Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Polychronis Dilaveris.
Structural remodelling refers to the development of atrial fibrosis, as well as to alterations in atrial size and cellular ultrastructure. The latter includes myolysis, the development of glycogen accumulation, altered Connexin expression, subcellular changes, and sinus rhythm (SR) alterations.
- atrial conduction
- structural remodeling
- fibrosis
- Connexins
1. Left Atrial Size and Strain
Atrial dilatation’s relationship to the incidence of AF was first described in mitral valve disease patients [63][1]. Twenty years later, another study showed that a LA diameter above 45 mm would predict AF recurrence over the 6 months following cardioversion in patients with valve disease or septal hypertrophy [64][2]. Left atrial enlargement was established as an independent risk factor of AF in two large prospective trials [65,66][3][4]. Animal models of acute atrial dilatation in both isolated hearts and in situ showed a decrease in the atrial refractory period [67,68][5][6]. On the other hand, no change [69][7] or even prolongation of atrial refractoriness was reported elsewhere [70,71][8][9]. All of them found an increment in the inducibility and persistence of AF, though.
A change in atrial structure and function is the background of atrial remodelling, which results in left atrial enlargement [72,73][10][11]. On the other hand, conditions such as hypertension, structural, and valve heart disease may also induce similar changes in atrial anatomy or function because of an increase in LA pressure and/or volume. Atrial remodelling provides the substrate for AF episodes to be triggered, which results in a vicious circle of further atrial remodelling as a consequence [74,75][12][13].
It is well established that LA diameter is an independent predictor of AF recurrence post-PVI [76][14]. Although used widely, the LA anterior–posterior diameter is not the most accurate index of its “true” size [77][15]. Left atrial enlargement occurs in an asymmetric way and is mainly oriented in the superior–inferior and medial–lateral directions [78][16]. In a study assessing LA volume by computed tomography, it was found to be related to the outcome of RF ablation, whereas the echo-derived LA anterior–posterior diameter was not [79][17]. Cardiac MRI is a more accurate modality compared to echo in order to assess LA volume and is also being used to assess the arrhythmic substrate in regard to catheter ablation [80][18]. Patients with AF have larger LA volumes, as assessed by MRI, compared to those with SR [81,82][19][20]. On the contrary, those with “lone AF” have similar LA volumes to healthy individuals [83][21]. Additionally, persistent versus paroxysmal AF is related to higher LA volumes [84][22].
Atrial contractile dysfunction, as assessed by echocardiography, is correlated with the duration of AF. After a 2-week period of AF and restoration of SR, 24 h was the time for the recovery of atrial contractile function. On the other hand, it took more than 1 month for recovery, while AF lasted more than 6 weeks [85,86][23][24]. A new atrial thrombus can be formed after cardioversion or even several days or weeks later [87][25]. Thus, the time needed for a full recovery of atrial contraction after the restoration of SR may affect the occurrence of thromboembolic events [88][26]. It is not clear what mechanisms are involved in post-fibrillatory contractile dysfunction. Initially, it was thought that electrical cardioversion caused “atrial stunning” [87][25], but soon it was revealed that, even after the pharmacological and spontaneous restoration of SR, the atrial contractile function was depressed [89,90][27][28].
In another animal model of AF [91][29], changes in atrial contractility, as expressed by a reduction of the atrial work index and a shift in atrial electrical properties with a shortening of the atrial effective refractory period, followed the exact same time course. It is known that the main cellular mechanism of electrical remodelling is the reduction of the L-type Ca2+ inward current (ICaL) [92][30]. As a result, it is thought that this reduction of ICaL induced the atrial contractile dysfunction noted during the first 5 days of AF. Interestingly, after the restoration of SR, atrial contractility recovered completely within 3 days. An increase in compliance and the diameter of the atrium was noted early, during the first days of AF, following the same time course as the loss of contractility, and were fully reversible within two days of SR. Loss of atrial contractility is one of the mechanisms of atrial dilatation during prolonged AF, while the stretch of the atrial wall might lead to the elongation of the collagen fibers. Cellular hypertrophy and possible synthesis of new collagen fibers may also contribute as well [93][31].
