1. Hydrogel and Silica Sol
Hydrogel is a substance with a 3D reticular structure, usually consisting of cross-linked tangles of hydrophilic polymers. Hydrogel coatings contain large amounts of water, are environmentally friendly, and are increasingly being used in flame-retardant applications
[1][37]. Hydrogels, such as montmorillonite/xylose hydrogel
[2][38], di-(triethoxysilylpropyl) phenylphosphamide hydrogel
[3][39], maize straw-co-2-acrylamide-2-methylpropanesulfonic acid-co-acrylic acid hydrogel
[4][40], poly (N-isopropylacrylamide) (PNIPAAm)/sodium alginate (SA)/silver nanoparticles (AgNPs) thermosensitive network hydrogel
[5][41], PNIPAAm/SA/polyvinyl alcohol hydrogel
[6][42], PNIPAAm/SA
[7][43], konjac glucomannan (KGM)/fly ash (FA) hydrogel
[8][44], and SA/sodium carboxymethylcellulose (CMC)/N-isopropyl acrylamide (PNIPAM) hydrogel
[9][45] are prepared and effectively improve the fire safety of various substrates.
Jiang et al.
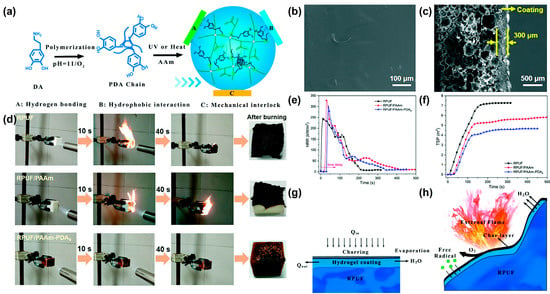
[10][29] introduced polydopamine (PDA) chains into polyacrylic-polydopamine (PAAm) hydrogels to form PAAm-PDA double network hydrogels to improve the toughness and the adhesion strength of the hydrogel coating. The schematic diagram of the synthesis route of PAAm-PDA coating is shown in
Figure 1a; an ultraviolet (UV) curing lamp or 60 °C oven is used to cure the precursor solution and form the hydrogel coating on the RPUF surface. The surface of the hydrogel-coated RPUF is uniform and smooth (
Figure 1b). The cross-section structure of the hydrogel-coated RPUF shows that the dense hydrogel coating is attached to the porous surface of the RPUF (
Figure 1c). Due to the introduction of PDA, the adhesion strength of PAAm-PDA hydrogel to RPUF increases significantly from 67.5 kPa to 191.25 kPa, compared to the PAAm hydrogel. After coating with the PAAm-PDA hydrogel, RPUF shows an excellent self-extinguishing performance (
Figure 1d), the time to ignition (TTI) increases from 6 s to 36 s, the heat release rate (HRR) decreases after reaching the peak values (
Figure 1e), and the total smoke production (TSP) decreases by 42.2% (
Figure 1f) in the cone test. As shown in
Figure 1g, the hydrogel coating releases a lot of water vapor when it is attacked by heat. Therefore, the mechanisms of the improvement of the PAAm-PDA coating for RPUF are speculated as (i) the heat absorption and cooling effect of water during evaporation after heating, (ii) the presence of hydrogel coating delaying the ignition of foam, (iii) the char layer that forms after carbonization of the hydrogel playing a protective role, and (iv) water vapor diluting the combustible gas and oxygen concentration (
Figure 1h).
Figure 1. (
a) Schematic diagram of the synthesis route of the PAAm–PDA hydrogel, (
b) SEM image of the surface of the hydrogel-coated RPUF, (
c) SEM image of the cross-sectional structure of the hydrogel-coated RPUF, (
d) video screenshot of the burning process of uncoated and hydrogel-coated RPUF under the propane flame for 10 s, (
e) HRR and (
f) TSP curves of uncoated and hydrogel-coated RPUF, (
g) and (
h) are schematic of the flame-retardant mechanisms of the hydrogel-coated RPUF
[10][29].
Yang et al.
