Trigonelline is a bioactive pyridine alkaloid that occurs naturally in high concentrations in coffee (up to 7.2 g/kg) and coffee by-products (up to 62.6 g/kg) such as coffee leaves, flowers, cherry husks or pulp, parchment, silver skin, and spent grounds. In the past, coffee by-products were mostly considered waste and discarded. HIn recent years, however, the use of coffee by-products as food has attracted interest because of their economic and nutritional value and the environmental benefits of sustainable resource use. Their authorization as so-called novel foods in the European Union may lead to increased oral exposure of the general population to trigonelline.
- trigonelline
- coffee
- coffee by-products
- toxicity
- risk assessment
1. Introduction

2. Estimation of Human Oral Exposure
The trigonelline contents vary greatly from 0.2 to 63 g/kg (factor ~200) depending on multiple factors, for instance: country of origin [48][89], coffee species and cultivar [49][50][74,90], part of the coffee plant [9], age of the plant part [19][51][52][53][19,76,77,79], applied industrial production processes [6][54][55][6,91,92], used preparation methods [56][57][93,94], water content of the sample [58][84], pH condition of the sample media [59][95], and analytical method used [59][95]. Due to the distinct water solubility and thermolability of trigonelline, concentrations in green coffee beans were shown to be markedly reduced during industrial (a) washing and blanching, leading to losses via leaching [6], and (b) roasting and steaming, causing losses via thermally induced decomposition [14][60][61][14,40,96]. Consequently, profoundly lower concentrations were found in roasted than in green (i.e., unroasted) coffee beans. Numerous studies have demonstrated that both water- and heat-induced reduction depend on the time-temperature profile applied, with 15–90% of trigonelline being degraded with increasing processing time and/or temperature [6][17][59][60][62][63][64][6,17,40,95,97,98,99]. Fast roasting is therefore associated with higher trigonelline contents than slower roasting approaches [49][74]. Furthermore, decaffeination was shown to lower the trigonelline content of coffee beverages by up to 17.1% [49][61][63][74,96,98]. Besides industrial processes, the characteristics of the method used for preparing the final beverage have proven to be a crucial factor. In order to estimate the oral exposure of the EU general population to trigonelline from coffee beverages, knowledge of the daily consumption of coffee beverages and the mass of ground, roasted coffee beans weighed-in per volume of water is necessary. Yet, most sources use the term “cup of coffee” without specifying the amount of ground beans used in the preparation of the final drink. Thus, according to Romualdo et al., the most reliable data are represented by estimates of the annual consumption of ground beans per capita (which are typically expressed in kilograms) [65][55]. However, from such data, actual consumption habits regarding daily intake of coffee beverages can only be vaguely derived. Thus, data on the daily consumption of coffee beverages in the EU were adopted from the comprehensive European Food Consumption Database of the European Food Safety Authority (EFSA), as published in the 2018 International Agency for Research on Cancer (IARC) monograph, Drinking Coffee, Mate, and Very Hot Beverages [66][100], selecting the highest estimate reported. Accordingly, a daily maximum of 1914 mL of coffee was drunk per capita in Denmark (sex and year unspecified) [66][100].3. Absorption, Distribution, Metabolism, and Excretion
