Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 1 by César Ozuna.
Volatile organic compounds (VOCs) are promising alternatives to synthetic pesticides in pest and disease management. VOCs are gaining interest due to the various advantages of their application, such as the reduction in residuals in the environment and their ease of application in different agricultural systems.
- defense
- plant
- volatiles
1. General Aspects of Volatile OCrganic Compounds and Possible Biotechnological Applications
In nature, volatile organic VOCscompounds (VOCs) are emitted by all living organisms and occur as a complex mixture called “volatilome” [15][1]. For years, VOCs were considered non-essential to the functioning of the organisms that produced them. However, in the last decades, the scientific community has elucidated the important role of VOCs at the ecosystem level because they mediate intra- and interspecific interactions among all organisms [16][2]. VOCs typically occur as a complex mixture produced by four major metabolic pathways, namely the shikimate/phenylalanine, the mevalonic acid (MVA), the methylerythritol phosphate (MEP), and lipoxygenase (LOX) pathways [17,18][3][4] (Figure 1).
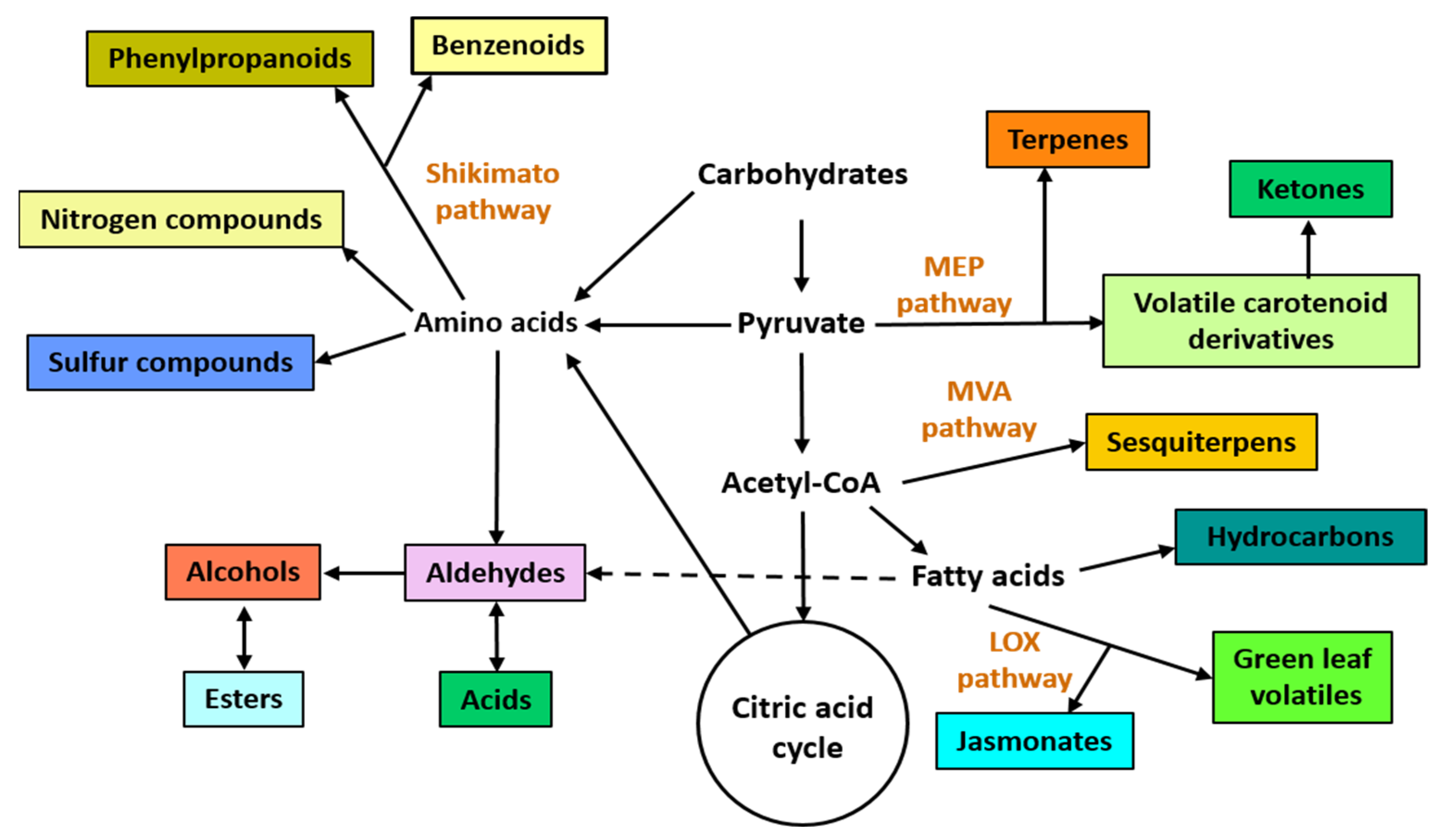
Figure 1. Overview of main biosynthetic pathways to produce plant and microbial volatile organic compounds. Different chemical classes of VOCs are depicted in colored rectangles. The four principal biosynthetic pathways are: the shikimate, the methylerythritol phosphate (MEP), the mevalonic acid (MVA), and the lipoxygenase (LOX) pathways.
