You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Maria felice Brizzi and Version 2 by Conner Chen.
The correlation between diabetes mellitus and infectious diseases is widely recognized. DM patients are characterized by the impaired function of the immune system. This translates into the occurrence of a variety of infections, including urinary tract, skin and surgical site infections, pneumonia, tuberculosis, and, more recently, SARS-CoV-2.
- Diabetes Mellitus
- infection
- tuberculosis
1. Introduction
Diabetes mellitus (DM) is a chronic disease characterized by abnormal blood glucose levels resulting from impaired insulin action and/or insulin secretion, usually both [1]. DM can be classified as type 1 diabetes, type 2, and gestational diabetes [2]. According to the 10th edition of the IDF Diabetes Atlas, 536.6 million people are currently diagnosed with DM worldwide with a prevalence of 10.5%, and this number is expected to increase to 783.2 million in 2045. The prevalence of DM increases with age, while the incidence of type 2 diabetes is expected to decrease or remain stable in high-income countries [3]. In addition to macro- and microvascular complications, an increased risk of infection is commonly associated with DM [4]. Individuals with DM are at a greater risk of hospitalization and mortality due to viral, bacterial, and fungal infections [5]. However, recent evidence indicates that DM does not represent a significant risk factor for poor survival in patients with sepsis, regardless of intensive care unit (ICU) admission [6][7][6,7]. Nevertheless, DM and sepsis remain important causes of morbidity and mortality worldwide, and DM patients represent the largest population experiencing post-sepsis complications [8]. This is mainly due to immunosuppression and uncontrolled hyperglycemia. In fact, high blood glucose impairs innate and adaptive immunity through various mechanisms [9].
Poor glycemic control increases the risk for skin, bone, eye, ear, gastrointestinal, urinary tract, and respiratory infections [10]. Moreover, impaired healing of diabetic wounds, which affects approximately 25% of all DM patients, is associated with an increased risk of limb amputation, thereby representing a crucial economic and psychosocial issue [11]. Uncommon life-threatening infections are also more frequent among DM patients. These include invasive otitis externa, rhino-cerebral mucormycosis, and emphysematous infections of the gall bladder, kidney, and urinary bladder [12]. Evidence has also been provided on the prevalence of drug resistance in DM patients [13], however, an increased prevalence of resistance to commonly used antibiotics in DM patients is still debated [14]. More recently, several infections have been supposed to rely on newly introduced therapies. As an example, sodium-glucose co-transport 2 inhibitors (SGLT2-i) are associated with the occurrence of urinary and genital tract infections [15][16][17][15,16,17]. Moreover, among infections commonly associated with hospitalization, including urinary tract and skin infections, pneumonias, and surgical infections, the presence of DM confers an increased risk (Figure 1).
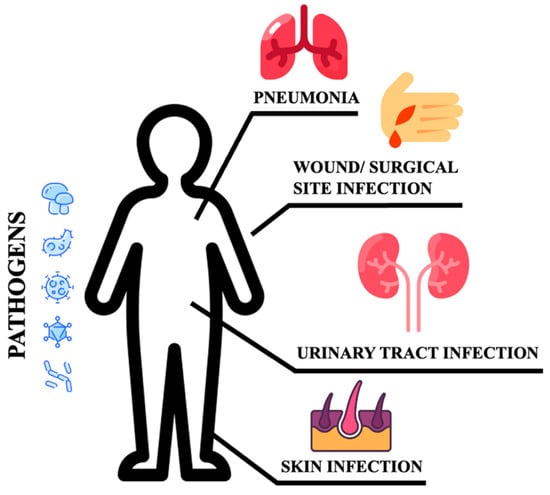
Figure 1.
Sites of infections.
