Intraocular pressure (IOP) is an important measurement that needs to be taken during ophthalmic examinations, especially in ocular hypertension subjects, glaucoma patients, and patients with risk factors for developing glaucoma. The gold standard technique in measuring IOP is still Goldmann applanation tonometry (GAT); however, this procedure requires local anesthetics, can be difficult in patients with scarce compliance, surgical patients, and children, and is influenced by several corneal parameters. Numerous tonometers have been proposed in the past to address the problems related to GAT. The authoresearch describess review the various devices currently in use for the measurement of intraocular pressure (IOP), highlighting the main advantages and limits of the various tools. The continuous monitoring of IOP, which is still under evaluation, will be an important step for a more complete and reliable management of patients affected by glaucoma.
1. Introduction
Intraocular pressure (IOP) is an important measurement, which should be taken in every patient over the age of 40 that undergoes a complete ophthalmic examination and in all patients with ocular hypertension (OHT) or with risk factors for developing primary open-angle glaucoma (POAG) (i.e., family history, myopia, increased cup-to-disc ratio, etc.). IOP measurement is obviously a fundamental tool in subjects with diagnosed ocular hypertension or glaucoma. Even if the IOP measurement in vivo is only an estimate of the true IOP (which is only possible with invasive manometry), this value, rightly or wrongly, is often taken as an indicator of the efficacy of any treatment for glaucoma and to assess glaucoma severity and progression in patient management. It is thus of great importance to acquire accurate and precise IOP measurements in clinical practice.
2. Indentation Tonometry
The prototype of the indentation tonometers is the Schiøtz tonometer that was introduced many years ago
[1][4] and is no longer currently used (
Figure 1).
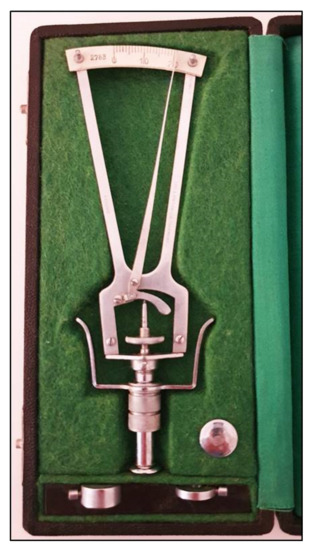
Figure 1.
Schiøtz tonometer with different weights.
Using this instrument, the cornea is indented by a plunger loaded with different weights. The IOP is based on the depth of indentation. The values are shown on a scale ranging from 0 to 20 units, in which the protrusion of the plunger of 0.05 mm represents each unit of measurement. The value indicated on the handle needs to be converted in mmHg using a conversion scale. The coefficient of ocular rigidity, which can differ amongst eyes, should be taken into consideration to obtain corrected measurements of IOP. The Schiøtz tonometer is a simple and relatively inexpensive instrument. It is still sometimes used in developing countries
[2][3][5,6] and in children under general anesthesia
[4][7]. This tonometer, however, is subject to several sources of error, which include improper positioning on the eye, defective or dirty instruments, high variability in comparison with other devices and measurements influenced by individual ocular rigidity
[5][8]. Moreover, patients must be in a supine position when taking measurements with this tonometer.
3. Applanation Tonometry
Applanation tonometers are currently considered the most reliable instruments for an accurate IOP measurement. Such tonometers use the Imbert–Fick law: P = F/S, in which P is pressure, S represents the surface of the flattened area, and F is the force needed to flatten a fixed corneal area. Apart from the tonometer by Maklakoff and several other instruments that are no longer currently in use, in which the force is provided by the weight of the tonometer itself, applanation tonometry is based on the area of flattened cornea that is calculated and converted in mmHg
[6][2]. In almost all instruments of this type, the F value is varied to get the proper corneal applanation for a predetermined area. The Goldmann applanation tonometer (GAT) was first invented in 1948 by Hans Goldmann
[7][9] and is still considered the gold standard to date. The tonometer needs to be positioned on a slit lamp.
