Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Hichem Moulahoum.
Minimally invasive approaches for cancer diagnosis are an integral step in the quest to improve cancer survival. Liquid biopsies such as blood samples are matrices explored to extract valuable information about the tumor and its state through various indicators, such as proteins, peptides, tumor DNA, or circulating tumor cells. Although these markers are scarce, making their isolation and detection in complex matrices challenging, the development in polymer chemistry producing interesting structures, including molecularly imprinted polymers, branched polymers, nanopolymer composites, and hybrids, allowed the development of enhanced platforms with impressive performance for liquid biopsies analysis.
- liquid biopsy
- biosensor
- polymers
1. Non-Fouling Agents
One of the critical challenges in liquid biopsy diagnosis is the potential of biological material accumulation (e.g., such as cells and proteins) on the medical devices used in the procedure. This is known as fouling, which can interfere with the accuracy and reliability of the test results. To address this issue, non-fouling coatings can be applied to the surface of the medical devices used in liquid biopsy analysis. These coatings are designed to minimize the interaction between the device and the biological sample, which helps prevent the accumulation of fouling materials.
Polymers are a common choice for non-fouling coatings in liquid biopsy analysis, as they can be easily modified to optimize their performance and tailor them to specific applications. Polyethylene glycol (PEG) is a pioneer antifouling polymer whose ability to inhibit protein adsorption due to its extensive hydrated layer, flexibility, and ability to be functionalized [9][1]. PEG and some other similar antifouling polymers (e.g., polyvinyl alcohol (PVA)) with suitable surface packing density create a resistant coating layer with great resistance toward non-specific adsorption. Such a layer that omits non-specific adsorption can significantly enhance the selectivity and specificity of the method for its own targeted materials. The antifouling phenomenon is typically achieved via the assistance of several mechanisms including hydration, steric hindrance, ionic solvation, and charge balance [10,11][2][3]. The physicochemical properties of the polymers such as the flexibility of the polymer chain, molecular weight, and packing density have direct and critical impact on the antifouling performance of hydrophilic polymers such as PEG chains [12][4]. This performance of hydrophilic polymer chains is closely related to the formation of a hydrate layer by hydrogen bonds. On the contrary, the implication of charged polymers, such as zwitterionic, results in a strong electrostatic component that positively affects the thickness of the water layer [13][5]. When using hydrophilic polymer chains as antifouling agents, different surface modification methods such as physical adsorption or covalent grafting can be applicable. The covalently grafted polymer chain or even chemisorbed to the surface can cause a random coil conformation at low density. The increased density makes the random coil state unstable and enlarges the polymer chains to have an arrangement in the “brush regime” [14][6]. Even though PEG compounds have shown great potential as antifouling agents, their application can be limited by drawbacks such as the vulnerability to oxidative damage and the high molecular weight requirement for creating a stable colloidal state [15][7]. Other than linear polymers, including PEG (polymer comb), several polymers with different structures, such as brush, dendritic, and hyperbranched polymers, have been utilized as next-generation antifouling coatings that form tightly compact structures required for protein repulsion (Figure 21) [9][1]. These types of polymers are either zwitterionic polymers or natural-based polymer chains with different morphologies and sizes.