Different studies demonstrated that VOCs modulate (suppress or stimulate) microbial and plant growth [13,19][5][6], induce systemic resistance in plants against biotic and abiotic stresses [20][7], and act as attractants or repellents of insects [16][2]. For these reasons, developing effective VOCs formulations for their biotechnological application in the field could facilitate the emergence of strategies for sustainable plant disease and pest control and productivity improvement [21][8]. However, wresearchers must consider that VOC emissions’ composition and quantity can be affected by different factors. For example, VOC emissions in bacteria and fungi depend on microbial taxa, life stage, growth phase, substrate type, and temperature [22][9]. For plants, high temperatures, high light intensities, and herbivore attacks increase VOC emissions [23][10]. This issue could be solved by using pure volatiles, thus improving reproducibility. However, the high vapor pressure at which VOCs would have to be stored and their high diffusion rate make them unstable, shortening their helpful half-life under normal conditions [24][11]. These characteristics, as well as the long-term exposure needed to obtain the beneficial effects of VOCs, are the main challenges for the production of VOC formulations [25][12].
2. Volatile OCrganic Compounds as Inductors of Resistance in Plants against Abiotic and Biotic Stress
Biotic and abiotic stresses are the two main factors that affect crop production [107][13], causing losses to approximately 25% and 50% of the world’s crop production, respectively [108,109,110][14][15][16]. The various biotic agents (viruses, bacteria, fungi, nematodes, weeds, insects, and arachnids) and abiotic factors (extreme temperatures, drought, salinity, and heavy metals) can deprive the plants of nutrients, limit growth, and lead to their death, thus reducing and limiting crop productivity and agriculture sustainability worldwide [108,109][14][15]. Moreover, factors such as pests’ resistance to pesticides, the