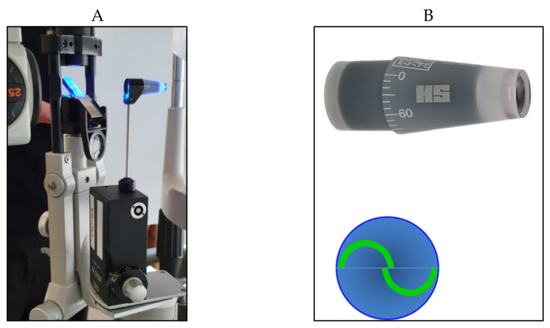
A truncated cone, with a 7.35 mm
2 surface area and a dimeter of 3.06 mm, illuminated by a blue light, is pushed on the center of the anaesthetized cornea. A doubling prism embedded in the cone divides the circular meniscus on the surface of the flattened cornea e into two arcs, which need to be aligned in order to obtain a precise and standardized applanation (
Figure 2).
Figure 2. Goldmann applanation tonometer positioned on the slit lamp (A) with its cone prism (B) (on the top right); the two arcs appear correctly aligned (B) (on the bottom right).
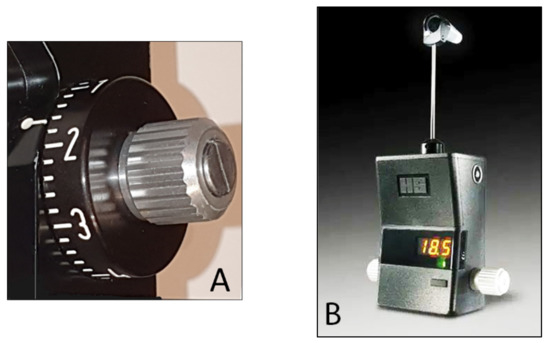
The force used needed to flatten the corresponding surface of the cornea is directly proportional to the IOP, expressed in mmHg that can be directly read in the scale of the measuring drum or in the posterior window for the digital version (
Figure 3A,B).
Figure 3.
(
A
) Scale with IOP values in the Goldmann tonometer; (
B
) digital Goldmann tonometer (posterior view).
4. Non-Contact Tonometry (Air-Puff Tonometry)
Non-contact tonometry (NCT) was first designed by Zeiss and developed by Grolman in 1972
[8][33]. Several models have been proposed in the past few decades that use a pulse of air to flatten the cornea without the need for touching the eye (
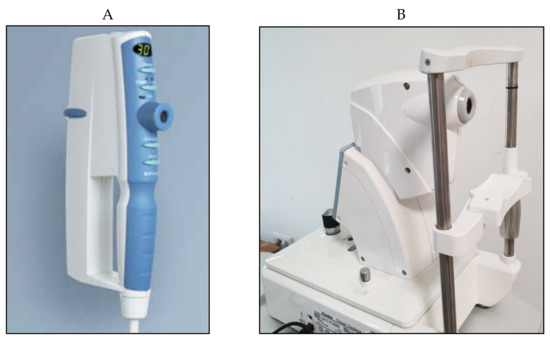
Figure 46); such models, therefore, do not require anesthesia or fluorescein drops. In the Pulsair tonometer, a light beam is used in combination with a sensor that stops the production of air and measures the force used at the moment of corneal flattening.
Figure 46.
Pulsair EasyEye handheld (A
) and Pulsair desktop (B
) non-contact tonometers.
Numerous studies have examined the differences in IOP measured with various types of NCT instruments and other non-conventional tonometers compared to GAT
[9][10][34,35]. Demirci et al. showed that IOP measurements with NCT were significantly higher than those obtained with both GAT and rebound tonometry, with significant differences (
p < 0.001) in all age groups
[11][36]. A recent study confirmed that NCT tends to overestimate IOP GAT measurements in patients with IOP > 16 mmHg, which was more evident when IOP > 20 mmHg
[12][37], showing a decrease in accuracy at higher values.