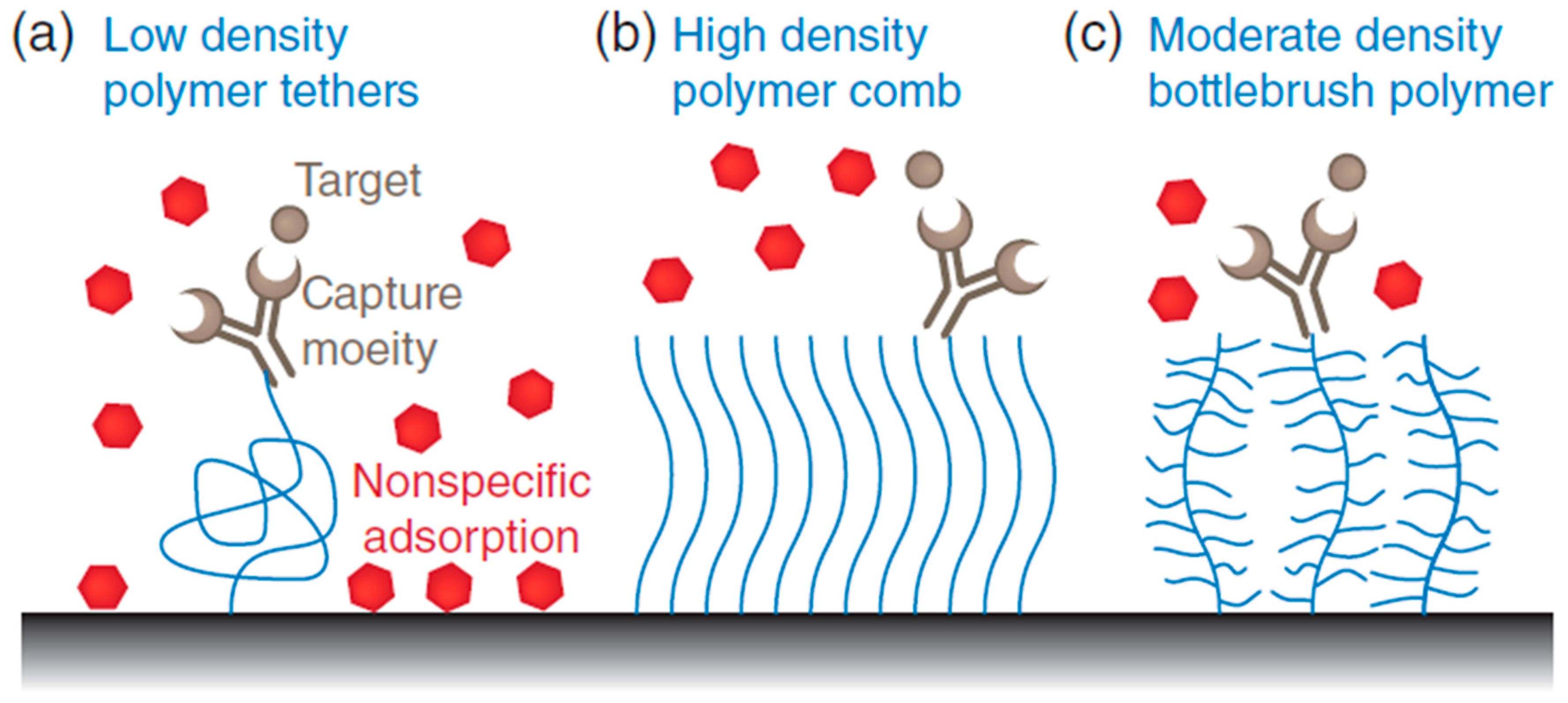
Figure 21. Schematic demonstration of the various surface grafting types of polymers. (a) Antibody grafted on a linear polymer and attached to a surface (at low density) enhances flexibility and allows access for capturing target molecules. There is a risk for non-specific attachment of other molecules on the empty gaps (fouling). (b) Polymer comb structure made from densely packed linear and hydrophilic polymers to produce antifouling effects. The attachment of targeting molecules will have better specificity. (c) Branched bottlebrush polymers are used at lower densities and demonstrate a better antifouling feature than linear polymers.
A bottle brush polymer is a type of polymer that involves a main chain (or backbone) that is densely packed with secondary chains attached. These secondary chains are made from monomers that have antifouling properties. A basic example of bottle brush polymers is poly[oligo(ethylene glycol) methyl methacrylate], which has the backbone and the secondary chains made from ethylene glycol units [16][8]. Other examples of bottle brush polymers include those with backbones made from poly(acrylic acid) [17][9] or poly(L-lysine) [18][10], and secondary chains made from poly(2-methyl-2-oxazoline) [19][11], poly(2-ethyl-2-oxazoline) [18][10], or poly(N-vinylpyrrolidone) [20][12].
Dendrimers are a type of polymer with highly organized branching moieties, which gives them a tree-like structure. They are synthesized through a process called divergent synthesis, in which branches are added to a small central molecule in a stepwise fashion [21][13]. Dendrimers are characterized by their low polydispersity (i.e., uniform size and shape distribution) with a broad application range in liquid biopsy diagnostics. They are often synthesized from an ethylenediamine core [22][14]. In addition, dendrimers can be created by building inwards (outside-in) via a convergent synthesis process involving attaching branches called “dendrons” to a central structure.
On the other hand, a single-step polycondensation can be used to synthesize hyperbranched polymers with a high density of functional groups [23][15]. They are commonly used in biosensor applications, and include hyperbranched polyglycerol [24][16], polyethylene imine [25][17], polyester [26][18], and aromatic polyamide [27][19].
Despite the morphology of the polymer chains, the uniform charge distribution along with the neutral total charge of two oppositely charged moieties on the surface make zwitterionic polymers promising candidates for advanced antifouling-biocompatible materials. Moreover, there are several strategies such as designing new monomers with better hydrophilicity and charge balance, optimizing polymerization conditions, and modifying surface properties by incorporating functional groups that can be used to further enhance the antifouling performance of zwitterionic polymers. On the other hand, natural-based polymers due to their exceptional features including protease resistance, precise control of molecular weight, and broad range of side-chain compositions are regularly utilized in the development of antifouling materials.
While antifouling strategies offer promising solutions to combat fouling, there are several challenges associated with their use. Biocompatibility, biodegradability, and toxicity are important considerations that should be meticulously taken into account, to ensure their safe and effective use.
