 nm.
This effort is also supported by the wide range of applications using photopolymerization. Notably, 3D and 4D printing, microelectronics, dentistry, coatings, solvent-free paints, adhesives and varnishes can be cited as the main applications of photopolymerization [1][2][3][4][5][6][7][8][9][10][1,2,3,4,5,6,7,8,9,10]. Another drawback of visible light photopolymerization is that visible light photons are less energetic than UV photons, so more reactive photo-initiating systems have to be developed in order to overcome this lower energy. In the search for highly reactive photo-initiating systems, numerous structures have been examined as potential candidates capable of addressing the reactivity issue and carbazoles [34][35][36][37][38][39][40][41][42][43][44][45][46][47][34,35,36,37,38,39,40,41,42,43,44,45,46,47], dihydroanthraquinones [48], camphorquinone [49][50][49,50], chalcones [9][51][52][53][54][55][56][57][58][59][60][61][62][63][9,51,52,53,54,55,56,57,58,59,60,61,62,63], naphthalimides [64][65][66][67][68][69][70][71][72][73][74][75][76][77][78][79][80][81][82][64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82], benzophenones [83][84][85][86][87][88][89][90][83,84,85,86,87,88,89,90], silyl glyoximides [91], phenothiazines [92][93][94][95][96][97][98][99][100][101][102][103][92,93,94,95,96,97,98,99,100,101,102,103], thioxanthones [28][104][105][106][107][108][109][110][111][112][113][114][115][116][28,104,105,106,107,108,109,110,111,112,113,114,115,116], curcumin [117][118][119][120][117,118,119,120], pyrenes [121][122][123][124][125][126][127][128][129][121,122,123,124,125,126,127,128,129], iodonium salts [64][130][131][132][133][134][135][136][64,130,131,132,133,134,135,136], push-pull dyes [137][138][139][137,138,139], copper complexes [140][141][142][143][140,141,142,143], iron complexes [144][145][144,145] zinc complexes [146] iridium complexes [147][148][147,148] and N-heterocyclic carbene boranes [29] can be cited among the most extensively studied structures of the past decade. Beyond the simple selection of the chromophore, the way how to generate initiating species is important. Notably, photoinitiators can be divided into two main categories, namely, Type I and Type II photoinitiators. In the case of Type I photoinitiators, these structures can generate reactive species by homolytic cleavage of a specific bond (See Scheme 1) [149][150][151][152][153][154][155][156][157][158][149,150,151,152,153,154,155,156,157,158].
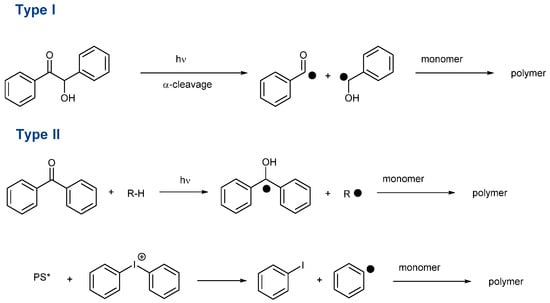
Scheme 1. Formation of initiating species with Type I and Type II photoinitiators. (PS* means that PS is in the excited state).
In this first family, O-acyl-α-oximino ketones, acetophenones, acylgermanes, benzoin ether derivatives, α-aminoketones benzyl ketals, acylphosphine oxides, aminoalkyl phenones, α-hydroxyalkyl ketones, hydroxylalkylphenones and oxime esters can cleave homolytically, generating initiating radicals [103][151][153][158][159][160][161][103,151,153,158,159,160,161]. As the main interest of these structures, Type I photoinitiators can be used as mono-component systems. As a drawback, the photodecomposition is irreversible, so the concentration continuously decreases during the polymerization process. Conversely, Type II photoinitiators are unable to generate initiating species without additives. However, a few exceptions exist. For instance, free radical polymerization of ether acrylates (such as poly(ethylene glycol) diacrylates) is possible with benzophenone, thioxanthone and different chalcones, the monomer itself acting as a co-initiator because -CH2O- groups are good hydrogen donors [162]. Type II photoinitiators are typically used for the sensitization of onium salts [163][164][165][166][167][168][163,164,165,166,167,168]. A photoinduced electron transfer from the photosensitizer towards the onium salts is the key step to generating initiating radicals. However, Type II photoinitiators are also combined with hydrogen donors, leading to the formation of a ketyl radical with hydrogen abstraction and an additional radical issued from the hydrogen donor (See Scheme 1) [105][110][169][170][171][172][173][174][105,110,169,170,171,172,173,174]. Considering that Type II photoinitiators are bimolecular photoinitiators, the introduction of a sacrificial amine can contribute to regenerating the photosensitizer in its initial redox state during the polymerization process so that this latter can be introduced in a catalytic amount. This point is important, considering that the photosensitizer is responsible for the final color of the polymer. By decreasing its