Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Sirius Huang and Version 1 by José A. Fernández-López.
Nitrate (NO3−) pollution of surface and groundwater bodies is a global problem of increasing concern, which has stimulated significant research interest. Nitrogen is crucial for life as a macronutrient for living organisms on Earth, but the global nitrogen cycle has been seriously altered by intensification of human activities, leading to eutrophication and hypoxic conditions of aquatic ecosystems. Due to nitrogen overfertilization, intensive agricultural practices generate huge nitrate fluxes that inadvertently deteriorate water quality. Different industrial processes also contribute to NO3− pollution in the environment.
- nitrate pollution
- waterbodies
- nitrification
- diffuse contamination
1. Introduction
Water is essential for economic growth, human health, and the environment. In terms of quantity and quality, freshwater is a limited resource on Earth. The pollution of surface and groundwater poses a threat to aquatic ecosystems and society. The challenge of providing quality water, both for human consumption and for food production and recreational use, is undoubtedly one of the most pressing of the 21st century. Global warming, desertification, and the contamination of surface and groundwater have drastically reduced the availability of freshwater [1], and it is a key issue in meeting the Sustainable Development Goals (SDG) included in the 2030 Agenda for Sustainable Development, adopted by all United Nations Member States [2,3][2][3].
Nitrogen compounds in the soil may have a natural origin where they are subsequently oxidized to nitrates. Nitrate ions, driven by its extreme water solubility and resistance to fixation by soil colloids, are very susceptible to contamination by leakage. They can be easily washed into deep areas of the soil by rainfall and/or overirrigation, reaching groundwater bodies [4].
Diffuse agricultural pollution is the contamination of water, soil, and air, as a result of farming practices. This pollution depends on what happens on the Earth’s surface and practically affects large areas of our planet. It is very significant in many world regions because of the cumulative effect that certain discharges can have on the environment. Agricultural practices such as seedbed preparation, ploughing, fertilization, slurry application, and crop spraying are all possible contributors to diffuse pollution [5,6][5][6]. Thus, there is a wide range of potential sources of diffuse pollution that are linked to agricultural practices and that can damage the environment. This diffuse pollution is particularly evident in water bodies, when due to rainfalls and the way the land is managed, nutrients, pesticides, faecal bacteria, chemicals, and fine sediments are leached from the land into streams, rivers, lakes, and groundwater.
2. The Biological Nitrogen Cycle
