Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by Jessica Roscher.
A reference electrode is a half-cell (an electrode) with a stable, well-defined and highly reproducible electrode potential. A vast number of electrodes have been developed for different applications. They are briefly presented. For the common types, the advantages and drawbacks are discussed. Practical hints for daily use are provided.
- electrode
- reference electrode
- non-polarizable electrode
In electrochemistry, an electronically (n-) conducting material (a metal, graphite, n-doped semiconductor, etc.) or hole-conducting (p-doped semiconductor) material brought into contact with ionically conducting material (an electrolyte solution, an ionic liquid, a solid ion conductor) forms an electrode. At the plane of contact, an electrochemical double layer (sometimes a slightly oversimplified electric double layer) is formed. Because the two phases are immiscible and have vastly different properties strongly related to the mobile charge carriers in both phases at the phase boundary, strong electric fields (with a field strength of up to 109 V·m−1) are established. Upon the initial contact, both phases may not be in thermodynamic equilibrium. Such equilibrium can be established by suitable redox reactions. Some species from the electronically conducting phase are oxidized, leaving negative charges behind, or some species from the ionically conducting phase may be reduced, taking up electrons from the other phase. In both cases (and more options are conceivable), differences in the electrostatic potentials in both phases (the Galvani potential difference) are established. The formation of the electrode potential is the result [1]. This definition of an electrode as being essentially equivalent to an electrochemical half cell is only one option [2]. Another one focuses entirely on the electronically conducting phase (a platinum electrode or a lead electrode), as specified in [3]. For reasons discussed elsewhere [4], this certainly simpler approach (because of shortness and convenience) is less precise, although more popular.
Measurement of a single electrode’s potential (or absolute potential) is not feasible, as has been discussed extensively in a vast body of literature conclusively reviewed in [5]; an update has been provided in [6]. An electric voltage U defined as the difference between two electrode potentials (in this text, the highly recommended but frequently ignored distinction between potential and voltage is maintained; the use of the same unit, which is possibly the cause of frequent confusions, is no contradiction) can nevertheless be measured (in units of voltage V) easily with high precision. The obtained results obviously do not enable the assignment of the electrode potentials to the electrodes used. To resolve this problem, one electrode potential under well-defined conditions has arbitrarily been defined as being zero at all temperatures. This electrode is the standard hydrogen electrode. In this electrode, as an electronically conducting phase, a piece of inert metal, most commonly platinized platinum, is brought into contact with an ionically conducting phase composed of an aqueous solution with a unit of proton activity saturated with hydrogen gas at a unit of pressure activity. The electrode’s reaction establishing the electrode is the hydrogen electrode’s reaction with a zero net current at equilibrium, i.e., hydrogen evolution and hydrogen oxidation proceed at the same rate:
H2 + 2 H2O ⇄ 2 H3O+ + 2 e−
This SHE is sometimes called the normal hydrogen electrode (NHE) as initially suggested 1898 by Nernst with a hydrogen pressure of 1 atm and 1 M sulfuric acid. Sometimes, it has been proposed that because the term refers to “normal” as obsolete concentration information, it should be avoided. The SHE is the primary standard for determining the electrode potential. A typical design is shown in Figure 1. Further electrodes combined with the SHE yield an electrochemical cell (combined of two half-cells) with two electric connectors enabling the measurement of voltage, specifically the cell’s voltage. The electric connectors of both terminals should be identical in order to avoid any electric contributions from metallic contacts. Measurement must be performed with zero current in order to avoid any shift in the electrode potentials; for details see, e.g., [7,8,9,10][7][8][9][10]. Because the electrode potential of the SHE has been set to zero, the measured cell voltage is equal to the electrode potential of the second electrode. The voltage of this cell may be called the cell potential because the numerical value is equivalent to the value of the electrode potential of the other electrode. This usage does not help; it only results in confusion and should be avoided.