These patients are more susceptible to urinary tract infections than non-DM individuals. They have a higher risk of developing asymptomatic bacteriuria, cystitis, acute pyelonephritis, and complications such as emphysematous pyelonephritis [9][18][9,18]. Bacteria isolated from DM patients with an urinary tract infection (UTI) do not differ from those found in non-DM patients with complicated UTI. E. coli are the most common pathogens, while Klebsiella spp., Enterobacter spp., Proteus spp., Group B Streptococci, and Enterococcus faecalis are the most frequently isolated pathogens [19]. DM patients are more likely to develop skin and soft tissue infections, including cellulitis and osteomyelitis [14]. Staphylococcus aureus and Pseudomonas [20] are the most common isolated gram-positive and negative bacteria respectively. Methicillin-resistant Staphylococcus aureus (MRSA) and other antibiotic-resistant pathogens generally account for skin and soft tissue infections in the diabetic foot compared to other tissue sites and populations [14]. DM increases the susceptibility to different respiratory infections, thereby representing an independent risk factor for lower respiratory tract infections, particularly influenza and pneumonia [20][21][20,21]. More importantly, DM individuals are at higher risk of pulmonary infections caused by microorganisms such as Mycobacterium tuberculosis, Staphylococcus aureus, gram-negative bacteria, and fungi, and have a high risk of hospitalization upon influenza or flu-like infections. Additionally, infections caused by Streptococcus pneumonia or influenza virus are characterized by high morbidity and mortality rates [20]. Furthermore, pulmonary infections in elderly DM patients remain occult. Advanced age, comorbidities (senile dementia, hypothyroidism), and prolonged bed rest are indeed considered independent risk factors for occult pneumonia [22], resulting in long-term hospitalization and increased mortality. DM also increases community-acquired pneumonia (CAP) compared to non-diabetic individuals, and gram-negative bacteria such as K. pneumoniae and S. aureus are much more commonly isolated [9]. The susceptibility to fungal infections caused by Mucorales has been estimated at 75% in this population. Aspergillus is an additional microorganism causing infections in these patients [23]. It has been reported that S. pneumoniae, Enterobacter, K. pneumoniae, Serratia, E. coli, S. aureus, Proteus, and Haemophilus influenzae are the most common bacteria causing hospital-acquired pneumonia (HAP) during the first four days of hospitalization, while Acinetobacter, MRSA, E. coli, L. pneumophila, Pseudomonas aeruginosa, and K. pneumonia are more prevalent after day five [20][24][20,24]. Tuberculosis infections are also frequent and increased with a mortality rate corresponding to 50% [25]. Hyperglycemia also predisposes to superinfection of the surgical site following surgery (SSI), and the association between pre- and post-surgery hyperglycemia remains a significant risk factor for SSI [26]. In conclusion, the risk and mortality associated with infectious diseases are high in DM patients, implying that infections should be considered among the most common DM complications [12].
2. Immunity Impairment in DM
Several pre-clinical and clinical studies have revealed a significant defect in both innate and adaptive immunity. Although some mechanisms are glycemia-independent [27], most of them rely on hyperglycemia and its metabolic effects, such as non-enzymatic glycation, generation of reactive oxygen species, and hyperactivity of the polyol pathway [28].2.1. Neutrophils
Neutrophils are recognized as key elements to counteract infection, and DM impairs their recruitment as well as their killing capability. Several mechanisms also account for the dysfunction of adhesion, rolling, and chemotaxis [29][30][31][29,30,31]. CXCR2, a chemokine receptor expressed on neutrophils, was found downregulated during sepsis, thereby impairing neutrophil recruitment [27][32][27,32]. Moreover, since CXCR2 also controls the expression of the intracellular adhesion molecule-1 (ICAM) on endothelial cells (ECs), its decreased expression further weakens neutrophil recruitment at the inflammatory site [33]. Regarding phagocytosis, the main recognized abnormality is related to C3-mediated opsonization owing to complement glycation [34]. Both intracellular and extracellular killing mechanisms (involving the production of intracellular ROS [35][36][35,36], enzymatic degranulation [37], and inhibition of neutrophil extracellular traps (NET) formation [38]) are impaired in the hyperglycemic condition.2.2. Macrophages
Chronic hyperglycemia also weakens macrophage adhesion and chemotaxis, antibacterial activity, and phagocytosis, damaging both the FCy receptor and the complement cascade [39][40][39,40]. Furthermore, the low-grade inflammation caused by hyperglycemia, insulin resistance, and obesity promotes macrophage differentiation towards their anti-inflammatory M2 phenotype, activating the IL-6 signaling pathway [41]. The homeostatic action of IL-6 in limiting inflammation represents a relevant impediment to the control of infections [42]. Figure 2 summarizes the most relevant DM-associated immune cell impairment.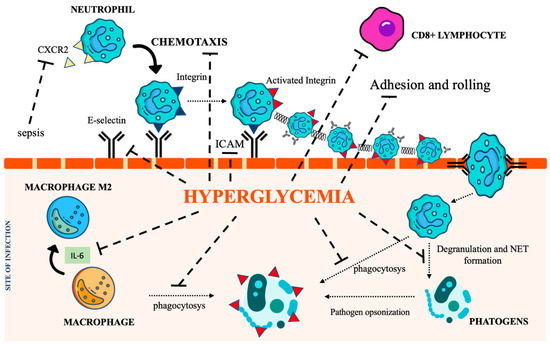
Figure 2. The immunological pathways under the control of hyperglycemia. CXCR2 (C-X-C Motif Chemokine Receptor 2), IL-6 (interleukin 6), NET (Neutrophil extracellular traps), ICAM (intercellular adhesion molecules). Icon design by icons8.