The nitrogen cycle is the biogeochemical loop that supplies nitrogen to living organisms and keeps it recirculating in the biosphere (Figure 1). Nitrogen undergoes a variety of chemical reactions (oxidations and reductions) (Table 1) that produce N-compounds with oxidation states ranging from +5 (NO3−) to −3 (NH4+) [24][7].
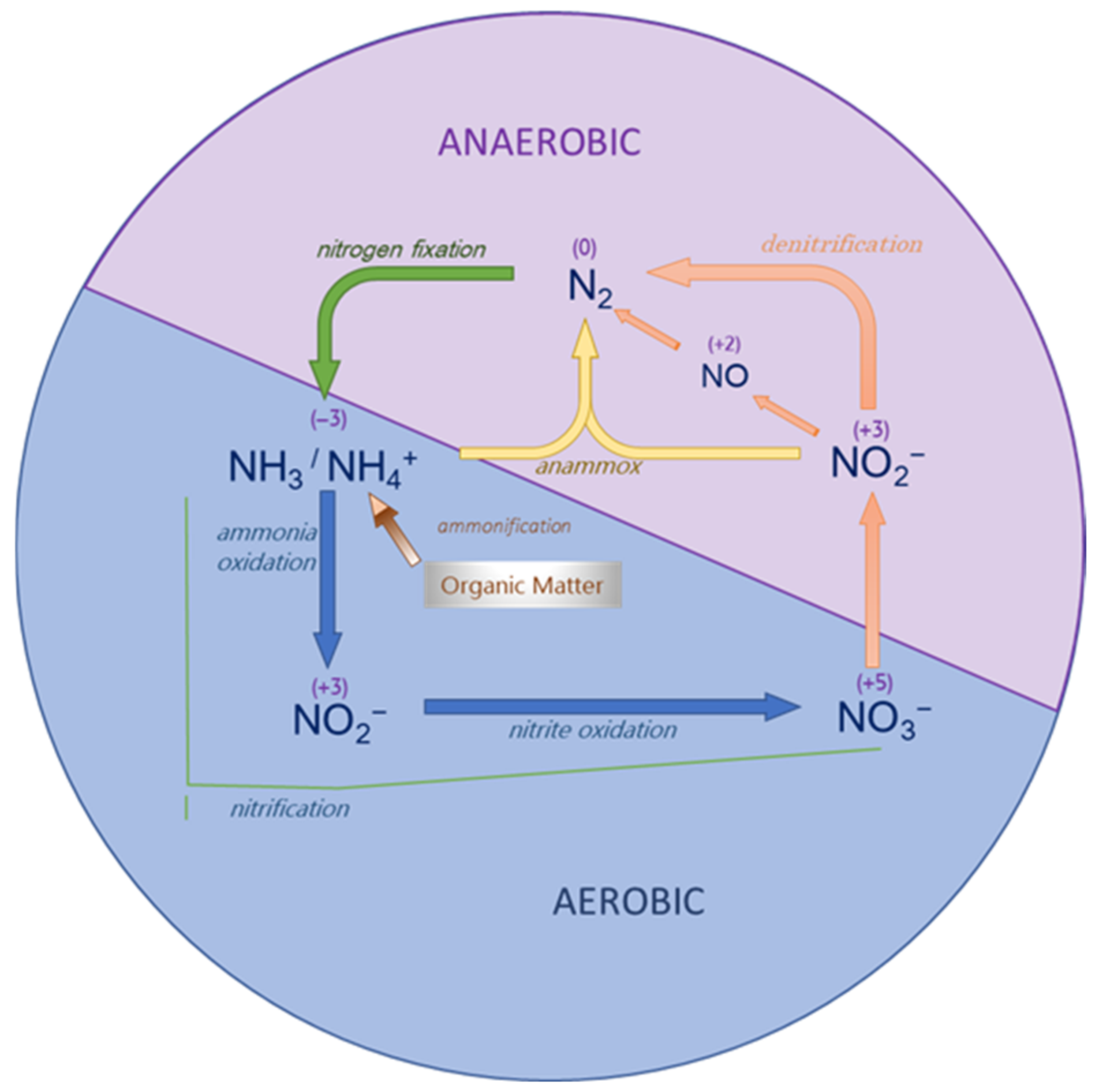
Figure 1.
The biological nitrogen cycle.
Table 1.
Chemical reactions in the nitrogen cycle.
| Process | Chemical Reaction | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 2 | + 8 H | + | + 8 e | − | → 2 NH | 3 | + H | 2 | |||
| NH | 3 | + H | 2 | O → NH | 4+ | + OH | − | |||||
| NH | 4+ | + 3/2 O | 2 | → NO | 2− | + H | 2 | O + 2 H | + | |||
| NO | 2− | + 1/2 O | 2 | → NO | 3− | |||||||
| NH | 4+ | + 2 O | 2 | → NO | 3− | + H | 2 | O + 2 H | + | |||
| 2 NO | 3− | + 10 e | − | + 12 H | + | → N | 2 | + 6 H | 2 | O | ||
| NO | 2− | + NH | 4+ | → N | 2 | + 2 H | 2 | O |
The main and most abundant source of nitrogen is the atmosphere, where it is found in molecular form (N2). In this form, it is generally not available to plants, which limits primary productivity in most ecosystems [25][8]. Only a small group of highly specialized prokaryotes are capable of using nitrogen from the atmosphere, taking N2 from the air and reducing it to ammonium (NH4+), a nitrogenous form that can be uptaken by plants. This process is known as biological nitrogen fixation. While certain nitrogen-fixing organisms are free-living, others need to form symbiotic associations with a host to complete the process. [26][9]. Among the latter, the rhizobial and actinorhizal symbiotic systems stand out for their high efficiency [27][10]. All nitrogen-fixing organisms are characterised by the nitrogenase enzyme complex, which is responsible for the reduction reaction of N2 to NH3/NH4+. In this way, the soil is enriched in nitrogenous compounds that can be assimilated by the plants that serve as food for herbivores, and through them for carnivores, thus incorporating nitrogen into their organism. Later, through excretion and the decomposition of plant and animal residues, nitrogen returns to the soil, this time in the form of ammonium (NH4+); this process is named mineralization. This NH4+ remains in the soil to be absorbed by plants that do not have the nitrogenase system [28][11].
During nitrification process, a series of aerobic nitrifying bacteria transform the NH3/NH4+ produced during mineralization initially into nitrite (NO2−), and finally, it is oxidized to nitrate (NO3−), the nitrogenous form primarily assimilated by the radicular system of plants. The nitrification process is particularly important as it produces an extra supply of nitrogen available to plants. Finally, NO3− is transformed by denitrifying bacteria into gaseous nitrogen (N2), which returns to the atmosphere, completing the cycle [29][12].
The anammox (anaerobic ammonium oxidation) process, also makes a significant contribution to the nitrogen cycle, turning over NO2− and NH4+ to N2 gas. This conversion is performed by bacteria of the phylum Planctomycetes, which are present in anoxic freshwater and marine environments [30][13].