content, less colored coatings can be obtained. The search for new structures is also motivated by the recent interest in developing photo-initiating systems activable with sunlight [175][176][177][178][179][180][181][182][183][184][175,176,177,178,179,180,181,182,183,184] or capable of initiating a polymerization process in water [22][60][116][185][186][187][188][189][190][191][192][193][194][195][196][197][198][199][200][22,60,116,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200]. With the aim of polymerizing in energy-saving conditions, the use of light-emitting diodes (LEDs) is now generalized in photopolymerization due to their low costs, long operating lifetimes, compactness and their precise emission wavelengths. In the search for photo-initiating systems that can be activated under low light intensity, i.e., LEDs, quinoxalines have been identified as potential candidates for visible light photopolymerization. Quinoxalines are heterocyclic compounds in which two nitrogen atoms replace two carbons in the ring of naphthalene. Quinoxalines have been extensively studied for their biological properties. Indeed, quinoxalines are biologically active against bacteria, viruses, cancer, leishmania, tuberculosis, malaria, depression and fungi [201]. Nevertheless, quinoxalines were also used for the design of light-emitting materials for OLEDs [202][203][202,203], semiconductors and charge transport materials for solar cells [204][205][206][207][204,205,206,207], the design of building blocks for covalent organic frameworks [208]. Recently, different quinoxaline derivatives were also proposed as fluorescent probes for near-infrared II (NIR-II, 1000–1700 nm) fluorescent imaging [209].
The first report mentioning the use of quinoxalines as photoinitiators of polymerization was reported in 1999 by Pączkowski and coworkers. By using 3-benzoyl-7-diethylamino-5-methyl-1-phenyl-1H-quinoxalin-2-one (ChAD) as an electron acceptor and N-phenylglycine derivatives (NPG) as electron donors, the free radical polymerization of trimethylolpropane triacrylate (TMPTA) was carried out, using an argon ion laser (emission between 351 and 361 nm, 25.5 mW/cm2) or a He/Ne laser as the light sources (See Figure 2) [210][211][210,211]. The best monomer conversion was obtained using (4-methoxyphenyl)glycine as the electron donor. Noticeably, efficiencies of the different photo-initiating systems based on quinoxalines were lower than that of a Rose Bengal derivative, namely RBAX, previously reported in the literature.
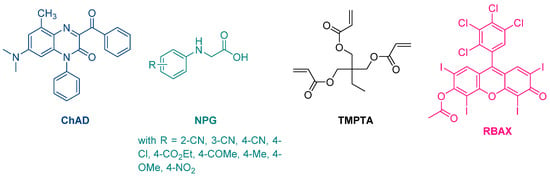
Figure 2. Chemical structures of the chromophore ChAD, the electron donors NPG, the monomer TMPTA and the reference compound RBAX.
The same year, Aydin and coworkers proposed a series of quinoxalines (QNX-1-QNX-5) that proved to be excellent photoinitiators in combination with electron donors such as N-methyldiethanolamine (MDEA) and 2-(N-methyl-N-phenylamino)-1-phenylethanol (MPAPE). By using these two co-initiators, the polymerization of methyl methacrylate (MMA) was carried out upon irradiation with a UV light (λ = 350 nm) (See Figure 3) [212]. A few years later, this strategy was extended to an unusual co-initiator in photopolymerization, namely benzaldehyde [213].
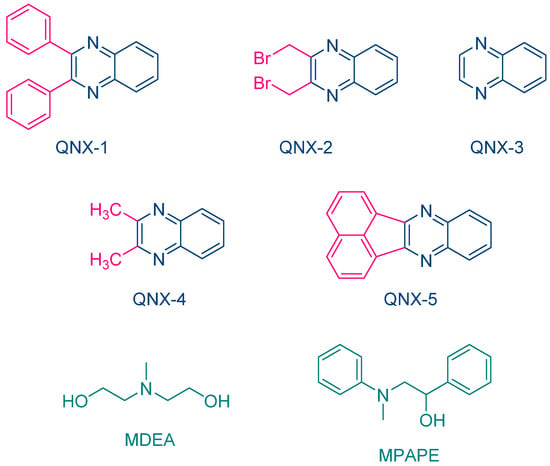
Figure 3. Chemical structures of different quinoxaline derivatives investigated by Aydin and coworkers as photoinitiators of polymerization in combination with MDEA and MPAPE.
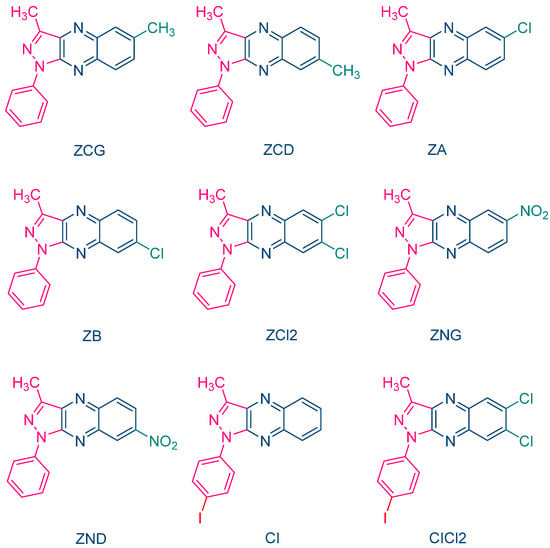
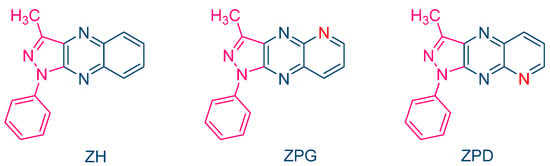 The occurrence of a back electron transfer was confirmed with a series of ten pyrazoloquinoxalines, including the previously studied quinazoline dye ZH (See Figure 5) [216][217]. Examination of their UV-visible absorption spectra in ethyl acetate revealed that these structures were relatively insensitive to the substitution pattern. Thus, absorption maxima ranging between 404 nm for ZCD and 435 nm for ZND were determined. It has to be noticed that the positions of the absorption maxima were affected by the substitution pattern. Thus, for the nitro-substituted quinoxaline, positions of the absorption maxima varied from 417 nm for ZNG and up to 435 nm for ZND, in which the nitro group was in a conjugated position with the rest of the molecule (See Table 1). In this series of dyes, the authors could establish a linear relationship between the monomer conversion and the efficiency of singlet oxygen formation, evidencing that the electron transfer between NPG and the different dyes was occurring via the triplet state. By introducing heavy atom in ZCl2, CI and CICl2, quantum yields of the triplet state formation were greatly improved, enhancing the photo-initiating ability.