NCT could be helpful in a day-to-day clinical setting that involves dealing mostly with normal patients undergoing routine checkups. This type of tonometry can be ideal as a screening tool, which can easily be performed by non-medical staff. Although studies have shown that NCT tends to overestimate GAT measurements, NCT can prove to be useful for post-operative patients with lid edema, limited collaboration, ocular pain, discomfort and increased tear film meniscus size, which are all factors that influence proper GAT measurements. NCT can be a useful screening tool, but should never replace or be interchanged with GAT, especially in the management of patients with risk factors, ocular hypertension, suspect patients and glaucoma.
Other types of non-contact tonometers, with new interesting features, have recently been introduced. In addition to the traditional tonometers, these devices show IOP values that take CCT and corneal biomechanics into account, claiming to provide more accurate IOP measurements
[13][14][19,46].
This instrument provides IOP measurements based on the indentation principle, in addition to pachymetry taken by on optical device and other biomechanical parameters of the cornea obtained by registering the surface deformation due to an applied air pulse, similar to an ORA device. A Scheimpflug camera visualizes an 8.5 mm diameter of the center of the surface of the cornea and precisely records the corneal deformation induced by the air-jet and its return to its normal shape with a high resolution and more than 4300 frames per second. A biomechanically corrected IOP value (bIOP), which takes the individual corneal deformation parameters into account, is also provided by the device.
The Corvis ST precision for the CCT and IOP values has been shown to be excellent; however, it is moderate for the corneal deformation parameters
[15][16][17][62,63,64]. Previous studies demonstrated that Corvis ST tends to underestimate IOP readings obtained with GAT
[15][18][19][20][61,62,65,66]. The Corvis ST biomechanically corrected IOP values (bIOP) have been shown to be less influenced by the CCT and corneal biomechanics and to be more effective in measuring the IOP in subjects who underwent refractive surgery
[21][67]. Moreover, the Corvis ST corneal deformation parameters have been shown to be effective in discriminating between normal and keratoconic eyes
[22][68].
5. Pneumotonometry
Pneumotonometers are devices based on the applanation principle, which use a different technology
[23][24][69,70]: the tonometer probe consists of a hollow central tube flanked by a side exhaust, and the sensor is air pressure, which is dependent on the resistance of the exhaust. During the cornea applanation, the pressure within the central tubes increases to match the force generated by the IOP. A pneumatic electronic transducer converts the air pressure to a tracer on a strip of paper (
Figure 59).
Figure 59.
Pneumotonometer.
In several studies, pneumotonometry proved to be quite accurate and reliable in glaucoma screening and showed a greater reliability compared to GAT after PRK and LASIK
[25][26][27][71,72,73]. Pneumotonometers such as the Pulsatile Ocular Blood Flow (OBF,
Figure 610) have been used in the past to measure the pulse fluctuation and thereby give indirect information regarding the ocular blood pulse
[28][29][30][31][74,75,76,77]. OBF measurements, however, appear to be more influenced by CCT and more variable than GAT readings, with a significant overestimation
[32][33][34][78,79,80]. The clinical usefulness of this instrument in clinics still remains controversial.
Figure 610.
Langham Ocular Blood Flow pneumotonometer.
6. Rebound Tonometry
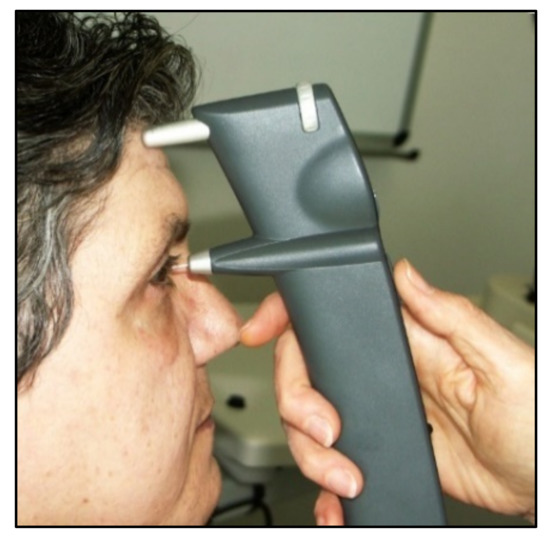
From a clinical point of view, the iCare rebound tonometer, introduced in 2000 by Kontiola
[35][81], is currently one of the most interesting and widespread instruments used in practice (
Figure 711).