emergence of new insect pests and diseases, and the loss of soil fertility, among others, improve the severity of crop loss and favor pest infestations and diseases [111][17]. To defend against these stresses, plants synthesize secondary metabolites that act directly by acting on the pathogen or indirectly by inducing the necessary defensive or resistance/tolerance response of the plant [112][18]. These secondary metabolites include VOCs, which play different roles in the defense against biotic stresses and the resistance/tolerance to abiotic stresses; therefore, they have received particular attention because they constitute one of the most promising alternatives for pest and disease management preharvest [112,113][18][19]. Different trials have demonstrated that specific single VOCs and mixtures of VOCs can induce a defense response in plants against pathogens [114[20][21][22],115,116], nematodes [117][23], insects [114][20], and viruses [118[24][25][26],119,120], which allows preparations to start beforehand and be present when at risk of attack [121][27]. In addition, some VOCs can attract beneficial insects, such as predatory arthropods and parasitoids (an organism whose larvae feed and develop inside or on the body surface of another organism), that serve as a defense against herbivores and weeds [71][28] (Figure 2). Indeed, various studies demonstrated the efficacy of VOCs in attracting beneficial insects such as parasitoids wasps [91[29][30][31],94,101], lady beetles [93][32], hoverflies, predatory mites [95][33], and lacewing larvae [93][32], among others. Similarly, VOCs are capable of inducing systemic resistance/tolerance to different abiotic stresses such as drought [122[34][35][36],123,124], cold [124[36][37],125], and salinity [126][38] (Table 1).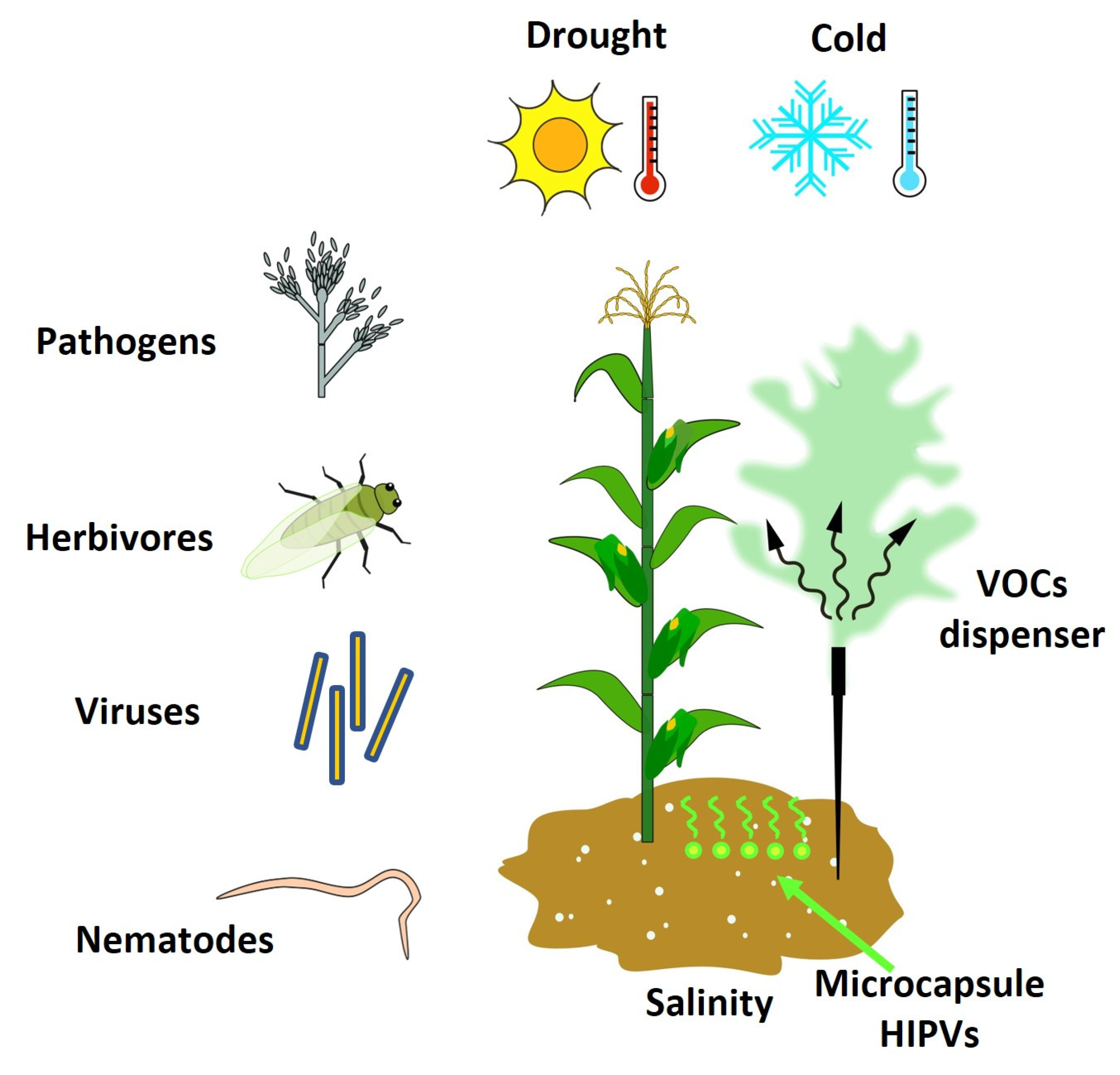
Figure 2. The HIPVs as inductors of resistance against biotic and abiotic stresses.
The herbivore-induced plant volatiles (HIPVs) as inductors of resistance against biotic and abiotic stresses.
Table 1.