The occurrence of a back electron transfer was confirmed with a series of ten pyrazoloquinoxalines, including the previously studied quinazoline dye ZH (See Figure 5) [216][217]. Examination of their UV-visible absorption spectra in ethyl acetate revealed that these structures were relatively insensitive to the substitution pattern. Thus, absorption maxima ranging between 404 nm for ZCD and 435 nm for ZND were determined. It has to be noticed that the positions of the absorption maxima were affected by the substitution pattern. Thus, for the nitro-substituted quinoxaline, positions of the absorption maxima varied from 417 nm for ZNG and up to 435 nm for ZND, in which the nitro group was in a conjugated position with the rest of the molecule (See Table 1). In this series of dyes, the authors could establish a linear relationship between the monomer conversion and the efficiency of singlet oxygen formation, evidencing that the electron transfer between NPG and the different dyes was occurring via the triplet state. By introducing heavy atom in ZCl2, CI and CICl2, quantum yields of the triplet state formation were greatly improved, enhancing the photo-initiating ability.
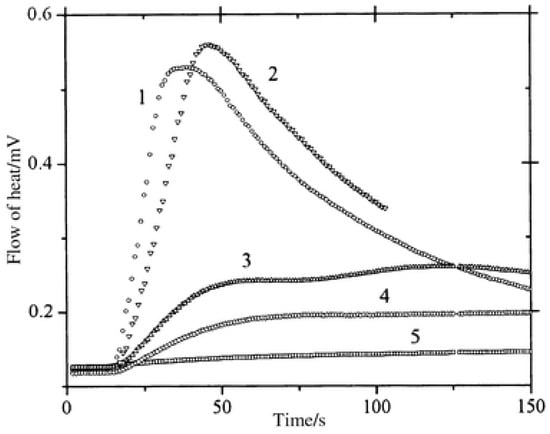
 In benefiting from these elongated excited state lifetimes, interactions between the dyes and NPG in the excited state were favored, improving the polymerization efficiency. As shown in Figure 6, halogenated quinoxalines polymerized TMPTA within 50 s, contrarily to the non-halogenated dyes for which a three-fold elongation of the polymerization time was necessary. To monitor the polymerization process, photo-DSC was used. In this case, access to the monomer conversion was not directly possible. Using photo-DSC, only the heat flow can be determined. Heat flow is proportional to the polymerization rate, which is the derivative of the conversion versus time function. In the present case, the highest heat flows were obtained for CICl2 and CI, the two compounds bearing halogens.
In benefiting from these elongated excited state lifetimes, interactions between the dyes and NPG in the excited state were favored, improving the polymerization efficiency. As shown in Figure 6, halogenated quinoxalines polymerized TMPTA within 50 s, contrarily to the non-halogenated dyes for which a three-fold elongation of the polymerization time was necessary. To monitor the polymerization process, photo-DSC was used. In this case, access to the monomer conversion was not directly possible. Using photo-DSC, only the heat flow can be determined. Heat flow is proportional to the polymerization rate, which is the derivative of the conversion versus time function. In the present case, the highest heat flows were obtained for CICl2 and CI, the two compounds bearing halogens.
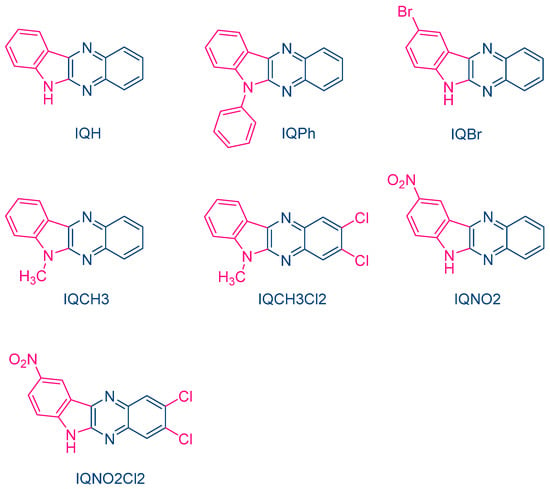
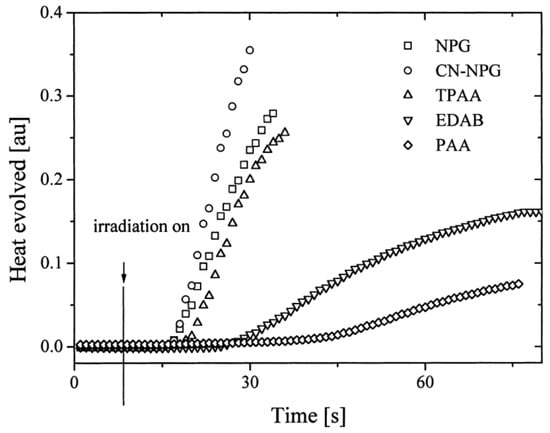
 In 2004, quinoxaline derivatives were tested for the first time in lower light intensity (no use of lasers as the light sources as in the previous works) since dental lamps were used for the polymerization experiments [217][218]. A series of seven dyes were examined, differing by the substitution pattern and the alkylation or not of the nitrogen groups (See Figure 7). All dyes exhibited an absorption centered in the near UV-visible range, the absorption maxima peaking between 386 nm for IQH and up to 409 nm for IQNO2Cl2. Noticeably, in this series of dyes, the lowest monomer conversions were obtained for the nitro derivatives, namely IQNO2 and IQNO2Cl2. These counter-performances were assigned to the photoreduction of the nitro groups to nitroso groups, constituting efficient free radical scavengers and good inhibitors of free radical polymerization [218][219]. Polymerization efficiency was also strongly related to the electron donors used. Among the five electron donors tested, namely N-phenylglycine (NPG), N-(4-cyanophenyl)glycine (CN-NPG), (phenylthio)acetic acid (TPAA), ethyl 4-dimethylaminobenzoate (EDAB) and phenoxyacetic acid (PAA), the best monomer conversions were obtained with NPG and CN-NPG while using IQCH3 as the dye (See Figure 8). Using the best electron donors (NPG and CN-NPG), the highest monomer conversions were obtained with IQBr and IQCH3Cl2 bearing halogens. Here again, the beneficial effect of heavy atoms on quinoxalines was demonstrated. Noticeably, no direct correlations could be established between the rates of the electron transfer and the polymerization rates determined for the different dyes. This unexpected result was assigned to differences in molar extinction coefficients between dyes and the diffusion effects of radicals within the resins affecting the polymerization efficiency.