Figure 711.
iCare rebound tonometer.
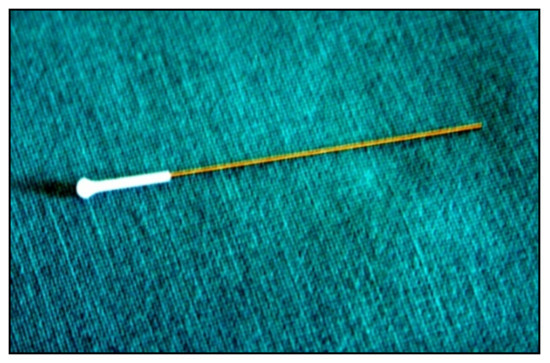
A subtle probe impacts onto the cornea and then rebounds from the eye with a different velocity, which varies according to the IOP (
Figure 812).
Figure 812.
Disposable iCare probe.
The movement of the probe causes a voltage in the internal solenoid that is then amplified and digitally changed by a microprocessor. The IOP value is averaged from six consecutive measurements. The reliability of the final value is also displayed. The iCare tonometer is a reliable and precise instrument. It is rapid and easy to use, which is particularly helpful in busy clinics and with children, considering that there is no need for topic anesthesia
[36][37][38][39][82,83,84,85]. The small surface contact makes it suitable to measure IOP after keratoplasty and in damaged corneas
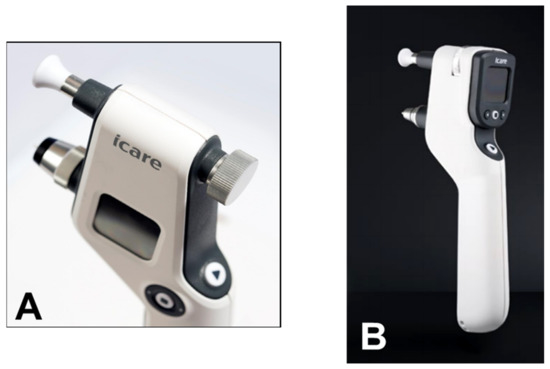
[39][40][85,86]. The iCare PRO version released in 2011 uses a shorter probe, which can also be used to measure IOP in a supine position. The most recent versions of this instrument, which are updated versions of the iCare PRO with a long probe (iCare IC100 and IC200) (
Figure 913A,B), provide new features, such as a red or green light to show if the position of the probe is correct, in addition to providing the possibility of measuring IOP in a supine position
[41][42][87,88].
Figure 913.
(A
) iCare 100; (B
) iCare 200 version.
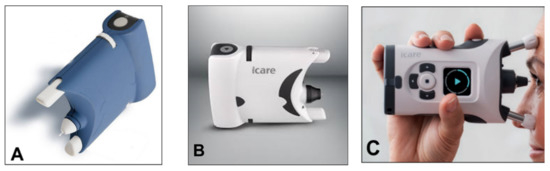
A simplified version (iCare One, replaced at first by the iCare Home and, recently, by the iCare Home2 (
Figure 104A–C)), which can autonomously be used by patients, has recently been introduced for at-home auto-tonometry. It can be helpful for detecting IOP peaks, especially in suspect glaucoma and in normal tension glaucoma subjects, when IOP measurements appear to be normal during office hours
[43][44][45][46][89,90,91,92].
Figure 104.
(A
) iCare One; (B
) iCare Home; (C
) iCare Home2.
Numerous studies have compared the different versions of iCare with GAT and other non-conventional tonometers. When compared to gold standard GAT, clinical results report a good correlation of tonometry readings, with r values greater than 0.8 for low-to-moderate GAT readings
[47][93]. A recent study showed agreement between GAT readings and iCare to be good, with a <2 mmHg mean difference for all ranges of IOP
[41][87]. For IOP > 23 mmHg, rebound tonometry tends to underestimate IOP compared to GAT, showing readings that are significantly lower
[47][93].