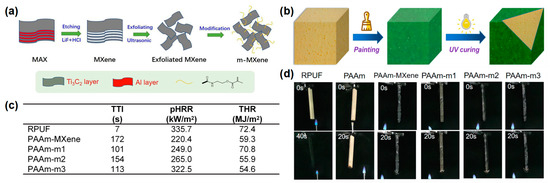
[11][30] reported an organic-inorganic hydrogel modified with MXene nanosheets for RPUF coating. To improve the dispersion of MXene nanosheets in the hydrogel, MXene is first modified by grafting with double bonds, and then introduced into an polyacrylamide (PAAm) hydrogel by radical polymerization to prepare MXene-based hydrogel coating (PAAm-MXene) (
Figure 2a,b). The RPUFs coated with PAAm hydrogels, appended with 10 mg of MXene and 5 mg, 10 mg, and 15 mg of modified MXene, were named as PAAm-MXene, PAAm-m1, PAAm-m2, and PAAm-m3, respectively. From the cone result, the TTI was significantly delayed after coating with the PAAm-MXene hydrogel. Compared to the pure RPUF, the p-HRR and THR of PAAm-m2 decreased from 335.7 kW/m
2 and 72.4 MJ/m
2 to 265 kW/m
2 and 55.9 MJ/m
2, respectively (
Figure 2c). In the UL-94 test, the pure RPUF was easily ignited, and the flame quickly burned to the top of the specimen, while the coated RPUF could not be ignited after 2 10-s of flame applications (
Figure 2d). The above results showed that the construction of the hydrogel coating on the RPUF surface made the RPUF more difficult to ignite, prolonging the ignition time, providing time for the personnel to escape, and in fact significantly reducing the fire hazard of the RPUF.
Figure 2. (
a) Schematic diagram of preparation route of m-MXene nanosheets; (
b) UV curing process of coated RPUF, (
c) TTI, pHRR, and THR data of samples in cone test; (
d) video screenshot of pure RPUF and coated RPUF samples during UL-94 testing
[11][30].
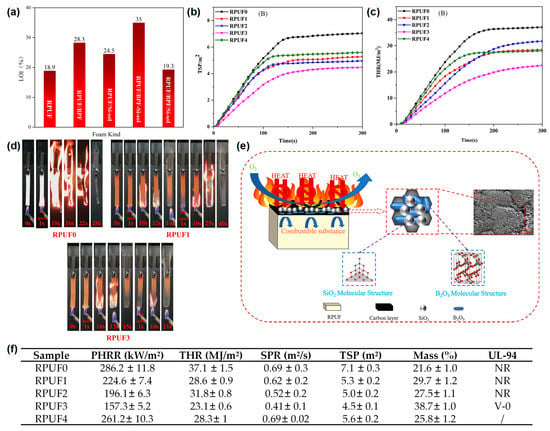
In addition to hydrogels, silica sol (Si-sol) has also been used to prepare flame-retardant coatings for RPUF. Xu and Zhong
[12][31] reported a boron phenolic resin (BPR)/Si-sol coated RPUF with a high flame-retardant efficiency. From the LOI test results, the BPR/Si-sol hybrid coating reached a 35 vol % and was higher than those of RPUF coated with BPR (28.3 vol %) or Si-sol (24.5 vol %), indicating that the synergistic effect exists between BPR and Si-sol exists during the combustion process (
Figure 3a). The RPUFs coated with different BPR and/or Si-sol were designated as RPUF 0–4 in the cone test and the UL-94 test. The peak smoke production rate (p-SPR) and TSP of the RPUF coated with BPR were significantly reduced compared to that of the pure RPUF, indicating that the presence of BPR could effectively inhibit the smoke production in the process of combustion. For the sample of RPUF coated with BPR and Si-sol, the p-SPR and THR were further reduced compared to that of the RPUF coated with BPR, indicating the synergistic effect between BPR and Si-sol on the smoke suppression of RPUF (
Figure 3b,f). The p-HRR and THR of the RPUFs coated with BPR/Si-sol hybrid coating decreased by 45% (from 286.2 kW/m
2 to 157.3 kW/m
2) and 37.7% (from 37.1 MJ/m
2 to 23.1 MJ/m
2), respectively, compared to the pure RPUF (
Figure 3c,f). From the video screenshot during the UL-94 test in
Figure 3d, the pure RPUF was easily ignited as shown in other reports. The sample of RPUF coated with BPR could not be ignited in the first 10 s flame application but burned violently after the second 10 s flame applications, indicating that this sample could not pass any rating in the UL-94 test. When BPR was combined with Si-sol, the sample could quickly self-extinguish after both of the 2 10 s flame applications and could pass the V-0 rating in the UL-94 test, indicating that the BPR/Si-sol hybrid coating could observably prevent flame propagation along the surface of the RPUF. The flame-retardant mechanisms are speculated as follows: (i) BPR can form a char layer on the surface of the RPUF due to the rich benzene ring structure and (ii) the Si-O-Si bond formed from the Si-sol can further improve the high-temperature ablation resistance of the char layer (
Figure 3e).