LA reservoir strain, as a surrogate of LA compliance, is a predictor of AF-related outcomes, including stroke [94,95][32][33]. In a study with 1361 patients, impaired LA function, as measured by reduced LA reservoir strain and higher echocardiography-derived total atrial conduction time (PA-TDI duration) at the time of AF diagnosis, was shown to be related to stroke in addition to CHA2DS2-VASc scoring [96][34]. Both LA volume and function in “lone AF” are predictors of cardiovascular events, including cerebral infarction or haemorrhage, hospitalization, and death [97,98][35][36]. In addition, LA deformation imaging has proved as a more robust factor of prognostication for cardiovascular outcomes, including thromboembolic events, and demonstrates the LA substrate properties more accurately than morphological parameters such as LA volume and LA ejection fraction [98,99,100][36][37][38]. Finally, atrial fibrosis appears to be common in IAB, and LA strain is associated with both partial and advanced IAB [101][39].
2. Total Atrial Conduction Time (PA-TDI Duration)
Total atrial conduction time (PA-TDI duration) is an echocardiography-derived parameter that reflects LA electrical and structural changes. By obtaining a Tissue-Doppler Imaging (TDI) tracing of the lateral LA wall just below the mitral annulus level in the apical 4-chamber view, PA-TDI duration is calculated by measuring the time interval from the P-wave onset on the ECG to the peak of the A’-wave (Figure 41). This is the time needed for the atrial depolarization to occur and result in active atrial contraction, as assessed with TDI. Thus, PA-TDI duration represents a more complete measure of the extent of atrial remodelling than other indices [95][33].
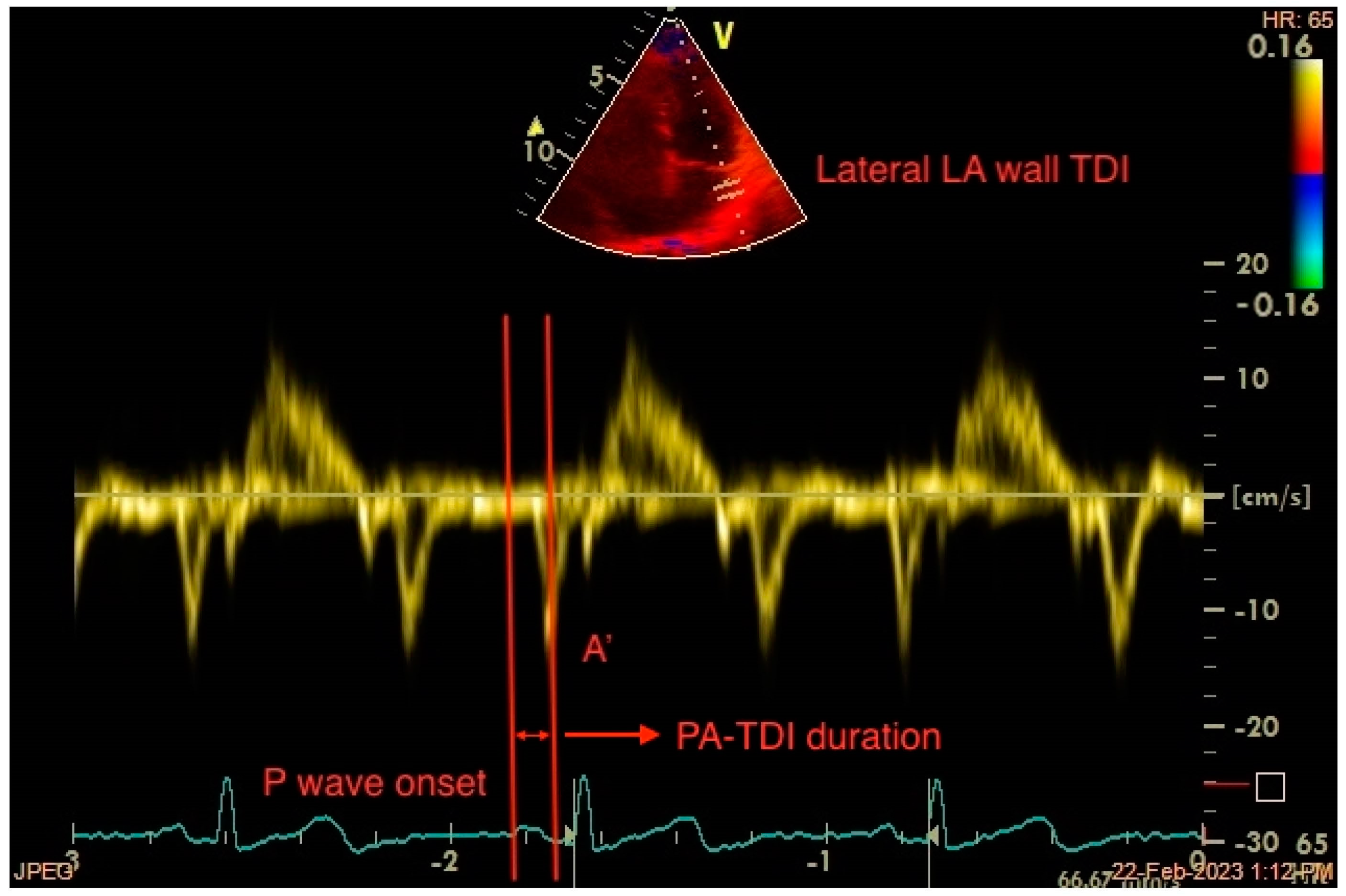
Figure 41. Measurement of the echocardiography-derived total atrial conduction time (PA-TDI duration) between the onset of the atrial electrical activation (P wave on ECG) and the peak of atrial mechanical contraction (A’ wave on TDI). LA: left atrium, TDI: tissue Doppler imaging.