Volatile organic compounds with application in biocontrol against biotic and abiotic stress.
| Volatile Compound | Organism Target | Effect | Crop | Reference |
|---|---|---|---|---|
| Dimethyl disulfide, methyl isovalerate, 2-undecanone |
Nematode (Meloidogyne incognita Kofoid and White) |
Induce defense response and growth promotion |
Tomato (Solanum lycopersicum L.) |
[117][23] |
| (E)-nerolidol | Leafhopper (Empoasca onukii Matsuda), Fungus (Colletotrichum fructicola Prihast et al.) |
Induce defense response |
Tea plant (Camellia sinensis L.) |
[114][20] |
| Z-3-hexenol | Tomato yellow leaf curl virus |
Induces defense response |
Tomato (Solanum lycopersicum L.) |
[118][24] |
| 2R,3R-butanediol, 2R,3S- butanediol | Cucumber mosaic virus, Tobacco mosaic virus |
Induce defense response |
Pepper (Capsicum annum L. cv. Bukwang) |
[119][25] |
| 6-pentyl-α-pyrone (6PP) |
Tobacco mosaic virus | Induces systemic resistance | Tobacco (Nicotiana tabacum cv. White Burley) |
[120][26] |
| Dimethyl disulfide (DMDS) |
Fungus (Sclerotinia minor Jagger) |
Induces systemic resistance | Tomato (Solanum lycopersicum L.) |
[115][21] |
| Nonanal, limonene |
Fungus (Colletotrichum lindemuthianum Sacc. and Magnus) |
Induce systemic resistance | Common bean (Phaseolus vulgaris L. Sp. Pl.) |
[116][22] |
| Dimethyl disulfide, 2,3-butanediol, 2-pentylfuran |
Induces systemic drought tolerance |
Maize (Zea mays L.) |
[122][34] | |
| (Z)-3-hexen-1-yl acetate | Induces tolerance against cold stress |
Maize (Zea mays L.) |
[125][37] | |
| Induces drought resistance |
Wheat (Triticum spp. L.) |
[123][35] | ||
| Protects against salinity stress |
Peanut Arachis hypogaea L.) |
[126][38] | ||
| Eugenol | Induces cold and drought tolerance |
Tea plant (Camellia sinensis L.) |
[124][36] | |
3. Application of Volatile OCrganic Compounds in Agricultural Systems
Currently, alternatives that exploit the potential of VOCs in agricultural systems have been increasing, such as dispensers for the application of single or a mixture of VOCs, as well as the use of genetically modified (GM) crops with altered VOC emissions. Recent studies have demonstrated the success of HIPVs in the biocontrol of pests, for example, the continuous application of (Z)-3-hexenyl propanoate ((Z)-3-HP) by a polymeric dispenser in tomato plants in commercial greenhouse conditions. These dispensers maintained the defenses of commercial tomato plants activated for over two months, reducing the attack of economically significant tomato pests Tetranychus urticae and Tuta absoluta without lowering productivity. The induction of tomato plants with (Z)-3-HP increased the production of fatty acids, the activation of the lipoxygenase pathway, the accumulation of specific defense compounds, and the upregulation of genes involved in the antiherbivore defense [150][48]. Another case is the use of HIPV (sabinene, n-heptanal, α-pinene, and (Z)-3-hexenyl acetate) dispensers to attract the Cotesia vestalis larval parasitoid to control the diamondback moth (DBM) (Plutella xylostella) larvae, which are an important pest of cruciferous crops in greenhouses. The dispensers successfully attracted C. vestalis and honey feeders, which reduced the presence of DBM in the greenhouse [151][49]. Similar results were shown with the dispenser application of β-caryophyllene and β- myrcene which enhanced the attraction of the parasitic wasp Encarsia formosa, resulting in the feeding of Bemicia tabaci adults. The use of dispensers enhanced the efficacy of E. formosa as a biological agent to control the B. tabaci pest in glasshouse production systems [152][50]. Limonene applied in the dispenser system acts as a repellent and plant defense elicitor to control the whitefly (Trialeurodes vaporariorum) pest on tomatoes in a commercial glasshouse. In addition, MeSA reduces whitefly population development, elevates peroxidase (POD) activity, and increases the thioredoxin peroxidase (TPX1) and pathogenesis-related protein 1 (PR1) transcripts and both volatiles [153][51]. On the other hand, the use of genetically modified (GM) crops with altered VOC emissions provides enhanced resistance against pests and abiotic stress. The hypersensitive GM crops could