Nitrogen and its cycle are the key for different forms of life as its multiple oxidation states confer to nitrogen a significant biological relevance in the context of microbial metabolism [31][14].
3. Processes Responsible for Nitrate Pollution in Water
Nitrogen is an essential macronutrient that enables plants and crops to grow, but excessive concentrations in water are detrimental to people and nature. The nitrate ion (NO3−) is the most thermodynamically stable nitrogenous compound present in aqueous and oxygenated earth systems, so there is a tendency for all nitrogenous materials to be readily converted to nitrate in these environments. The release of massive levels of nitrate ions into waterbodies causes cumulative impacts on living organisms and ecosystems. Nitrate pollution is widespread throughout the world in recent decades because of anthropogenic actions and natural processes, and a number of studies have been recently published warning of its consequences [31,32,33,34][14][15][16][17].
The processes responsible for the accumulation of nitrates in different ecosystems are many and varied, and it is recognized that they can be divided broadly according to their origin as geogenic or anthropogenic processes, depending on whether the source is natural or directly related to human action [34,35][17][18].
Geogenic sources of nitrogen compounds in the environment include weathering and erosion of igneous rocks and minerals [36][19], lightning storms through different precipitation and rainfall modes [37][20], and atmospheric deposition of nitrogen oxides and symbiotic fixation by selected plants and cyanobacteria [38][21]. All these processes are responsible for the input of nitrogenous forms into the soil, which enter the biogeochemical cycle, and under normally oxidizing conditions lead to their accumulation in the form of NO3−, which are easily leached to deep layers and reach groundwater bodies.
Runoff processes that displace large quantities of soil, due to torrential rainfall, are increasingly frequent in certain areas of the planet and also entail the entrainment and incorporation of significant levels of NO3− and other nutrients into waterbodies.
The main environmental contamination with NO3− has typically been attributed to anthropogenic activities. Due to anthropogenic actions, the natural nitrogen (N) cycle has been significantly altered by a 100% increase in the rate of N influx into the global N cycle. The abuse of organic and inorganic N-based fertilizers in agriculture is a major source of diffuse pollution, contributing to the increase of NO3− contamination in waterbodies and to the increase of nitrous oxide in the atmosphere [39,40][22][23]. Nitrogen is one of the three macronutrients for plants (N–P–K), and its application has a direct influence on the development of plants, which is why it tends to be abused to ensure maximum agricultural yields. Intensive agricultural practices also lead to a high demand for nitrogen fertilizers. Inorganic nitrogen is normally added to the soil as NH4+ and NO3− in quantities above the nutritional needs of plants, and is therefore not partially assimilated by crops and leaches from agricultural irrigation and rainfall to be discharged into surrounding waterbodies [41][24]. Although there is a direct correlation between the higher consumption of nitrogen fertilizers and the increase in crop production to supply the world’s growing population, concerns have been expressed about the environmental sustainability of these activities in terms of the impairment they cause to water resources [40][23].
Intensive livestock production, and the proliferation of large-scale farms, is also a potential source of NO3− pollution. Livestock waste contains nitrogen in the form of both organic and inorganic compounds. Microbial action breaks down waste containing organic nitrogen into NH4+, which is then transformed into NO2− and NO3− [42][25]. NO2− is further readily converted to NO3−. Thus, organic waste generated from livestock farming is a potent source of NO3− inputs that pollute surrounding waterbodies.
Population growth and the development of densely populated urban centres are also responsible for the increase of NO3− in wastewater. Urban runoff also pollutes surface waters in many parts of the world. In some scenarios, urban landfills and poorly insulated septic tanks are also involved in nitrate seepage and contamination [34][17].
Different industrial processes also contribute to NO3− pollution in the environment. Chemical industries using nitrogenous commodities (ammonia, nitric acid, urea, and ammonium nitrate) may cause nitrate contamination problems [37][20]. In these plants, the inappropriate handling and waste-mismanagement may also contribute to the leaching of nitrates into aquifer systems. Nitrate is often present in waters associated with mining because of ammonium nitrate-blasting activities [43][26].
Deforestation and forest fires also lead to a deterioration of the soil structure and a decrease in its capacity to retain and assimilate NO3−, which will therefore be easily washed into deep layers and migrate to the groundwater.
References
- Moss, B. Water pollution by agriculture. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 659–666.
- Sachs, J.D. From millennium development goals to sustainable development goals. Lancet 2012, 379, 2206–2211.
- Griggs, D.; Stafford-Smith, M.; Gaffney, O.; Rockström, J.; Öhman, M.C.; Shyamsundar, P.; Steffen, W.; Glaser, G.; Kanie, N.; Noble, I. Policy: Sustainable development goals for people and planet. Nature 2013, 495, 305–307.