The correlation between PA-TDI duration and the degree of right atrial appendage fibrosis was presented by Müller et al. in a histological validation study [102][40]. PA-TDI duration was also shown to be affected by factors known to play a significant role in atrial remodelling, such as age, hypertension, increased body mass index, valvular disease, the presence of diastolic dysfunction, and sleep apnoea [95][33]. Additionally, it was found to be inversely related to LA reservoir strain, a marker of reduced LA compliance [103][41]. A prolonged PA-TDI duration was associated with a larger LA volume index and a reduced LA reservoir strain [104][42]. It is an independent predictor of newly diagnosed AF [105][43], AF incidence after cardiac surgery [106][44], AF recurrence after electrical cardioversion [107][45], and catheter ablation, and it provides higher accuracy compared to LA volume [108][46]. In a prospective study of anticoagulant-naïve patients who were free of AF episodes following successful catheter ablation, prolonged PA-TDI duration was associated with an increased incidence of stroke and improved CHA2DS2-VASc score performance [109][47]. Another study demonstrated an independent association of PA-TDI duration with new-onset AF in hypertrophic cardiomyopathy patients, apart from other factors, such as LA dilatation and decreased LA reservoir strain [110][48]. Lastly, a good agreement between PA-TDI duration and total atrial conduction time obtained with an electrophysiological study was demonstrated by Erdem et al. in a study of healthy individuals [111][49].
References
- Fraser, H.R.L.; Turner, R.W.D. Auricular Fibrillation. BMJ 1955, 2, 1414–1418.
- Henry, W.L.; Morganroth, J.; Pearlman, A.S.; Clark, C.E.; Redwood, D.R.; Itscoitz, S.B.; Epstein, S.E. Relation between Echocardiographically Determined Left Atrial Size and Atrial Fibrillation. Circulation 1976, 53, 273–279.
- Vasan, R.S.; Larson, M.G.; Levy, D.; Evans, J.C.; Benjamin, E.J. Distribution and Categorization of Echocardiographic Measurements in Relation to Reference Limits: The Framingham Heart Study: Formulation of a Height- and Sex-Specific Classification and Its Prospective Validation. Circulation 1997, 96, 1863–1873.