be used as an attractant to trap and kill herbivores, as a repellent of herbivores, or as a lure to attract natural enemies [154][52]. For example, the overexpression of the protein OsCYP92C21, which is known to be responsible for homoterpene biosynthesis in rice, enhanced the emission of DMNT and TMTT, which attract the parasitic wasp Cotesia chilonis, the natural enemy of the rice pest striped stemborer Chilo suppressalis [155][53]. In addition, the overexpression of the caryophyllene synthase gene GhTPS1 in cotton enhanced the emission of (E)-β-caryophyllene, which reduces pests, such as Apolygu slucorum, Aphis gossypii, and Helicoverpa armigera, through the attraction of parasitoids, such as Peristenus spretus and Aphidius gifuensis [57][54]. The overexpression of enzymes responsible for the emission of specific volatiles could be an excellent tool to improve pest management. In agricultural systems, GM crops can favor the enhanced resistance to pests and abiotic stresses [85][55]. However, GM crops can also favor the presence of non-target species due to the reduction in chemical pesticides; for example, GM cotton that has been cultivated in China for more than two decades and that promotes the presence of mirid bugs, such as Adelphocoris suturalis, Apolygus lucorum, and Lygus pratensis. These bugs are pests that affect a broad range of important crops including cotton, jujube, and grape [156][56]. Recent studies demonstrated that VOCs obtained from plant extracts such as Allium tuberosum had a significantly higher attractive effect on A. suturalis and A. lucorum; among the volatiles responsible for this effect are diethyl phthalate and methyl levulinate. Therefore, applying these volatile as attractants has a potential to control mirid bugs in agriculture [157,158][57][58].References
- Maffei, M.E.; Gertsch, J.; Appendino, G. Plant Volatiles: Production, Function and Pharmacology. Nat. Prod. Rep. 2011, 28, 1359–1380.
- Veselova, M.A.; Plyuta, V.A.; Khmel, I.A. Volatile Compounds of Bacterial Origin: Structure, Biosynthesis, and Biological Activity. Microbiology 2019, 88, 261–274.
- Kaddes, A.; Fauconnier, M.L.; Jijakli, M.H.; Sassi, K.; Nasraoui, B. Antifungal Properties of Two Volatile Organic Compounds on Barley Pathogens and Introduction to Their Mechanism of Action. Int. J. Environ. Res. Public Health 2019, 16, 2866.
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, Function and Metabolic Engineering of Plant Volatile Organic Compounds. New Phytol. 2013, 198, 16–32.
- Gomes, A.; Queiroz, M.; Pereira, O. Mycofumigation for the Biological Control of Postharvest Diseases in Fruits and Vegetables: A Review. Austin J. Biotechnol. Bioeng. 2015, 2, 1–8.
- Elsherbiny, E.A.; Amin, B.H.; Aleem, B.; Kingsley, K.L.; Bennett, J.W. Trichoderma Volatile Organic Compounds as a Biofumigation Tool against Late Blight Pathogen Phytophthora Infestans in Postharvest Potato Tubers. J. Agric. Food Chem. 2020, 68, 8163–8171.
- Raza, W.; Ling, N.; Yang, L.; Huang, Q.; Shen, Q. Response of Tomato Wilt Pathogen Ralstonia Solanacearum to the Volatile Organic Compounds Produced by a Biocontrol Strain Bacillus Amyloliquefaciens SQR-9. Sci. Rep. 2016, 6, 24856.
- Sharifi, R.; Ryu, C.M. Biogenic Volatile Compounds for Plant Disease Diagnosis and Health Improvement. Plant Pathol. J. 2018, 34, 459–469.
- Misztal, P.K.; Lymperopoulou, D.S.; Adams, R.I.; Scott, R.A.; Lindow, S.E.; Bruns, T.; Taylor, J.W.; Uehling, J.; Bonito, G.; Vilgalys, R.; et al. Emission Factors of Microbial Volatile Organic Compounds from Environmental Bacteria and Fungi. Environ. Sci. Technol. 2018, 52, 8272–8282.
- Holopainen, J.K.; Gershenzon, J. Multiple Stress Factors and the Emission of Plant VOCs. Trends Plant Sci. 2010, 15, 176–184.
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2016, 15, 143–182.
- Xie, X.; Zhang, H.; Paré, P.W. Sustained Growth Promotion in Arabidopsis with Long-Term Exposure to the Beneficial Soil Bacterium Bacillus Subtilis (GB03). Plant Signal. Behav. 2009, 4, 948–953.
- Hashem, A.; Tabassum, B.; Fathi Abd_Allah, E. Bacillus Subtilis: A Plant-Growth Promoting Rhizobacterium that also Impacts Biotic Stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297.