In 2004, quinoxaline derivatives were tested for the first time in lower light intensity (no use of lasers as the light sources as in the previous works) since dental lamps were used for the polymerization experiments [217][218]. A series of seven dyes were examined, differing by the substitution pattern and the alkylation or not of the nitrogen groups (See Figure 7). All dyes exhibited an absorption centered in the near UV-visible range, the absorption maxima peaking between 386 nm for IQH and up to 409 nm for IQNO2Cl2. Noticeably, in this series of dyes, the lowest monomer conversions were obtained for the nitro derivatives, namely IQNO2 and IQNO2Cl2. These counter-performances were assigned to the photoreduction of the nitro groups to nitroso groups, constituting efficient free radical scavengers and good inhibitors of free radical polymerization [218][219]. Polymerization efficiency was also strongly related to the electron donors used. Among the five electron donors tested, namely N-phenylglycine (NPG), N-(4-cyanophenyl)glycine (CN-NPG), (phenylthio)acetic acid (TPAA), ethyl 4-dimethylaminobenzoate (EDAB) and phenoxyacetic acid (PAA), the best monomer conversions were obtained with NPG and CN-NPG while using IQCH3 as the dye (See Figure 8). Using the best electron donors (NPG and CN-NPG), the highest monomer conversions were obtained with IQBr and IQCH3Cl2 bearing halogens. Here again, the beneficial effect of heavy atoms on quinoxalines was demonstrated. Noticeably, no direct correlations could be established between the rates of the electron transfer and the polymerization rates determined for the different dyes. This unexpected result was assigned to differences in molar extinction coefficients between dyes and the diffusion effects of radicals within the resins affecting the polymerization efficiency.
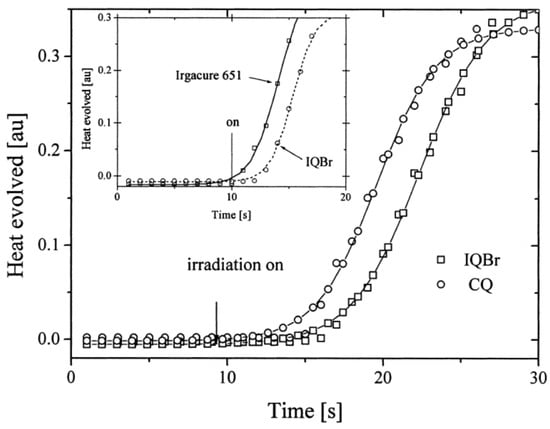
 Finally, a comparison of the photo-initiating ability of IQBr with that of camphorquinone (CQ) and 2,2-dimethoxy-2-phenylacetophenone (DMPA, Irgacure 651) revealed IQBr furnished similar polymerization rates to these benchmark photoinitiators (See Figure 9).
Finally, a comparison of the photo-initiating ability of IQBr with that of camphorquinone (CQ) and 2,2-dimethoxy-2-phenylacetophenone (DMPA, Irgacure 651) revealed IQBr furnished similar polymerization rates to these benchmark photoinitiators (See Figure 9).
Since these pioneering works, numerous quinoxaline derivatives have been proposed, enabling the initiation of free radical polymerizations and cationic polymerizations in the UV and visible range. It has to be noticed that quinoxalines were also investigated as chromophores for two-photon polymerization [214]. Triphenylamine-modified quinoxalines were notably reported as exhibiting a two-photon value higher than 160 GM in the 780–820 nm range, greatly higher than the values reported for most of the benzil derivatives investigated as photoinitiators for two-photon initiated polymerization.
2. Quinoxalines as Photoinitiators of Polymerization
In 2000, Pączkowski and coworkers examined two dyes containing pyrazoloquinoxaline moieties, i.e., ZPG and ZPD, and investigated the photochemical mechanism involved during the free radical polymerization (FRP) of TMPTA (See Figure 4) [215][216]. Noticeably, similar absorption maxima were found for ZH (λmax = 409 nm in ethyl acetate) and ZPG/ZPD (λmax = 415 nm in ethyl acetate). The photo-initiating abilities of these two dyes were compared with that of the quinoxaline derivative ZH. While the FRP of TMPTA was carried out upon irradiation with an argon ion laser, ZPG and ZPD greatly outperformed the monomer conversion obtained with ZH. To support this, the authors evidenced that the intersystem crossing between the singlet excited state and the triplet excited state was more efficient for ZPG and ZPD than for ZH, enabling these dyes to interact more efficiently with the electron donor N-phenylglycine (NPG) and facilitating the generation of radicals. Application of the Rehm–Weller equation also revealed the free energy change to be more positive for ZH than for ZPD and ZPG so that the rate constant of electron transfer between ZH and NPG was expected to be slower than with ZPD and ZPG. However, the authors also suggested a competitive back electron transfer between ZH and NPG, adversely affecting the photo-initiating ability of this dye.
Figure 4. Chemical structures of ZH, ZPG and ZPD.