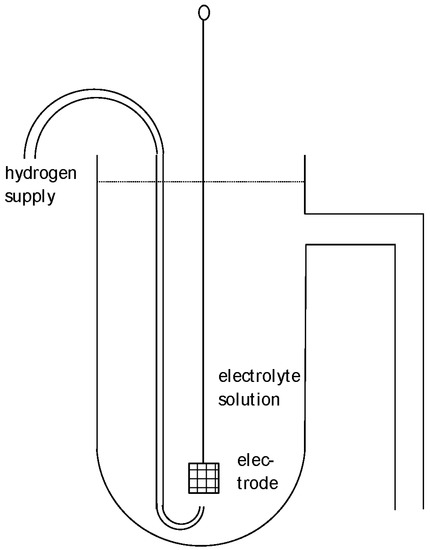
Figure 1. Schematic design of a hydrogen reference electrode.
Because the use of a reference electrode needing a continuous supply of high-purity hydrogen gas is a rather unwelcome experimental detail in many applications [7[7][8][9][10][11][12],8,9,10,11,12], alternative options have been suggested. A setup initially suggested by Giner [13] (for further developments and simplification details, see [8,9][8][9]) is depicted below in Figure 2. This electrode has also been called a dynamic hydrogen reference electrode (DHRE).
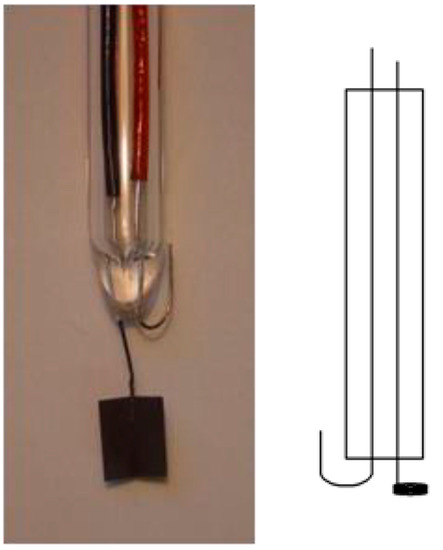
Figure 2. Schematic cross-section (right) and typical sample of a hydrogen electrode according to Giner.
At the large area of a platinized platinum electrode, hydrogen is formed by applying a small electric current supplied by an external power source [8], with the wire electrode serving as an oxygen-developing electrode. Because the current density j0 at the hydrogen electrode is negligibly small, its electrode potential hardly deviates from the other potential. Thus the electrode potential of hydrogen depending only on the proton activity in the surrounding electrolyte solution is controlled. Accordingly, the electrode is called a relative hydrogen electrode RHE (with an electrode potential relative to the proton activity). The pressure activity of hydrogen depends on the surrounding pressure; in most cases, it will be unity. Frequently, RHE is spelled out as “reversible hydrogen electrode”. This does not make sense for two obvious reasons: To be useful as a reference electrode, it must be reversible (in whatever meaning of this term); in addition, this term lacks information about the pH dependence of this electrode. Nevertheless, even a plain hydrogen electrode is sometimes called a reversible hydrogen electrode—an obvious pleonasm [14]. This report is also noteworthy because it describes the technology of hydrogen electrodes from before 1964 as desirable laboratory equipment.
The need for an external power supply with the Giner electrode (even only a single D-size alkaline cell with a simple Ohmic resistor of 15 kΩ [8]) has stimulated further developments, resulting in the self-contained reference electrode described first by Will [15,16][15][16]. A schematic cross-section and a typical sample are shown in Figure 3. The hydrogen gas is formed by the electrolyte solution already present in the glass tube and the metal wick inside as the cathode, with an external second electrode as the anode. Hydrogen is formed, the gas bubble establishes a pressure activity of unity, whereas the proton activity controls the electrode potential of the RHE. This electrode has sometimes been called a convenient hydrogen electrode (CHE). Depending on the glass-to-metal seal atop the gas bubble (e.g., its sealing quality), the gas bubble may stay in place for many weeks. With proper storage preventing the access of dioxygen, the electrode is ready for use for the said period of time.

Figure 3. Schematic cross-section (left) and practical sample of an RHE according to Will.