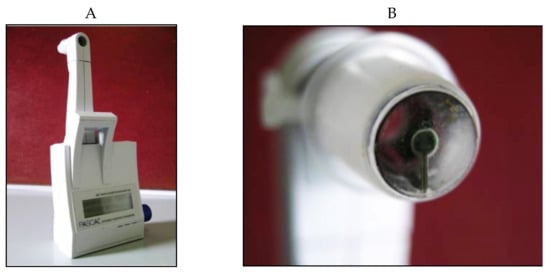
7. Dynamic Contour Tonometry
The Dynamic Contour Tonometer (PASCAL, DCT) (SMT Swiss Microtechnology AG, Port, Switzerland) is a relatively new device developed by Kaufmann et al. in 2003
[48][105] and implemented by Kanngiesser et al. in 2005
[49][106]. The DCT, which is not based on the applanation principle, calculates the IOP using the Pascal principle, according to which the pressure change is applied to all parts of a fluid in a contained enclosed space. The tonometer is positioned on the slit-lamp, requires the use of anesthetic drops (no fluorescein) and is automatically calibrated. It uses a concave contour tip that is equipped with a tiny sensor in the center of the contact surface (
Figure 115).
Figure 115.
Dynamic Contour Pascal (A
) with its sensor tip (B
).
8. Applanation Resonance Tonometry
The Applanation Resonance Tonometer (ART), known in the current commercial version as BioResonator ART (BioResonator AB, Umea, Sweden) (
Figure 126), was developed by Eklund et al. in 2003
[50][120]. It was released as both a manual and automatic version in 2012
[51][121]. This tonometer uses the applanation tonometry principle combined with the resonance technique. The device needs must be mounted on a slit lamp, requires the use of local anesthetic drops before IOP measurement and uses a concave surface sensor tip, which is positioned on the cornea. The sensor tip is manually pushed towards the cornea in the manual version of the instrument, whereas the automatic version provides a tiny motor for movement of the tip. A resonance piezoelectric device is found in the tip of the sensor that generates a shift in frequency which is proportional to the area of contact. The IOP is based on the contact area and force measurement parameters, which are taken continuously throughout the test
[50][51][120,121].
Figure 126.
The BioResonator ART tonometer.
The ART probe must be carefully disinfected before each subject. This tonometer is self-calibrated and gives the repeated IOP measurement median and a quality index reflecting the standard deviation of the IOP values. The IOP measurement provided by the BioResonator ART is claimed to be more accurate than that of GAT considering that it represents the median of repeated measures; however, the precision of the instrument has been questioned
[52][53][54][122,123,124].
9. Continuous IOP Monitoring
All the aforementioned devices can be usefully employed for taking spot IOP measurements during office time. This can be acceptable in a screening setting, but, unfortunately, undetected elevated IOP spikes tend to occur during the night in many glaucomatous patients
[55][127]. IOP readings during clinical office hours fail to detect these peaks in more than 50% of cases with a significant underestimation of IOP
[56][57][58][59][128,129,130,131]. Hughes et al. reported that data obtained with continuous monitoring in IOP using a 24 h device had an influence in therapeutic decisions in 79.3% of enrolled subjects
[60][132]. Keeping these data in mind, it can be inferred that our current standards in clinics with regard to taking IOP measurements may not suffice and thus need to be modified
[61][133].
An important step towards a more precise management of patients affected by ocular hypertension and chronic glaucoma would be the possibility of continuously monitoring IOP values not only during the day but also in the night, as occurs with the 24 h blood pressure Holter. This information could be particularly useful in the so-called normal tension glaucoma patients, which show significant damage progression despite an apparently normalized IOP. In these cases, an elevated IOP can sometimes be found during the night, especially early in the morning, outside office hours
[62][63][134,135]. A number of devices, most of them only experimental, have been proposed for this purpose over the past 20 years
[64][65][66][136,137,138]. Some of them need to be surgically inserted into the eye, either during a cataract extraction procedure, usually embedded in an intraocular lens
[67][68][69][139,140,141], or positioned in the anterior chamber
[70][71][142,143], or in the suprachoroidal space
[72][144]. A non-invasive continuous IOP measurement is also possible using special contact lenses with different types of miniaturized sensors and a wireless power transmission of data to a recorder.