Figure 5. Chemical structures of various pyrazoloquinoxaline dyes.

Figure 6. Polymerization profiles obtained by photo-DSC using (1) CICl2, (2) CI, (3) ZB, (4) ZH, (5) CNH2 in combination with NPG.

Figure 7. Chemical structures of different 6H-indolo[2,3-b]quinoxalines.

Figure 8. Polymerization profiles obtained by photo-DSC using IQCH3 as the dye and different electron donors (NPG, CN-NPG, TPAA, EDAB and PAA).

Figure 9. Polymerization profiles obtained during the FRP of TMPTA using IQBr, CQ and Irgacure 651 as the dyes and CN-NPG as the electron donor using a dental lamp as the light source.
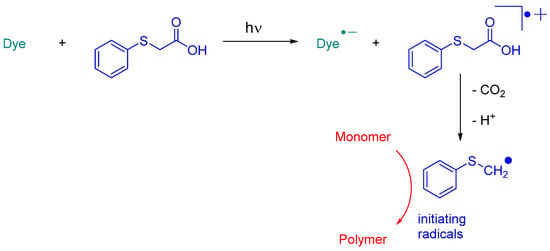
In 2023, Jędrzejewska and coworkers revisited IQH in the context of a comparative study between indenono- and indoloquinoxaline derivatives IN1-IN5, IQH, IN7 and IN8 (See Figure 10) [219][220][220,221]. The different dyes were used as electron acceptors for (phenylthio)acetic acid (PTAA) [221][222], and the resulting photoredox pairs were used as photo-initiating systems for dental applications. The mechanism of radical generation is presented in Scheme 2.
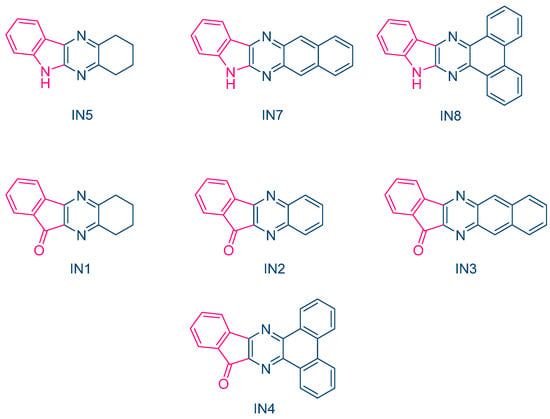
 From the absorption viewpoint, all dyes IN1-IN5, IQH, IN7 and IN8 showed an intense absorption band centered in the near UV range. Only IN8 exhibited an absorption peak located in the visible range, peaking at 417 nm and attributable to the presence of the phenanthrene moiety extending the aromaticity of this dye. In fact, IN4 and IN8 exhibited the most red-shifted absorption for the two series of dyes, namely the indenono- and indoloquinoxaline series. This redshift was beneficial for the FRP experiments. Indeed, when paired with PTAA, the highest polymerization rates were obtained with these two dyes due to a better match between the emission of the dental lamp emitting at 400 nm and the absorption maxima of these chromophores. In fact, the photo-initiating abilities of these photoredox pairs were comparable to that of camphorquinone (CQ), a benchmark photoinitiator commonly used in dentistry. Interestingly, in the context of dental fillings, an increase of the temperature lower than 5 °C was evidenced, which did not exceed the temperature tolerance threshold for the tooth pulp.
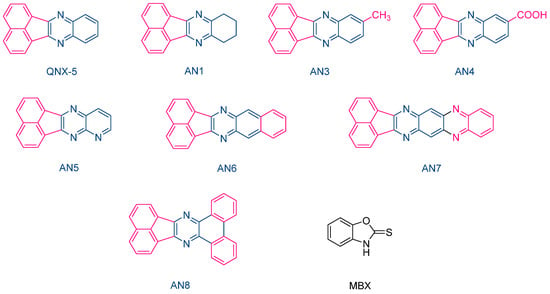
The same performances were also obtained with another series of photoredox pairs based on acenaphthoquinoxalines and 2-mercaptobenzoxazole (MBX) used as the co-initiator (See Figure 11) [222][223]. In this series of dyes (QNX5, AN1, AN3-AN-8) and during the FRP of TMPTA, the lowest heat increase was obtained with the AN6/MBX combination. In addition, the temperature increase with the other dyes remained in the tolerance threshold for dental applications. Due to their strong absorption located in the UV range, colorless dental fillings could be obtained with these different acenaphthoquinoxalines, making these dyes suitable candidates for dental applications.
From the absorption viewpoint, all dyes IN1-IN5, IQH, IN7 and IN8 showed an intense absorption band centered in the near UV range. Only IN8 exhibited an absorption peak located in the visible range, peaking at 417 nm and attributable to the presence of the phenanthrene moiety extending the aromaticity of this dye. In fact, IN4 and IN8 exhibited the most red-shifted absorption for the two series of dyes, namely the indenono- and indoloquinoxaline series. This redshift was beneficial for the FRP experiments. Indeed, when paired with PTAA, the highest polymerization rates were obtained with these two dyes due to a better match between the emission of the dental lamp emitting at 400 nm and the absorption maxima of these chromophores. In fact, the photo-initiating abilities of these photoredox pairs were comparable to that of camphorquinone (CQ), a benchmark photoinitiator commonly used in dentistry. Interestingly, in the context of dental fillings, an increase of the temperature lower than 5 °C was evidenced, which did not exceed the temperature tolerance threshold for the tooth pulp.