- Vaziri, S.M.; Larson, M.G.; Benjamin, E.J.; Levy, D. Echocardiographic Predictors of Nonrheumatic Atrial Fibrillation. The Framingham Heart Study. Circulation 1994, 89, 724–730.
- Ravelli, F.; Allessie, M. Effects of Atrial Dilatation on Refractory Period and Vulnerability to Atrial Fibrillation in the Isolated Langendorff-Perfused Rabbit Heart. Circulation 1997, 96, 1686–1695.
- Solti, F.; Vecsey, T.; Kekesi, V.; Juhasz-Nagy, A. The Effect of Atrial Dilatation on the Genesis of Atrial Arrhythmias. Cardiovasc. Res. 1989, 23, 882–886.
- Wijffels, M.C.E.F.; Kirchhof, C.J.H.J.; Dorland, R.; Power, J.; Allessie, M.A. Electrical Remodeling Due to Atrial Fibrillation in Chronically Instrumented Conscious Goats: Roles of Neurohumoral Changes, Ischemia, Atrial Stretch, and High Rate of Electrical Activation. Circulation 1997, 96, 3710–3720.
- Sideris, D.A.; Toumanidis, S.T.; Thodorakis, M.; Kostopoulos, K.; Tselepatiotis, E.; Langoura, C.; Stringli, T.; Moulopoulos, S.D. Some Observations on the Mechanism of Pressure Related Atrial Fibrillation. Eur. Heart J. 1994, 15, 1585–1589.
- Satoh, T.; Zipes, D.P. Unequal Atrial Stretch in Dogs Increases Dispersion of Refractoriness Conducive to Developing Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 1996, 7, 833–842.
- Nattel, S.; Burstein, B.; Dobrev, D. Atrial Remodeling and Atrial Fibrillation: Mechanisms and Implications. Circ. Arrhythmia Electrophysiol. 2008, 1, 62–73.
- Casaclang-Verzosa, G.; Gersh, B.J.; Tsang, T.S.M. Structural and Functional Remodeling of the Left Atrium. J. Am. Coll. Cardiol. 2008, 51, 1–11.
- Allessie, M.A. Atrial Electrophysiologic Remodeling: Another Vicious Circle? J. Cardiovasc. Electrophysiol. 1998, 9, 1378–1393.
- Wijffels, M.C.E.F.; Kirchhof, C.J.H.J.; Dorland, R.; Allessie, M.A. Atrial Fibrillation Begets Atrial Fibrillation: A Study in Awake Chronically Instrumented Goats. Circulation 1995, 92, 1954–1968.
- Berruezo, A.; Tamborero, D.; Mont, L.; Benito, B.; Tolosana, J.M.; Sitges, M.; Vidal, B.; Arriagada, G.; Mendez, F.; Matiello, M.; et al. Pre-Procedural Predictors of Atrial Fibrillation Recurrence after Circumferential Pulmonary Vein Ablation. Eur. Heart J. 2007, 28, 836–841.
- Hof, I.; Arbab-Zadeh, A.; Scherr, D.; Chilukuri, K.; Dalal, D.; Abraham, T.; Lima, J.; Calkins, H. Correlation of Left Atrial Diameter by Echocardiography and Left Atrial Volume by Computed Tomography. J. Cardiovasc. Electrophysiol. 2009, 20, 159–163.
- den Uijl, D.W.; Bax, J.J. Left Atrial Size as a Predictor of Successful Radiofrequency Catheter Ablation for Atrial Fibrillation. Europace 2009, 11, 1255–1256.
- Abecasis, J.; Dourado, R.; Ferreira, A.; Saraiva, C.; Cavaco, D.; Santos, K.R.; Morgado, F.B.; Adragao, P.; Silva, A. Left Atrial Volume Calculated by Multi-Detector Computed Tomography May Predict Successful Pulmonary Vein Isolation in Catheter Ablation of Atrial Fibrillation. Europace 2009, 11, 1289–1294.
- Beinart, R.; Nazarian, S. Role of Magnetic Resonance Imaging in Atrial Fibrillation Ablation. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 316.