Figure 3. (
a) The histogram of LOI values of pure RPUF and treated RPUF samples, (
b) THR, and (
c) TSP curves of pure RPUF and treated RPUF samples; (
d) video screenshot of pure RPUF, RPUF coated with BPR, and RPUF coated with BPR/Si-sol during UL-94 test; (
e) schematic diagram of the flame-retardant mechanism of treated RPUF samples; (
f) the data of p-HRR, THR, SPR, TSP, residual mass in cone test, and UL-94 test of pure RPUF and treated RPUF samples
[12][31].
2. Aerogel
The aqueous gels can be converted into aerogels using advanced drying technology, replacing the internal liquid with gas. The aerogel consists of a microporous solid and is made from inorganic compounds or organic polymers
[13][32]. Among them, silica aerogels, an ideal candidate for both thermal insulation and flame-retardant applications, have been extensively studied
[13][14][15][16][17][18][32,46,47,48,49,50]. The unique nanoporous network skeleton of the aerogel confers special properties, such as low density, low thermal conductivity, and small average pore diameter. Therefore, many aerogels have been used as coatings to improve the flame retardancy of organic matrices
[19][20][21][22][22,51,52,53].
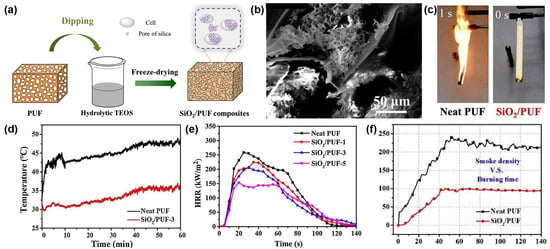
Zhao et al.
[13][32] reported a novel method to prepare silica aerogel on the surface of RPUF. The results show that it significantly improves the flame retardancy and the smoke suppression of RPUF while maintaining the inherent properties. The SiO
2/PUF composites are prepared by a series of processes, as shown in
Figure 4a. The neat RPUF with specific dimensions is dipped in a hydrolysate of tetraethyl orthosilicate (TEOS); during the dipping process, the TEOS can attach to the bubble wall of the RPUF and form a gel in situ. After ageing, the samples are soaked and washed with water and then freeze-dried to obtain the final products. After the freeze-drying process, the silica aerogel with nanopores is formed on the surface of the RPUF with macropores (
Figure 4b). The SiO
2/PUFs with 1 mL, 3 mL, and 5 mL TEOS are named as SiO
2/PUF-1, SiO
2/PUF-2, and SiO
2/PUF-3, respectively. In the UL-94 test, pure RPUF is easily ignited with no rating, while the SiO
2/PUF passes the UL-94 V-0 rating and self-extinguishes immediately after ignition, demonstrating its excellent fire safety (
Figure 4c). Infrared thermal imaging tests are carried out to illustrate the good thermal insulation of SiO
2 aerogel-coated RPUF. Remarkably, the temperatures of the upper surface points of SiO
2/PUF-3 are always much lower than those of pure RPUF for the same heating time, indicating the better thermal insulation property of SiO
2/PUF composites (
Figure 4d). From the cone test, the pure RPUF has the highest p-HRR value (260 kW/m
2), while the p-HRR value of the SiO
2 aerogel coated RPUF gradually decreases with the increase in the SiO
2 aerogel amount, which means that the effective protective barrier effect of silica aerogels can significantly retard the combustion of RPUF (
Figure 4e). From the smoke density curve in
Figure 4f, the specific optical density of SiO
2/PUF is reduced by 55.7%, which may be due to the barrier effect of the SiO
2 aerogel and the SiO
2 aerogel does not produce smoke when heated. The flame-retardant mechanism can be speculated as follows: a SiO
2-rich hybrid char layer forms during the combustion process and acts as a barrier to protect the RPUF matrix from further combustion.
Figure 4. (
a) Preparation route of SiO
2/RPUF composites, (
b) SEM image of SiO
2/RPUF, (
c) digital images of pure and SiO
2 aerogel-coated RPUF, (
d) temperature vs. time curves of the upper surface points in thermal conductivity test, (
e) HRR in cone test of pure PUF and SiO
2 aerogel-coated RPUF, and (
f) specific density of neat and SiO
2 aerogel-coated RPUF in smoke chamber test
[13][32].