The same performances were also obtained with another series of photoredox pairs based on acenaphthoquinoxalines and 2-mercaptobenzoxazole (MBX) used as the co-initiator (See Figure 11) [222][223]. In this series of dyes (QNX5, AN1, AN3-AN-8) and during the FRP of TMPTA, the lowest heat increase was obtained with the AN6/MBX combination. In addition, the temperature increase with the other dyes remained in the tolerance threshold for dental applications. Due to their strong absorption located in the UV range, colorless dental fillings could be obtained with these different acenaphthoquinoxalines, making these dyes suitable candidates for dental applications.
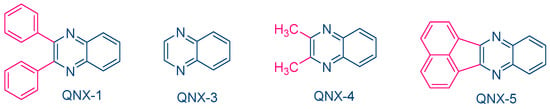
 While NPG was extensively used as an electron donor for quinoxaline derivatives, other compounds were also examined, as exemplified with N-methyldiethanolamine (MDEA) that was used as an electron donor for UV photopolymerization experiments (See Figure 12) [223][224]. 2,3-Diphenylquinoxaline (QNX-1), quinoxaline (QNX-3), 2,3-dimethylquinoxaline (QNX-4) and acenaphthoquinoxaline (QNX-5) were tested as UV photoinitiators.
While NPG was extensively used as an electron donor for quinoxaline derivatives, other compounds were also examined, as exemplified with N-methyldiethanolamine (MDEA) that was used as an electron donor for UV photopolymerization experiments (See Figure 12) [223][224]. 2,3-Diphenylquinoxaline (QNX-1), quinoxaline (QNX-3), 2,3-dimethylquinoxaline (QNX-4) and acenaphthoquinoxaline (QNX-5) were tested as UV photoinitiators.
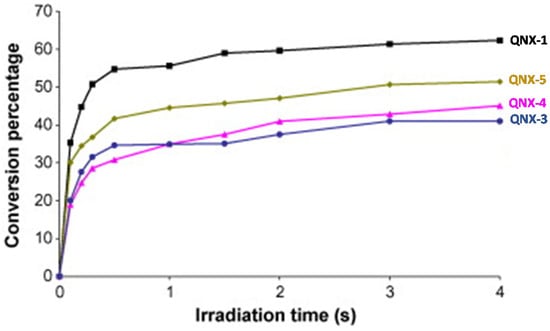
 Interestingly, by using a polychromatic light, very fast polymerization processes were evidenced since a full curing of TMPTA was obtained within 4 s of irradiation (See Figure 13). The highest final monomer conversion was obtained for QNX-1, with a conversion of 60% after 4 s. Noticeably, the highest monomer conversions were obtained for the most polyaromatic structures (QNX-1, QNX-5), certainly attributable to higher molar extinction coefficients in the visible range. TContrarily to the different work previously mentioned in this review, the TMPTA conversion could be monitored by Real-Time Fourier Transform Infrared (RT-FTIR) spectroscopy, giving direct access to the monomer conversion. To conduct this, modification of the IR peak at ca 6120 cm−1 was monitored, corresponding to a characteristic peak of TMPTA. The polymerization profiles were established from the difference between the initial peak area before irradiation and the peak area after irradiation for a given time t [224][225][225,226].
Interestingly, by using a polychromatic light, very fast polymerization processes were evidenced since a full curing of TMPTA was obtained within 4 s of irradiation (See Figure 13). The highest final monomer conversion was obtained for QNX-1, with a conversion of 60% after 4 s. Noticeably, the highest monomer conversions were obtained for the most polyaromatic structures (QNX-1, QNX-5), certainly attributable to higher molar extinction coefficients in the visible range. TContrarily to the different work previously mentioned in this review, the TMPTA conversion could be monitored by Real-Time Fourier Transform Infrared (RT-FTIR) spectroscopy, giving direct access to the monomer conversion. To conduct this, modification of the IR peak at ca 6120 cm−1 was monitored, corresponding to a characteristic peak of TMPTA. The polymerization profiles were established from the difference between the initial peak area before irradiation and the peak area after irradiation for a given time t [224][225][225,226].
 This trend of reactivity was confirmed during the FRP of other monomers, such as a 75% P-3038 epoxyacrylate (EA) and 25% tripropyleneglycol diacrylate (TPGDA) (3/1 v/v) blend (See Figure 14). However, by elongating the irradiation time to 180 s, QNX-5 furnished a similar monomer conversion to QNX-1. Clearly, the difference in monomer conversions is directly related to differences in polymerization rates at early irradiation time.
This trend of reactivity was confirmed during the FRP of other monomers, such as a 75% P-3038 epoxyacrylate (EA) and 25% tripropyleneglycol diacrylate (TPGDA) (3/1 v/v) blend (See Figure 14). However, by elongating the irradiation time to 180 s, QNX-5 furnished a similar monomer conversion to QNX-1. Clearly, the difference in monomer conversions is directly related to differences in polymerization rates at early irradiation time.
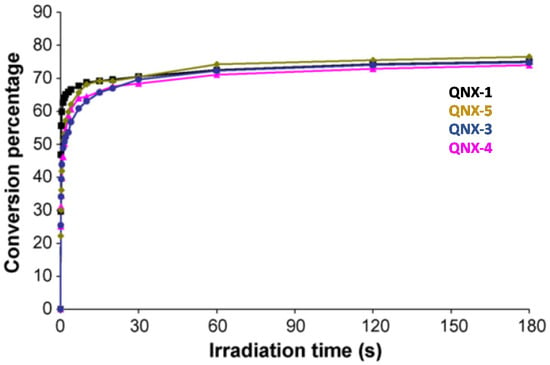