- Nori, D.; Raff, G.; Gupta, V.; Gentry, R.; Boura, J.; Haines, D.E. Cardiac Magnetic Resonance Imaging Assessment of Regional and Global Left Atrial Function before and after Catheter Ablation for Atrial Fibrillation. J. Interv. Card. Electrophysiol. 2009, 26, 109–117.
- Kato, R.; Lickfett, L.; Meininger, G.; Dickfeld, T.; Wu, R.; Juang, G.; Angkeow, P.; LaCorte, J.; Bluemke, D.; Berger, R.; et al. Pulmonary Vein Anatomy in Patients Undergoing Catheter Ablation of Atrial Fibrillation: Lessons Learned by Use of Magnetic Resonance Imaging. Circulation 2003, 107, 2004–2010.
- Ishimoto, N.; Ito, M.; Kinoshita, M. Signal-Averaged P-Wave Abnormalities and Atrial Size in Patients with and without Idiopathic Paroxysmal Atrial Fibrillation. Am. Heart J. 2000, 139, 684–689.
- Anselmino, M.; Blandino, A.; Beninati, S.; Rovera, C.; Boffano, C.; Belletti, M.; Caponi, D.; Scaglione, M.; Cesarani, F.; Gaita, F. Morphologic Analysis of Left Atrial Anatomy by Magnetic Resonance Angiography in Patients with Atrial Fibrillation: A Large Single Center Experience. J. Cardiovasc. Electrophysiol. 2011, 22, 1–7.
- Manning, W.J.; Silverman, D.I.; Katz, S.E.; Riley, M.F.; Come, P.C.; Doherty, R.M.; Munson, J.T.; Douglas, P.S. Impaired Left Atrial Mechanical Function after Cardioversion: Relation to the Duration of Atrial Fibrillation. J. Am. Coll. Cardiol. 1994, 23, 1535–1540.
- Manning, W.J.; Silverman, D.I.; Katz, S.E.; Riley, M.F.; Doherty, R.M.; Munson, J.T.; Douglas, P.S. Temporal Dependence of the Return of Atrial Mechanical Function on the Mode of Cardioversion of Atrial Fibrillation to Sinus Rhythm. Am. J. Cardiol. 1995, 75, 624–626.
- Fatkin, D.; Kuchar, D.L.; Thorburn, C.W.; Feneley, M.P. Transesophageal Echocardiography before and during Direct Current Cardioversion of Atrial Fibrillation: Evidence for “Atrial Stunning” as a Mechanism of Thromboembolic Complications. J. Am. Coll. Cardiol. 1994, 23, 307–316.
- Black, I.W.; Fatkin, D.; Sagar, K.B.; Khandheria, B.K.; Leung, D.Y.; Galloway, J.M.; Feneley, M.P.; Walsh, W.F.; Grimm, R.A.; Stollberger, C. Exclusion of Atrial Thrombus by Transesophageal Echocardiography Does Not Preclude Embolism after Cardioversion of Atrial Fibrillation. A Multicenter Study. Circulation 1994, 89, 2509–2513.
- Grimm, R.A.; Leung, D.Y.; Black, I.W.; Stewart, W.J.; Thomas, J.D.; Klein, A.L. Left Atrial Appendage “Stunning” after Spontaneous Conversion of Atrial Fibrillation Demonstrated by Transesophageal Doppler Echocardiography. Am. Heart J. 1995, 130, 174–176.
- Allessie, M. Electrical, Contractile and Structural Remodeling during Atrial Fibrillation. Cardiovasc. Res. 2002, 54, 230–246.
- Schotten, U.; Allessie, M.A. Electrical and Mechanical Remodeling of the Atria: What Are the Underlying Mechanisms, the Time Course and the Clinical Relevance? In Cardiac Arrhythmias 2001, Proceedings of the 7th International Workshop on Cardiac Arrhythmias, Venice, Italy, 7–10 October 2001; Springer: Milan, Italy, 2002.