- Gull, A.; Lone, A.A.; Wani, N.U.I. Biotic and Abiotic Stresses in Plants. In Abiotic and Biotic Stress in Plants; Oliveira, A.B., Ed.; IntechOpen: London, UK, 2019; pp. 3–9.
- Pimentel, D.; Peshin, R. (Eds.) Integrated Pest Management: Pesticide Problems; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2014; ISBN 9789400777958.
- Chattopadhyay, C.; Birah, A.; Jalali, B.L. Sustainability in Plant and Crop Protection Natural Resource Management: Ecological Perspectives. In Natural Resource Management: Ecological Perspectives; Peshin, R., Dhawan, A.K., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; pp. 133–146. ISBN 9783319997674.
- López-Gresa, M.P.; Payá, C.; Ozáez, M.; Rodrigo, I.; Conejero, V.; Klee, H.; Bellés, J.M.; Lisón, P. A New Role for Green Leaf Volatile Esters in Tomato Stomatal Defense against Pseudomonas syringe Pv. tomato. Front. Plant Sci. 2018, 871, 1855.
- Pappas, M.L.; Broekgaarden, C.; Broufas, G.D.; Kant, M.R.; Messelink, G.J.; Steppuhn, A.; Wäckers, F.; van Dam, N.M. Induced Plant Defences in Biological Control of Arthropod Pests: A Double-Edged Sword. Pest Manag. Sci. 2017, 73, 1780–1788.
- Chen, S.; Zhang, L.; Cai, X.; Li, X.; Bian, L.; Luo, Z.; Li, Z.; Chen, Z.; Xin, Z. (E)-Nerolidol Is a Volatile Signal That Induces Defenses against Insects and Pathogens in Tea Plants. Hortic. Res. 2020, 7, 52.
- Tyagi, S.; Lee, K.J.; Shukla, P.; Chae, J.C. Dimethyl Disulfide Exerts Antifungal Activity against Sclerotinia Minor by Damaging Its Membrane and Induces Systemic Resistance in Host Plants. Sci. Rep. 2020, 10, 6547.
- Quintana-Rodriguez, E.; Molina-Torres, J.; Ádame-Alvarez, R.-M.; Acosta-Gallegos, J.A.; Heil, M. Plant Volatiles Cause Direct, Induced and Associational Resistance in Common Bean to the Fungal Pathogen Colletotrichum lindemuthianum. J. Ecol. 2015, 103, 250–260.
- Ayaz, M.; Ali, Q.; Farzand, A.; Khan, A.R.; Ling, H.; Gao, X. Nematicidal Volatiles from Bacillus Atrophaeus GBSC56 Promote Growth and Stimulate Induced Systemic Resistance in Tomato against Meloidogyne incognita. Int. J. Mol. Sci. 2021, 22, 5049.
- Su, Q.; Yang, F.; Zhang, Q.; Tong, H.; Hu, Y.; Zhang, X.; Xie, W.; Wang, S.; Wu, Q.; Zhang, Y. Defence Priming in Tomato by the Green Leaf Volatile (Z)-3-Hexenol Reduces Whitefly Transmission of a Plant Virus. Plant Cell Environ. 2020, 43, 2797–2811.
- Kong, H.G.; Shin, T.S.; Kim, T.H.; Ryu, C.M. Stereoisomers of the Bacterial Volatile Compound 2,3-Butanediol Differently Elicit Systemic Defense Responses of Pepper against Multiple Viruses in the Field. Front. Plant Sci. 2018, 9, 90.
- Taha, M.A.; Ismaiel, A.A.; Ahmed, R.M. 6-Pentyl-α-Pyrone from Trichoderma Koningii Induces Systemic Resistance in Tobacco against Tobacco Mosaic Virus. Eur. J. Plant Pathol. 2021, 159, 81–93.
- Heil, M.; Silva Bueno, J.C. Within-Plant Signaling by Volatiles Leads to Induction and Priming of an Indirect Plant Defense in Nature. Proc. Natl. Acad. Sci. USA 2007, 104, 5467–5472.
- Yasmin, H.; Rashid, U.; Hassan, M.N.; Nosheen, A.; Naz, R.; Ilyas, N.; Sajjad, M.; Azmat, A.; Alyemeni, M.N. Volatile Organic Compounds Produced by Pseudomonas Pseudoalcaligenes Alleviated Drought Stress by Modulating Defense System in Maize (Zea mays L.). Physiol. Plant. 2020, 172, 896–911.