The pharmacokinetics of trigonelline have repeatedly been studied in humans [17][22][23][24][25][26][55][67][68][69][70][71][17,22,23,24,25,26,92,104,105,106,107,108]. Accordingly, absorption and oral ingestion are strongly correlated [22][23][24][26][68][70][71][22,23,24,26,105,107,108]. Since plasma concentrations responded rapidly as early as 15 min after administration, absorption is proposed to start in the stomach [26][55][26,92]. Peak plasma concentrations were reached at 3.00–8.48 h after administration [55][71][92,108], suggesting trigonelline to be predominantly absorbed in the small intestine [55][92]. In humans, differences in plasma concentrations between the sexes have been observed. Whilst Lang et al. found no differences in plasma concentrations between male and female participants within the first 2–4 h after ingestion, sex-dependent differences were observed in later phases, as peak plasma concentrations of females were 16.3% higher and reached considerably later (females: 3.17 h, males: 2.29 h) [26]. Coinciding with these results, Bresciani and colleagues found significantly higher Cmin, Cavg, Cmax, and AUC0–24 in females in relation to males [71][108]. These intersexual differences may be attributable to the lower body mass and blood volume of the recruited females, resulting in less dilution of trigonelline in the blood [26][71][26,108]. Further, plasma trigonelline was shown to be 20% lower in fasting than in non-fasting participants and increased by 9% per decade of age [70][107]. The volume of distribution (Vd), calculated on the basis of data from 13 volunteers, was described as comparatively small at 123 L [26] (for context: Holford and Yim [72][110] define the Vd as very small at 10 L and large at 500 L), suggesting that trigonelline remains primarily in the blood [17][22][23][26][17,22,23,26]. Yet, in transgenic mice, trigonelline entered the cerebral cortex [73][111], implying the ability of this compound to cross the blood-brain barrier. Up to 64.3% of the ingested amount is excreted unchanged via the urine within the first 8 h after ingestion [26][55][68][26,92,105], and additionally, about 10% as N1-methyl-2-pyridone-5-carboxylic acid [68][105], leaving the fate of the remaining ~30% unclear. Considering the good water solubility of trigonelline, it is likely that the vast majority of this proportion may also be excreted via the urine at later stages without undergoing any prior chemical changes [17][68][17,105]. As with absorption, excretion and oral ingestion are positively correlated [17][22][23][24][25][26][68][69][71][17,22,23,24,25,26,105,106,108]. It is noteworthy that trigonelline accumulated in human plasma at markedly elevated levels for an extended period of time following repeated ingestion of coffee beverages [26][55][26,92], due to the long biological elimination half-life of around 5.5 h [55][71][92,108].4. Nutritional Information
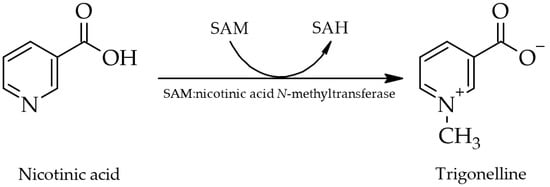
Trigonelline, as a constituent of green coffee beans, decomposes readily when heated at roasting temperatures above 180 °C [14], giving rise to various volatile derivatives such as pyrroles, alkylpyridines, and phenolic compounds, thereby influencing the flavor and aroma of coffee products. Additionally, nicotinic acid is produced as one of two non-volatile derivatives following N-demethylation (the second being N-methylpyridinium as a decarboxylation product) [17][55][74][17,39,92]. Serving as a precursor for essential nicotinic acid (niacin or vitamin B3) in coffee beans, trigonelline thus has nutritional value [8][17][74][8,17,39]. However, it is unclear whether intact trigonelline ingested via foods is metabolized to nicotinic acid in the human body and thus acts as a provitamin. Metabolic transformation to nicotinic acid is only assumed to potentially occur [26].
5. Toxicological Information
Trigonelline did not exert any cytotoxic effects in concentrations up to 100 µmol/L in human neuroblastoma SK-N-SH cells [75][114], human hepatocellular carcinoma (Hep3B) cells [76][115], human immortalized dermal keratinocytes (HaCat) and human foreskin fibroblasts (Hs68) [77][45] after treatment for six days, 48 and 24 h, respectively. In fact, in a recent study, trigonelline protected UV-B radiation-exposed Hs68 cells against photodamage by restoring the physiological function of the endoplasmic reticulum, decreasing oxidative stress, and positively modulating calcium homeostasis and apoptosis in a dose-responsive manner (10, 25, 50, and 100 µmol/L) [77][45]. It also did not alter the morphology of Madin–Darby bovine kidney cells and African green monkey kidney cells (Vero cells) at concentrations of 1.6 µg/mL each after 48 h of exposure [78][42].