A general drawback of RHEs is noteworthy. The actual exchange current density of the hydrogen electrode’s reaction established at the electrode depends on the proton activity. In the range between strongly acidic and alkaline values, it drops to low values [17,18][17][18]. Thus, a fundamental requirement for reference electrodes is the following. If a large exchange current density keeping the electrode unpolarized (or non-polarizable) vanishes to some extent, the hydrogen electrode potential may become instable. Accordingly, all types of hydrogen electrodes should preferably be used in strongly acidic or alkaline solutions. In other solutions, they may be used (because of their major advantage: they do not introduce any other ionic species into the electrolyte solution) only with frequent calibration.
Certainly, the hydrogen electrode as depicted in Figure 1 suffers from inherent practical limitations, whereas the latter designs described above are more or less convenient to handle with associated major advantages. Accordingly, the claim reported elsewhere [19] that the standard hydrogen electrode has lost its practical importance should be limited—if assumed to be valid at all—to the electrode shown in Figure 1. The other hydrogen electrodes (RHEs) enjoy considerable popularity because of their obvious advantages.
Nevertheless, there remain circumstances (neutral electrolyte solutions, long-term unattended use etc.) requiring other reference electrodes beyond SHEs and RHEs.
References
- Holze, R. Landolt-Börnstein, Numerical Data and Functional Relationships in Science and Technology, New Series, Group IV: Physical Chemistry, Electrochemistry, Subvolume A, Electrochemical Thermodynamics and Kinetics; Martienssen, W., Lechner, M.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 9.
- Bard, A.J.; Inzelt, G.; Scholz, F. (Eds.) Electrochemical Dictionary; Springer: Berlin/Heidelberg, Germany, 2012.
- Quack, M.; Stohner, J.; Strauss, H.L.; Takami, M.; Thor, A.J.; Cohen, E.R.; Cvitas, T.; Fry, J.; Holström, B.; Kuchitsu, K.; et al. (Eds.) Quantities, Units and Symbols in Physical Chemistry; RSC: Cambridge, UK, 2007.
- Wu, Y.; Holze, R. Electrochemical Energy Conversion and Storage; Wiley-VCH: Weinheim, Germany, 2022.
- Trasatti, S. The “absolute” electrode potential-the end of the story. Electrochim. Acta 1990, 35, 269–271.
- Inzelt, G.; Lewenstam, A.; Scholz, F. (Eds.) Handbook of Reference Electrodes; Springer: Berlin/Heidelberg, Germany, 2013.
- Holze, R. Experimental Electrochemistry: A Laboratory Textbook, 2nd ed.; VCH-Wiley: Weinheim, Germany, 2019.
- Caton, R.D., Jr. Topics in chemical instrumentation: LXXIII. Reference electrodes. J. Chem. Educ. 1973, 50, A571–A578.
- Pletcher, D. A First Course in Electrode Processes, 2nd ed.; The Royal Society of Chemistry: Cambridge, UK, 2009.
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods, 2nd ed.; Wiley: New York, NY, USA, 2001.
- Holze, R. Eine einfache Wasserstoffbezugselektrode. CHEMKON 2003, 10, 87–88.
- Roscher, J.; Holze, R. Messungen mit Wasserstoffelektroden ohne Druckgas—Measurements with a hydrogen electrode without pressured gas. CHEMKON 2022, 29, 408–411.
- Giner, J. A practical reference electrode. J. Electrochem. Soc. 1964, 111, 376–377.
- Zamora Zeledón, J.A.; Jackson, A.; Stevens, M.B.; Kamat, G.A.; Jaramillo, T.F. Methods—A Practical Approach to the Reversible Hydrogen Electrode Scale. J. Electrochem. Soc. 2022, 169, 066505.
- Will, F.G.; Hess, H.J. Morphology and Capacity of a Cadmium Electrode. J. Electrochem. Soc. 1973, 120, 1–11.
- Will, F.G. A self-contained miniature hydrogen reference electrode. J. Electrochem. Soc. 1986, 133, 454–455.
- Vielstich, W. Brennstoffelemente; Verlag Chemie: Weinheim, Germany, 1965.
- Ernst, S.; Hamann, C.H. The pH-dependence of the hydrogen exchange current density at smooth platinum in alkaline solution (KOH). J. Electroanal. Chem. 1975, 60, 97–100.
- Kahlert, H. Reference electrodes. In Electroanalytical Methods; Scholz, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 291–308.
More