- Bhatnagar, A.; Sillanpää, M. A review of emerging adsorbents for nitrate removal from water. Chem. Eng. J. 2011, 168, 493–504.
- Stevens, C.J.; Quinton, J.N. Diffuse pollution swapping in arable agricultural systems. Crit. Rev. Environ. Sci. Technol. 2009, 39, 478–520.
- Dunn, S.M.; Brown, I.; Sample, J.; Post, H. Relationships between climate, water resources, land use and diffuse pollution and the significance of uncertainty in climate change. J. Hydrol. 2012, 434–435, 19–35.
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276.
- Thamdrup, B. New pathways and processes in the global nitrogen cycle. Annu. Rev. Ecol. Evol. Syst. 2012, 43, 407–428.
- Bernhard, A. The nitrogen cycle: Processes, players, and human impact. Nat. Ed. 2010, 3, 25.
- Vitousek, P.M.; Cassman, K.; Cleveland, C.; Crews, T.I.; Field, C.B.; Grimm, N.; Howarth, R.; Marino, R.; Martinelli, L.; Rastetter, E.; et al. Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry 2002, 57, 1–45.
- Martínez-Espinosa, R.M.; Cole, J.A.; Richardson, D.J.; Watmough, N.J. Enzymology and ecology of the nitrogen cycle. Biochem. Soc. Trans. 2011, 39, 175–178.
- Udvardi, M.; Poole, P.S. Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol. 2013, 64, 781–805.
- Kuenen, J.G. Anammox and beyond. Environ. Microbiol. 2020, 22, 525–536.
- Moloantoa, K.M.; Khetsha, Z.P.; van Heerden, E.; Castillo, J.C.; Cason, E.D. Nitrate water contamination from industrial activities and complete denitrification as a remediation option. Water 2022, 14, 799.
- Van der Hoek, J.P.; Duijff, R.; Reinstra, O. Nitrogen recovery from wastewater: Possibilities, competition with other resources, and adaptation pathways. Sustainability 2018, 10, 4605.
- Abascal, E.; Gómez-Coma, L.; Ortiz, I.; Ortiz, A. Global diagnosis of nitrate pollution in groundwater and review of removal technologies. Sci. Total Environ. 2022, 810, 152233.
- Singh, S.; Anil, A.G.; Kumar, V.; Kapoor, D.; Subramanian, S.; Singh, J.; Ramamurthy, P.C. Nitrates in the environment: A critical review of their distribution, sensing techniques, ecological effects and remediation. Chemosphere 2022, 287, 131996.
- Selck, B.J.; Carling, G.T.; Kirby, S.M.; Hansen, N.C.; Bickmore, B.R.; Tingey, D.G.; Rey, K.; Wallace, J.; Jordan, J.L. Investigating anthropogenic and geogenic sources of groundwater contamination in a semi-arid alluvial basin, Goshen Valley, UT, USA. Water Air Soil Pollut. 2018, 229, 186.
- Ayub, R.; Messier, K.P.; Serre, M.L.; Mahinthakumar, K. Non-point source evaluation of groundwater nitrate contamination from agriculture under geologic uncertainty. Stoch. Environ. Res. Risk Assess. 2019, 33, 939–956.
- Shukla, S.; Saxena, A. Sources and leaching of nitrate contamination in groundwater. Curr. Sci. 2020, 118, 883–891.
- Gutiérrez, M.; Biagioni, R.N.; Alarcón-Herrera, M.T.; Rivas-Lucero, B.A. An overview of nitrate sources and operating processes in arid and semiarid aquifer systems. Sci. Total Environ. 2018, 624, 1513–1522.
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving nitrogen use efficiency in crops for sustainable agriculture. Sustainability 2011, 3, 1452–1485.
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Alamri, S.A. Fertilizers and their contaminants in soils, surface and groundwater. Encycl. Anthrop. 2018, 5, 225–240.
- Ye, L.; Zhao, X.; Bao, E.; Li, J.; Zou, Z.; Cao, K. Bio-organic fertilizer with reduced rates of chemical fertilization improves soil fertility and enhances tomato yield and quality. Sci. Rep. 2020, 10, 177.
- Sahoo, P.K.; Kim, K.; Powell, M.A. Managing groundwater nitrate contamination from livestock farms: Implication for nitrate management guidelines. Curr. Pollut. Rep. 2016, 2, 178–187.
- Choudhary, M.; Muduli, M.; Ray, S. A comprehensive review on nitrate pollution and its remediation: Conventional and recent approaches. Sustain. Water Resour. Manag. 2022, 8, 113.
More