- Yue, L.; Feng, J.; Gaspo, R.; Li, G.-R.; Wang, Z.; Nattel, S. Ionic Remodeling Underlying Action Potential Changes in a Canine Model of Atrial Fibrillation. Circ. Res. 1997, 81, 512–525.
- Schotten, U.; Neuberger, H.-R.; Allessie, M.A. The Role of Atrial Dilatation in the Domestication of Atrial Fibrillation. Prog. Biophys. Mol. Biol. 2003, 82, 151–162.
- Koca, H.; Demirtas, A.O.; Kaypaklı, O.; Icen, Y.K.; Sahin, D.Y.; Koca, F.; Koseoglu, Z.; Baykan, A.O.; Guler, E.C.; Demirtas, D.; et al. Decreased Left Atrial Global Longitudinal Strain Predicts the Risk of Atrial Fibrillation Recurrence after Cryoablation in Paroxysmal Atrial Fibrillation. J. Interv. Card. Electrophysiol. 2020, 58, 51–59.
- Müller, P.; Weijs, B.; Bemelmans, N.M.A.A.; Mügge, A.; Eckardt, L.; Crijns, H.J.G.M.; Bax, J.J.; Linz, D.; den Uijl, D.W. Echocardiography-Derived Total Atrial Conduction Time (PA-TDI Duration): Risk Stratification and Guidance in Atrial Fibrillation Management. Clin. Res. Cardiol. 2021, 110, 1734–1742.
- Leung, M.; van Rosendael, P.J.; Abou, R.; Ajmone Marsan, N.; Leung, D.Y.; Delgado, V.; Bax, J.J. Left Atrial Function to Identify Patients with Atrial Fibrillation at High Risk of Stroke: New Insights from a Large Registry. Eur. Heart J. 2018, 39, 1416–1425.
- Osranek, M.; Bursi, F.; Bailey, K.R.; Grossardt, B.R.; Brown, R.D.; Kopecky, S.L.; Tsang, T.S.; Seward, J.B. Left Atrial Volume Predicts Cardiovascular Events in Patients Originally Diagnosed with Lone Atrial Fibrillation: Three-Decade Follow-Up. Eur. Heart J. 2005, 26, 2556–2561.
- Saha, S.K.; Anderson, P.L.; Caracciolo, G.; Kiotsekoglou, A.; Wilansky, S.; Govind, S.; Mori, N.; Sengupta, P.P. Global Left Atrial Strain Correlates with CHADS2 Risk Score in Patients with Atrial Fibrillation. J. Am. Soc. Echocardiogr. 2011, 24, 506–512.
- Miyoshi, H.; Mizuguchi, Y.; Oishi, Y.; Iuchi, A.; Nagase, N.; Ara, N.; Oki, T. Early Detection of Abnormal Left Atrial-Left Ventricular-Arterial Coupling in Preclinical Patients with Cardiovascular Risk Factors: Evaluation by Two-Dimensional Speckle-Tracking Echocardiography. Eur. J. Echocardiogr. 2011, 12, 431–439.
- Cameli, M.; Lisi, M.; Focardi, M.; Reccia, R.; Natali, B.M.; Sparla, S.; Mondillo, S. Left Atrial Deformation Analysis by Speckle Tracking Echocardiography for Prediction of Cardiovascular Outcomes. Am. J. Cardiol. 2012, 110, 264–269.
- Lacalzada-Almeida, J.; Izquierdo-Gómez, M.M.; Belleyo-Belkasem, C.; Barrio-Martínez, P.; García-Niebla, J.; Elosua, R.; Jiménez-Sosa, A.; Escobar-Robledo, L.A.; Bayés de Luna, A. Interatrial Block and Atrial Remodeling Assessed Using Speckle Tracking Echocardiography. BMC Cardiovasc. Disord. 2018, 18, 38.