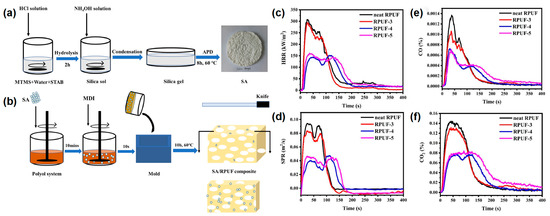
Yan et al.
[15][47] also reported a type of castor oil-based RPUF coated with silica aerogel by the sol-gel method of methyltrimethoxysilane (MTMS). In their work, the silica aerogel was successfully prepared by the sol-gel method of MTMS under the atmospheric drying process, as shown in
Figure 5a. Two types of silica aerogel/SA/RPUF composites were prepared by adding SA during the foaming process of RPUF and by coating SA on the RPUF surface, respectively (
Figure 5b). The RPUF with 0 wt% and 15 wt% SA powder were named as neat RPUF and RPUF-3. The dipped and dried neat RPUF and RPUF-3 were named as RPUF-4 and RPUF-5, respectively. From the cone test, the first and second p-HRR values of RPUF-4 were reduced by 52.43% and 40.78%, respectively, compared to the pure RPUF (
Figure 5c). The first and second p-SPR were reduced by 56.86% and 47.82%, respectively. The RPUF-4 and RPUF-5 showed a remarkable decrease in the first and second p-SRP values compared to those of the neat RPUF and RPUF-3 (
Figure 5d). The average CO production of RPUF-3, RPUF-4, and RPUF-5 decreased from 0.0029 kg/kg of pure RPUF to 2.3 g/kg, 1.5 g/kg, and 1.7 g/kg, respectively. (
Figure 5e). The average CO
2 production of RPUF-3, RPUF-4, and RPUF-5 decreased from 0.27 kg/kg of pure RPUF to 0.25 kg/kg, 0.16 kg/kg, and 0.19 kg/kg, respectively (
Figure 5f). From the above analysis, it can be seen that the formation of a silica aerogel layer on the surface of RPUF significantly improved the fire safety of RPUF and had a great application potential for improving the fire safety of the building insulation materials.
Figure 5. (
a) The schematic diagram of the SA manufacturing process; (
b) the preparation route of SA-coated RPUF, (
c) HRR, (
d) SPR, (
e) CO, and (
f) CO
2 curves in the cone test of pure RPUF and SA-coated RPUF
[15][47].
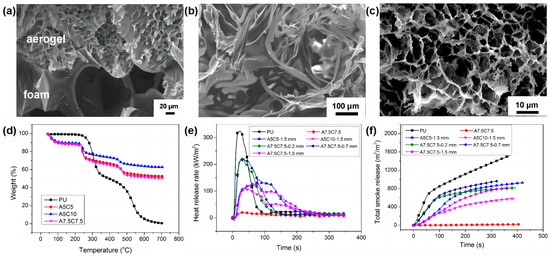
In addition to the silica aerogel, organic-inorganic aerogel has also been reported as a flame-retardant coating for RPUF. Chen and co-workers
[18][50] reported the flame-retardant application of the alginate/clay aerogel coating on the surface of RPUF by the freeze-drying method. In the preparation route of PU foam coated with alginate/clay aerogel coatings, the alginate/clay suspensions were coated on the surface of RPUF and then frozen and dried using a freeze-dryer. The alginate/clay aerogel with different thicknesses and different alginate and clay contents can be formed on the surface of the RPUF.