Figure 10. Chemical structures of indenono- and indoloquinoxaline derivatives.

Scheme 2. Mechanism of photoinitiation with dye/phenylthioacetic acid system.

Figure 11. Chemical structures of various acenaphthoquinoxalines AN1, AN3-AN8 and 2-mercaptobenzoxazole used as the hydrogen donor.

Figure 12. Quinoxalines investigated in combination with MDEA as the electron donor.

Figure 13. Polymerization profiles obtained during the FRP of TMPTA under air and using the two-component dyes/MDEA (1%/10% w/w).

Figure 14.
Polymerization profiles obtained during the FRP of an EA/TPGDA (3/1) blend under air and using the two-component dyes/MDEA (1%/10%
w
/
w
).
In the case of 5,12-dihydroquinoxalino[2,3b]quinoxalines (i.e., fluoflavins), these dyes were interesting candidates to induce the reductive decomposition of alkoxypyridinium salts (See Figure 15) [226][227]. The fluoflavin dyes/alkoxypyridinium salts combinations proved to be excellent two-component systems to initiate the FRP of TMPTA under visible light. It has to be noticed that previously to this work, cyanines [227][228], ketocoumarins [228][229] and acridinedione dyes [229][230] were also used as sensitizers capable of inducing the decomposition of alkoxypyridinium salts by photoinduced electron transfer. From the mechanistic viewpoint, upon excitation of the dye, a photoinduced electron transfer from fluoflavins towards the alkoxypyridinium salt can occur, generating an alkoxypyridinium radical that immediately decomposes, generating initiating alkoxy radicals (RO•) (See Scheme 3).
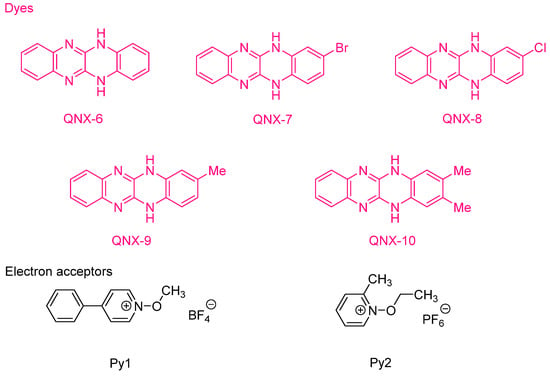
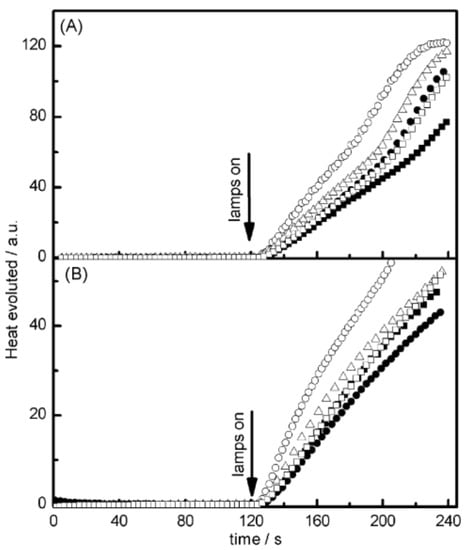
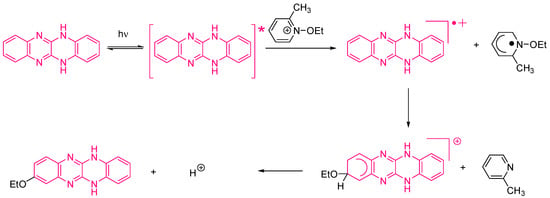
 Examination of their photo-initiating abilities during the FRP of TMPTA upon irradiation with a visible light revealed QNX-7 and QNX-8 furnished the best polymerization rates (See Figure 16). More precisely, QNX-7 was determined as outperforming all the other dyes, irrespective of the alkoxypyridinium salt. Experiments carried out with Py2 also furnish higher monomer conversion than Py1, once again evidencing the necessity to screen both the electron donors and acceptors in order to obtain the best combination. Interestingly, an efficient photobleaching of the TMPTA resins was observed during polymerization, assigned to the addition of alkoxy radicals on fluoflavins, according to the mechanism depicted in Scheme 4. Precisely, a reaction between the fluoflavin radical cation and the ethoxyl radicals at the 9-position of the phenyl ring was proposed by analogy to previous works performed on anthracene [230][231][231,232]. After proton release, an ethoxy derivative of the fluoflavin dyes can be formed, enabling the ability to obtain an efficient bleaching of the final polymers.
Examination of their photo-initiating abilities during the FRP of TMPTA upon irradiation with a visible light revealed QNX-7 and QNX-8 furnished the best polymerization rates (See Figure 16). More precisely, QNX-7 was determined as outperforming all the other dyes, irrespective of the alkoxypyridinium salt. Experiments carried out with Py2 also furnish higher monomer conversion than Py1, once again evidencing the necessity to screen both the electron donors and acceptors in order to obtain the best combination. Interestingly, an efficient photobleaching of the TMPTA resins was observed during polymerization, assigned to the addition of alkoxy radicals on fluoflavins, according to the mechanism depicted in Scheme 4. Precisely, a reaction between the fluoflavin radical cation and the ethoxyl radicals at the 9-position of the phenyl ring was proposed by analogy to previous works performed on anthracene [230][231][231,232]. After proton release, an ethoxy derivative of the fluoflavin dyes can be formed, enabling the ability to obtain an efficient bleaching of the final polymers.