- Müller, P.; Hars, C.; Schiedat, F.; Bösche, L.I.; Gotzmann, M.; Strauch, J.; Dietrich, J.W.; Vogt, M.; Tannapfel, A.; Deneke, T.; et al. Correlation Between Total Atrial Conduction Time Estimated via Tissue Doppler Imaging (PA-TDI Interval), Structural Atrial Remodeling and New-Onset of Atrial Fibrillation After Cardiac Surgery: The Role of Total Atrial Conduction Time in Patients Undergoing Cardiac Surgery. J. Cardiovasc. Electrophysiol. 2013, 24, 626–631.
- Abou, R.; Leung, M.; Tonsbeek, A.M.; Podlesnikar, T.; Maan, A.C.; Schalij, M.J.; Ajmone Marsan, N.; Delgado, V.; Bax, J.J. Effect of Aging on Left Atrial Compliance and Electromechanical Properties in Subjects without Structural Heart Disease. Am. J. Cardiol. 2017, 120, 140–147.
- Leung, M.; Abou, R.; van Rosendael, P.J.; van der Bijl, P.; van Wijngaarden, S.E.; Regeer, M.V.; Podlesnikar, T.; Ajmone Marsan, N.; Leung, D.Y.; Delgado, V.; et al. Relation of Echocardiographic Markers of Left Atrial Fibrosis to Atrial Fibrillation Burden. Am. J. Cardiol. 2018, 122, 584–591.
- De Vos, C.B.; Weijs, B.; Crijns, H.J.G.M.; Cheriex, E.C.; Palmans, A.; Habets, J.; Prins, M.H.; Pisters, R.; Nieuwlaat, R.; Tieleman, R.G. Atrial Tissue Doppler Imaging for Prediction of New-Onset Atrial Fibrillation. Heart 2009, 95, 835–840.
- Özlü, M.F.; Erdem, K.; Kırış, G.; Parlar, A.İ.; Demirhan, A.; Ayhan, S.S.; Erdem, A.; Öztürk, S.; Tekelioğlu, Ü.Y.; Yazıcı, M. Predictive Value of Total Atrial Conduction Time Measured with Tissue Doppler Imaging for Postoperative Atrial Fibrillation after Coronary Artery Bypass Surgery. J. Interv. Card. Electrophysiol. 2013, 37, 27–33.
- Müller, P.; Schiedat, F.; Dietrich, J.-W.; Shin, D.-I.; Kara, K.; Mügge, A.; Deneke, T. Reverse Atrial Remodeling in Patients Who Maintain Sinus Rhythm after Electrical Cardioversion: Evidence Derived from the Measurement of Total Atrial Conduction Time Assessed by PA-TDI Interval. J. Echocardiogr. 2014, 12, 142–150.
- den Uijl, D.W.; Delgado, V.; Bertini, M.; Tops, L.F.; Trines, S.A.; van de Veire, N.R.; Zeppenfeld, K.; Schalij, M.J.; Bax, J.J. Impact of Left Atrial Fibrosis and Left Atrial Size on the Outcome of Catheter Ablation for Atrial Fibrillation. Heart 2011, 97, 1847–1851.
- Chao, T.-F.; Lin, Y.-J.; Tsao, H.-M.; Chang, S.-L.; Lo, L.-W.; Hu, Y.-F.; Tuan, T.-C.; Li, C.-H.; Chang, H.-Y.; Wu, T.-J.; et al. Prolonged Atrium Electromechanical Interval Is Associated with Stroke in Patients with Atrial Fibrillation After Catheter Ablation. J. Cardiovasc. Electrophysiol. 2013, 24, 375–380.
- Tjahjadi, C.; Hiemstra, Y.L.; van der Bijl, P.; Pio, S.M.; Bootsma, M.; Ajmone Marsan, N.; Delgado, V.; Bax, J.J. Assessment of Left Atrial Electro-Mechanical Delay to Predict Atrial Fibrillation in Hypertrophic Cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 589–596.
- Erdem, F.H.; Erdem, A.; Özlü, F.; Ozturk, S.; Ayhan, S.S.; Çağlar, S.O.; Yazici, M. Electrophysiological Validation of Total Atrial Conduction Time Measurement by Tissue Doppler Echocardiography According to Age and Sex in Healthy Adults. J. Arrhythmia 2016, 32, 127–132.
More