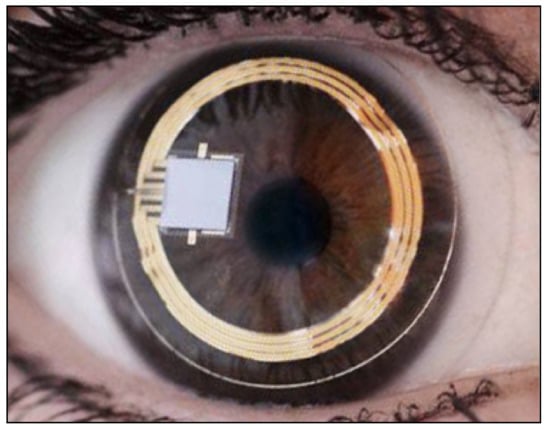
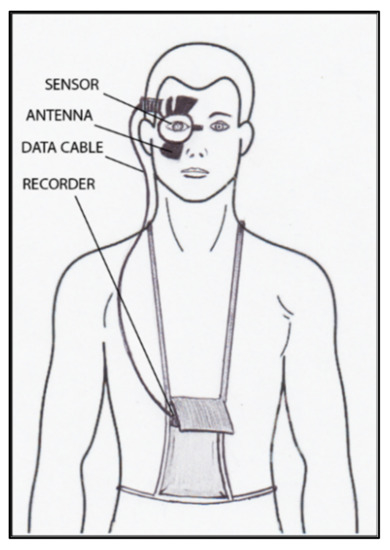
The contact lens sensor Sensimed Triggerfish (Triggerfish CLS, Sensimed AG, Lausanne, Switzerland) is a miniaturized electromechanical system with a microprocessor embedded in a disposable silicon contact lens, which transmits a signal to an external wireless antenna located in the periocular surface (
Figure 137 and
Figure 148). The data are then transferred to a portable recorder, for a total of 288 data sets in 24 h. This device can measure small modifications in the curvature of the cornea believed to be due to variations of IOP
[73][74][75][76][77][145,146,147,148,149].
Figure 137.
Sensimed Triggerfish.
Figure 148.
Schematic view of Triggerfish, wireless antenna and portable recorder.
Triggerfish CLS is usually well tolerated
[78][79][80][150,151,152] and has also been shown to have high reproducibility
[78][79][80][81][82][83][150,151,152,153,154,155]. The information obtained with these device parameters might be useful in assessing changes and IOP fluctuations in subjects with pseudoexfoliation syndrome, pigment dispersion, and in predicting the visual field loss progression rate
[84][85][156,157]. Several studies have shown the usefulness of this contact lens sensor for assessment of the risk of glaucoma, which may prove to be important in subjects with NTG, in which IOP tends to be normal with diurnal readings
[86][87][88][89][158,159,160,161].
The main problem with this device is that there is no direct correlation between corneal changes, expressed in millivolt equivalent (mVeq), and IOP values. Studies have shown that IOP measurements taken with GAT and Triggerfish values tend to have a high correlation at the beginning, after the insertion of CLS
[76][148]; however, the correlation becomes poor after 24 h
[81][82][153,154].
CLS is advantageous because it is not invasive, can be easily removed and dismantled
[83][155], readily available
[83][155], accepted and tolerated by patients
[78][79][80][150,151,152], and provides good reproducibility
[79][81][82][151,153,154]. The validity (i.e., considering the estimation accuracy of IOP readings) and relatively costly equipment of CLS are important drawbacks, which render the clinical usefulness of this instrument still debatable in literature
[81][82][153,154].
Other types of devices able to measure IOP, either implantable or non-invasive
[90][91][92][93][94][95][96][97][98][99][100][101][162,163,164,165,166,167,168,169,170,171,172,173], have been proposed, but almost all are still experimental and need further studies before being introduced into clinical practice.