- Turlings, T.C.; Ton, J. Exploiting Scents of Distress: The Prospect of Manipulating Herbivore-Induced Plant Odours to Enhance the Control of Agricultural Pests. Curr. Opin. Plant Biol. 2006, 9, 421–427.
- Kang, Z.W.; Liu, F.H.; Zhang, Z.F.; Tian, H.G.; Liu, T.X. Volatile β-Ocimene Can Regulate Developmental Performance of Peach Aphid Myzus Persicae through Activation of Defense Responses in Chinese Cabbage brasasica Pekinensis. Front. Plant Sci. 2018, 9, 708.
- Chen, C.S.; Zhao, C.; Wu, Z.Y.; Liu, G.F.; Yu, X.P.; Zhang, P.J. Whitefly-Induced Tomato Volatiles Mediate Host Habitat Location of the Parasitic Wasp Encarsia formosa, and Enhance Its Efficacy as a Bio-Control Agent. Pest Manag. Sci. 2021, 77, 749–757.
- Ali, M.Y.; Naseem, T.; Zhang, J.; Pan, M.; Zhang, F.; Liu, T.-X. Plant Volatiles and Herbivore Induced Plant Volatiles from Chili pepper Act as Attractant of the Aphid Parasitoid. Plants 2022, 11, 1350.
- Laznik, Ž.; Trdan, S. Are Synthetic Volatiles, Typically Emitted by Insect-Damaged Peach Cultivars, Navigation Signals for Two-Spotted Lady Beetle (Adalia bipunctata L.) and Green Lacewing (Chrysoperla carnea ) Larvae? J. Plant Dis. Prot. 2018, 125, 529–538.
- Salamanca, J.; Souza, B.; Kyryczenko-Roth, V.; Rodriguez-Saona, C. Methyl Salicylate Increases Attraction and Function of Beneficial Arthropods in Cranberries. Insects 2019, 10, 423.
- Li, X.; Ji, Y.; Sheng, Y.; Sheng, L.; Guo, W.; Wang, H.; Zhang, Y. Priming with the Green Leaf Volatile (Z)-3-Hexeny-1-Yl Acetate Enhances Drought Resistance in Wheat Seedlings. Res. Sq. 2021, 10, 785.
- Zhao, M.; Jin, J.; Wang, J.; Gao, T.; Luo, Y.; Jing, T.; Hu, Y.; Pan, Y.; Lu, M.; Schwab, W.; et al. Eugenol Functions as a Signal Mediating Cold and Drought Tolerance via UGT71A59-Mediated Glucosylation in Tea Plants. Plant J. 2022, 109, 1489–1506.
- Cofer, T.M.; Engelberth, M.; Engelberth, J. Green Leaf Volatiles Protect Maize (Zea mays) Seedlings against Damage from Cold Stress. Plant Cell Environ. 2018, 41, 1673–1682.
- Tian, S.; Guo, R.; Zou, X.; Zhang, X.; Yu, X.; Zhan, Y.; Ci, D.; Wang, M.; Wang, Y.; Si, T. Priming with the Green Leaf Volatile (Z)-3-Hexeny-1-Yl Acetate Enhances Salinity Stress Tolerance in Peanut (Arachis hypogaea L.) Seedlings. Front. Plant Sci. 2019, 10, 785.
- Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development. Ann. Bot. 2007, 100, 681–697.
- War, A.R.; Sharma, H.C.; Paulraj, M.G.; War, M.Y.; Ignacimuthu, S. Herbivore Induced Plant Volatiles: Their Role in Plant Defense for Pest Management. Plant Signal. Behav. 2011, 6, 1973–1978.
- Pieterse, M.J.; Does, D.; Zamioudis, C.; Leon-reyes, A.; Wees, S.C.M. Hormonal Modulation of Plant Immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521.
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of Jasmonate and Salicylate Signal Crosstalk. Trends Plant Sci. 2012, 17, 260–270.
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green Leaf Volatiles: A Plant’s Multifunctional Weapon against Herbivores and Pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811.
- Heil, M. Herbivore-Induced Plant Volatiles: Targets, Perception and Unanswered Questions. New Phytol. 2014, 204, 297–306.