 The possibility to initiate free-radical/cationic hybrid polymerizations (concomitant polymerization of TMPTA and 3,4-epoxycyclohexylmethyl 3′,4′-epoxycyclohexanecarboxylate (EPOX)) with fluoflavin derivatives was also examined. Using QNX-6-QNX-8 as the photosensitizers for triarylsulfonium hexafluoroantimonates, epoxide conversions higher than that of acrylates were observed with all photo-initiating systems [232][233]. The lower acrylate conversion determined during hybrid photopolymerization was assigned to oxygen inhibition favoring the cationic polymerization of epoxides. Indeed, contrarily to the cationic polymerization, which is insensitive to oxygen inhibition, free radicals can react with molecular oxygen, generating unreactive peroxyl radicals. In 2009, the same authors examined a series of dyes analogues to fluoflavins, namely QNX-11-QNX-19, that were used as electron donors for the reductive photosensitization of N-methoxy-4-phenylpyridinium tetrafluoroborate (Py1) and N-ethoxy-2-methylpyridinium hexafluorophosphate (Py2) examined in the previous work and for diphenyliodonium hexafluorophosphate (Ph2I+PF6−) (See Figure 17) [233][234]. These photoinitiators were advantageously used for the FRP of acrylates under visible light and the cationic polymerization (CP) of cyclohexene oxide (CHO).
The possibility to initiate free-radical/cationic hybrid polymerizations (concomitant polymerization of TMPTA and 3,4-epoxycyclohexylmethyl 3′,4′-epoxycyclohexanecarboxylate (EPOX)) with fluoflavin derivatives was also examined. Using QNX-6-QNX-8 as the photosensitizers for triarylsulfonium hexafluoroantimonates, epoxide conversions higher than that of acrylates were observed with all photo-initiating systems [232][233]. The lower acrylate conversion determined during hybrid photopolymerization was assigned to oxygen inhibition favoring the cationic polymerization of epoxides. Indeed, contrarily to the cationic polymerization, which is insensitive to oxygen inhibition, free radicals can react with molecular oxygen, generating unreactive peroxyl radicals. In 2009, the same authors examined a series of dyes analogues to fluoflavins, namely QNX-11-QNX-19, that were used as electron donors for the reductive photosensitization of N-methoxy-4-phenylpyridinium tetrafluoroborate (Py1) and N-ethoxy-2-methylpyridinium hexafluorophosphate (Py2) examined in the previous work and for diphenyliodonium hexafluorophosphate (Ph2I+PF6−) (See Figure 17) [233][234]. These photoinitiators were advantageously used for the FRP of acrylates under visible light and the cationic polymerization (CP) of cyclohexene oxide (CHO).
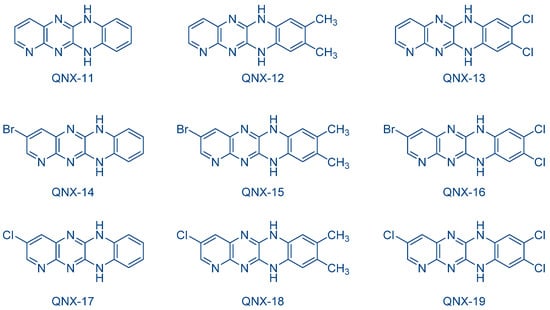

Figure 15. Chemical structures of 5,12-dihydroquinoxalino[2,3b]quinoxalines and alkoxypyridinium salts.
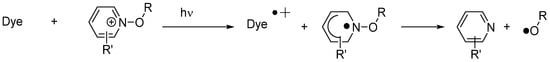
Scheme 3. Mechanism of photoinduced decomposition of alkoxypyridinium salts by electron transfer.

Figure 16. Photopolymerization profiles of TMPTA recorded for QNX-6 (▪); QNX-7 (O); QNX-8 (Δ); QNX-9 (•); QNX-10 (□) and (A) Py1 or (B) Py2.

Scheme 4. Mechanism of photobleaching occurring with the fluoflavin dyes/alkoxypyridinium salts combinations.

Figure 17. Chemical structures of QNX-11-QNX-19 examined as photosensitizers for iodonium salts.