Three animal studies were identified with designs appropriate for the investigation of subchronic toxicity. In cats (strain and sex not provided), trigonelline administered at doses up to 3500 mg/kg bw for 62–70 days did not induce any visible effects [79][121]. Additionally, when albino Sabra rats were fed 50 mg/kg bw of trigonelline daily for 21 days and sacrificed after 42 days, no changes in the weight of the thyroid, thymus, kidneys, liver, adrenals, ovaries, and uterus were observed [80][117].
The first in vitro experiments on the potential mutagenicity of trigonelline were conducted by Fung et al. in 1988. The scholars showed that pure trigonelline did not produce any mutagenic activity in an L5178Y TK+/− mouse lymphoma assay and several Salmonella typhimurium test systems (i.e., Ames tests) [10]. Contrarily, in the S. typhimurium assays conducted by Wu and colleagues, heated trigonelline, applied to the plates (a) alone and (b) combined with certain amino acids caused substantial amounts of revertants, especially when in binary combination with serine (12,740 revertants/mmol) or threonine (11,270/mmol). Of all the 13 single compounds examined in that study in strain TA98, heated trigonelline applied to the plates alone produced the most revertants (8160/mmol) and exhibited the highest mutagenicity [7].
Trigonelline has been examined for its potential role in cancer in several in vitro experiments. In only one study was it shown to have effects that could increase the risk of tumor formation, as it significantly induced the proliferation of MCF-7 breast cancer cells in a dose-responsive manner. Noticeably increased total cell numbers were observed at considerably low concentrations of 10 pmol/L, with the most potent proliferative effect yielded at 100 pmol/L. The growth-promoting effect was due to trigonelline binding the estrogen receptors (ER) of the MCF-7 cells in a way similar to estradiol, thereby altering ER-mediated transcription. As discussed by the scholars, trigonelline has no structural similarity to estradiol or phytoestrogens. Thus, the scholars suggested that trigonelline did not bind the active ligand binding site of the ER, but probably another domain, changing the receptors’ conformation and enabling binding of unspecified transcriptional co-regulators. Hence, trigonelline exerted sound estrogenic activity [81][123]. This is a significant finding as increased estrogenic activity—either induced by pathological overexpression of ER or mediated by estrogen-like compounds encountered, for instance, via the diet—has been shown to be an important factor in some types of cancer (e.g., breast, colorectal, endometrial, and ovarian cancer [82][124]), as it stimulates cell growth and proliferation, promoting cancer initiation [83][125].
Except for one in vivo study from 2009, no data on reproductive toxicity or teratogenic effects are available. In that study, Aswar et al. aimed to investigate the effects of trigonelline on the estrous cycle and fertility of female Wistar rats. To examine effects on the estrous cycle (endpoint: vaginal cornification), the scholars administered doses of 75 mg/kg bw of trigonelline orally to ovariectomized, immature female Wister rats weighing 55–60 g twice on the first two days and once on the third and fourth day of the treatment. Afterwards, vaginal smears were collected and microscopically searched for cornified or nucleated epithelial cells. As no such cells were noticed, it was concluded that trigonelline did not exert estrogenic activity.
Doses up to 10 µmol/L ameliorated the atrophy of neuronal dendrites and axons in amyloid β-peptide-treated female, hemizygous transgenic 5XFAD Alzheimer’s disease mice model [73][111]. Additionally, trigonelline (30 µmol/L) improved functional neurite outgrowth in human neuroblastoma SK-N-SH cells after treatment for six days [75][84][114,141]. Furthermore, daily oral administration of 500 mg/kg bw for 15 days was demonstrated to improve memory retention in six-week-old amyloid β-peptide-treated male ddY mice [85][136]. Thus, trigonelline is believed to improve cognition in Alzheimer’s disease model laboratory animals [84][86][141,142]. Furthermore, it has been discussed that trigonelline may protect against cerebral ischemia [87][140] by positively modulating neuronal spike frequency [88][143], stimulating the release of dopamine [89][139], competitively inhibiting γ-aminobutyric acid (GABA) A-receptors [90][138], and weakly hampering acetylcholine esterase [91][137].