Figure 6a shows that the pore sizes of the aerogel and the RPUF were very different. The pore sizes of the RPUF closed cells were several hundred microns, while the pore sizes of the aerogel were 10–30 μm. The thermal stability of the RPUF matrix and the alginate/clay aerogel coating were very different (
Figure 6d). Pure RPUF is a highly flammable material due to its LOI value of only 17 vol %, whereas the alginate/clay aerogel A7.5C7.5 can self-extinguish even in a pure oxygen atmosphere. With the increasing of the coating thickness and clay content, the LOI of the RPUF coated with alginate/clay aerogel gradually increased. The sample of RPUF coated with 1.5 mm of A7.5C7.5 could self-extinguish even in a pure oxygen atmosphere, indicating that the aerogel provides an excellent protection against the flame. From the SEM micrograph of the char residue of RPUF and aerogel (
Figure 6b,c), it can be seen that the shape of the char residue of the RPUF shrinks, while the shape of the aerogel remains after burning in the LOI test. The unaltered aerogel shell protects the RPUF from further combustion, resulting in self-extinguishing even in an atmosphere with a very high oxygen concentration. From the cone results, the alginate/clay aerogel-coated RPUF significantly reduced the p-HRR and TSR compared to those of pure RPUF (
Figure 6e,f), indicating that the alginate/clay aerogel coating had a very good fireproofing effect on RPUF. The above results indicate that this simple and inexpensive method has important practical significance for imparting higher fire safety to RPUF and has a potential application value.
Figure 6. SEM micrograph of (
a) RPUF/aerogel interface; Char residue of (
b) RPUF and (
c) aerogel after LOI test; (
d) TGA curves of pure RPUF and alginate/clay aerogels with different ratios; (
e) HRR, and (
f) TSR curves of samples in cone test
[18][50].
3. Ceramic
The flame-retardant mechanism can be either in the vapor phase or in the condensed phase. In the condensed phase, flame retardants accelerate the degradation of the polymer and form an insulating dense char layer on the polymer surface
[23][54]. The dense layers reduce the heat conduction, block the entry of oxygen, and reduce the concentration of combustible gas and the generation of consistent toxic smoke, thus protecting the RPUF
[24][55].
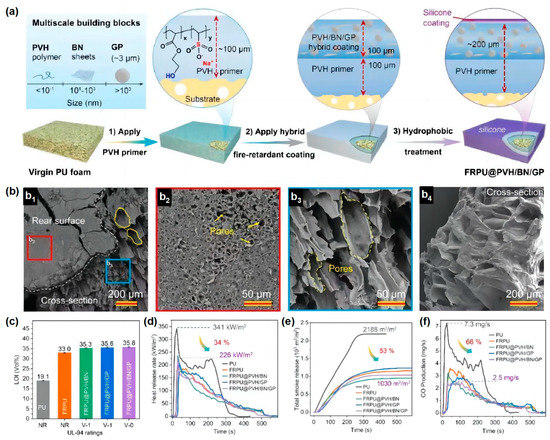
Similarly, inspired by the flame resistant, high-temperature flowable and low thermal conductivity properties of volcanic lava, a ceramizable multi-scale flame-retardant coating material has been developed by Song and co-workers to provide effective fire protection for many building materials
[25][36]. They prepared a multi-scale organic/inorganic hybrid PVH/BN/GP flame-retardant coating material using aqueous copolymer poly (hydroxyethyl acrylate sodium vinyl sulfonate) (PVH) as the coating matrix, introducing a low melting point glass powder (GP) as the ceramic precursor and boron nitride (BN) as the synergist. PVH (~100 μm) and PVH/BN/GP (~100 μm) were deposited on the substrate surface (
Figure 7a). The coating itself is highly flame retardant and can provide ideal fire protection for a wide range of base materials. At temperatures above 350 °C, the GP particles in the hybrid coating softened and eventually melted completely at high temperatures (>650 °C) to form a flowing melt. The GP melt can be used as a high-temperature adhesive to fill in the macroscopic cracks on the surface of the organic char layer, eventually forming a dense and complete ceramic protective layer (
Figure 7b). In addition, the thermal degradation of the organic PVH coating substrate can promote the formation of pores in the char layer, and its porous structure is very similar to the structure of volcanic lava, giving the char layer a thermal conductivity as low as 0.0897 W/m·K at 700 °C. The RPUF treated with ~200 μm coating showed a maximum LOI of 35.8 vol % and achieved the ideal V-0 rating in the UL-94 test. The total smoke release (TSR) and peak CO production (PCOP) were significantly reduced by 53% and 66%, respectively, while the compressive strength significantly increased by 41% (
Figure 7c–f). The overall performance of the material was superior to that of RPUF reported in the previous literature. This work provided a novel strategy for the production of an economical flame-retardant coating for the combustible substrate where fire protection is required.
Figure 7. (
a) The scheme of the manufacturing process of FRPU@PVH/BN/GP; (
b) porous structure of char layer; (
c) LOI value of neat RPUF and coated RPUF; (
d) HRR, (
e) TSR, and (
f) CO curves in cone calorimetry test
[25][36].