- Jing, T.; Qian, X.; Gao, T.; Li, D.; Schwab, W.; Guo, D.; He, F.; Yu, G.; Li, S.; Wan, X.; et al. Herbivore-Induced Volatiles Influence Moth Preference by Increasing the β -Ocimene Emission of Neighbouring Tea Plants. Plant Cell Environ. 2021, 44, 3667–3680.
- Bertini, L.; Proietti, S.; Focaracci, F.; Sabatini, B.; Caruso, C. Epigenetic Control of Defense Genes Following MeJA-Induced Priming in Rice. J. Plant Physiol. 2018, 228, 166–177.
- Martinez-medina, A.; Flors, V.; Heil, M.; Mauch-mani, B.; Pieterse, C.M.J. Recognizing Plant Defense Priming. Trends Plant Sci. 2016, 21, 818–822.
- Boudreau, M.A. Diseases in Intercropping Systems. Annu. Rev. Phytopathol. 2013, 51, 499–519.
- Uefune, M.; Abe, J.; Shiojiri, K.; Urano, S.; Nagasaka, K.; Takabayashi, J. Targeting Diamondback Moths in Greenhouses by Attracting Specific Native Parasitoids with Herbivory-Induced Plant Volatiles. R. Soc. Open Sci. 2020, 7, 20192.
- Conboy, N.J.A.; Mcdaniel, T.; George, D.; Ormerod, A.; Edwards, M.; Donohoe, P.; Gatehouse, A.M.R.; Tosh, C.R. Volatile Organic Compounds as Insect Repellents and Plant Elicitors: An Integrated Pest Management (IPM) Strategy for Glasshouse Whitefly (Trialeurodes vaporariorum). J. Chem. Ecol. 2020, 46, 1090–1104.
- Maurya, A.K.; Pazouki, L.; Frost, C. Plant Seeds Are Primed by Herbivore-Induced Plant Volatiles. bioRxiv 2019, 17, 522839.
- Li, W.; Wang, L.; Zhou, F.; Li, C.; Ma, W.; Chen, H.; Wang, G.; Pickett, J.A.; Zhou, J.-J.; Lin, Y. Overexpression of the Homoterpene Synthase Gene, OsCYP92C21, Increases Emissions of Volatiles Mediating Tritrophic Interactions in Rice. Plant, Cell Environ. 2021, 44, 948–963.
- Lu, Y.; Wu, K.; Jiang, Y.; Xia, B.; Li, P.; Feng, H.; Wyckhuys, K.A.G.; Guo, Y. Mirid Bug Outbreaks in Multiple Crops. Science 2010, 1151, 1151–1154.
- Yin, H.; Li, W.; Xu, M.; Xu, D.; Wan, P. The Olfactory Responses of Mirid Bugs to Six Plant Extracts and Their Volatiles. J. Appl. Entomol. 2021, 145, 125–133.
- Zhang, Y.; Li, T.; Liu, Y.; Li, X.; Zhang, C.; Feng, Z.; Peng, X.; Li, Z.; Qin, S.; Xing, K. Volatile Organic Compounds Produced by Pseudomonas Chlororaphis Subsp. Aureofaciens SPS-41 as Biological Fumigants to Control Ceratocystis Fimbriata in Postharvest Sweet Potatoes. J. Agric. Food Chem. 2019, 67, 3702–3710.
- Maurya, A.K. Application of Plant Volatile Organic Compounds (VOCs) in Agriculture. In New Frontiers in Stress Management for Durable Agriculture; Singh, H.B., Singh, A.K., Singh, U.S., Fraceto, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 369–388. ISBN 9789811513220.
- Murali-Baskaran, R.K.; Mooventhan, P.; Das, D.; Dixit, A.; Sharma, K.C.; Senthil-Nathan, S.; Kaushal, P.; Ghosh, P.K. The Future of Plant Volatile Organic Compounds (PVOCs) Research: Advances and Applications for Sustainable Agriculture. Environ. Exp. Bot. 2022, 200, 104912.
- Blande, J.D.; Holopainen, J.K.; Niinemets, Ü. Plant Volatiles in Polluted Atmospheres: Stress Responses and Signal Degradation. Plant Cell Environ. 2014, 37, 1892–1904.
- McFrederick, Q.S.; Fuentes, J.D.; Roulston, T.; Kathilankal, J.C.; Lerdau, M. Effects of Air Pollution on Biogenic Volatiles and Ecological Interactions. Oecologia 2009, 160, 411–420.
More