2. Isolation and Concentration of Analytes
One of the critical and practical points of liquid biopsy-based diagnosis is the efficient and effective separation of low-concentrated target analytes. Sensitivity and specificity are, therefore, essential performance characteristics of liquid biopsy diagnostics. Polymeric materials can capture different analytes through various mechanisms, depending on the polymer’s specific properties and the target analyte. One common method is affinity-based capture, in which the analyte is specifically attracted to a specific functional group on the polymer. For example, capillary-channeled polymer (C-CP) fibers made of poly(ethylene terephthalate) (PET) were used as a stationary phase in the separation and retrieval of exosomes from various sources, such as urine, buffer, and culture media [28][20].
Another valuable method for analyte isolation and pre-concentration is using size-based capture, in which the analyte is captured based on its size or shape. This can be accomplished by using a polymer with a porous structure, which allows the analyte to enter the pores and become trapped. Numerous materials have been designed for filtration-based isolation techniques. Polycarbonate-based membranes with pore sizes ranging from 30 to 50 nm are the most commonly used in EV collections [29,30][21][22]. Despite the benefits of this method, some significant drawbacks limit their performance. Hence, various contextual cells share comparable features, including the sizes of certain leukocytes and CTCs or the exceedingly large blood cell density that intervene with the efficiency and selectivity of the method. The deformability range is another factor that must be carefully considered in designing microstructures for primary tumor cells and CTCs.
In addition to these mechanisms, polymeric materials can be functionalized with specific ligands or antibodies to capture specific analytes selectively. This can be accomplished through chemical reactions or physical adsorption to attach the ligands or antibodies to the polymer. For example, a polymer with a high surface area and a functional group compatible with the ligand or antibody may be used to capture the analyte via hydrogen bonds or van der Waals forces (noncovalent interactions). Poly(amidoamine) (PAMAM) is a dendrimer frequently used for multivalent recognition and entrapment of CTCs [29,30,31,32,33,34][21][22][23][24][25][26]. The detachment of antibody-decorated PAMAM dendrimers from epithelial cell adhesion molecules (EpCAMs) compared to the antibody alone were found to be 106 times smaller, providing higher capture density and efficiency [33,35][25][27]. Jeon et al. developed a new platform using nanostructured conductive polymer polypyrrole (Ppy) to capture and release circulating cell-free DNA (cfDNA) efficiently in unprocessed plasma samples of breast and lung cancer [36][28]. The developed platform used a polymer-coated gold nanowires structure to trap and isolate cfDNA, followed by the induction of polymer breakage (via an electrical potential) to release the cfDNA.
Molecular imprinting is another promising and fast-growing bio-isolation technique. Using such a method, Takeuchi et al. created EV-shaped cavities by mimicking the structure of conjugating antibodies in polymer matrices where it has been used for the detection of breast cancer by analyzing EVs in tears [37][29].
The polymer-based precipitation method in liquid biopsy analysis is a technique that utilizes the ability of certain polymers to bind to specific biomolecules in a liquid sample selectively. This allows the targeted biomolecules to be separated and concentrated from the rest of the sample, enabling their analysis and detection. One example of this method is using magnetic nanoparticles (MNPs) coated with a specific polymer. These MNPs can be added to a liquid sample and then magnetically separated from the rest of the sample using a magnet. The biomolecules of interest will be bound to the polymer-coated MNPs and can be easily separated and purified for further analysis. Riethdorf et al. constructed layer-by-layer magnetic nanospheres (MNs) based on hydrophobic nano-γ-Fe2O3 coated with poly(styrene/acrylamide) copolymer nanospheres to obtain fast magnetic reaction [38][30]. Healthy human blood was spiked with breast cancer cells to examine the proposed platform, and the anti-EpCAM-functionalized MNs exhibited high efficiency with 94% capturing capacity in 5 min. The viability of CTCs was approximately 90% and could be transferred to culture, RT-PCR, and immunocytochemistry (ICC) analysis. In another attempt, Liu et al. manufactured a polymer brush and phenylboronic acid combination-based multi-reactive surface to capture and release cancer cells [39][31]. The polymeric structure can create links with sialic acid (overexpressed in cancer cell membranes) at pH 6.8. Adding glucose and simultaneously increasing the pH to 7.8 could exchange the sialic acid with glucose, creating a stable complex and releasing the caught cells. The created 3D topography could significantly enhance the responsiveness, and accelerate cell capture and the release response rate.
3. Probes for Enhancing the Analyte Detection and the Signal Amplification
Similar to other diagnostic methods, the most critical step is the accurate and sensitive detection of analytes in a liquid biopsy sample. In addition, the ability to amplify the signal produced by detecting an analyte can increase the sensitivity and reliability of the assay, making it possible to detect low levels of analytes in a sample. Several approaches can be used to detect and amplify the signal of analytes in a liquid biopsy sample, including immunoassays, PCR, and microarray analysis. Each of these approaches utilizes specific probes or reagents that bind to and detect target analytes and can be used in combination with signal amplification techniques to increase the sensitivity. Probes can be designed to bind specifically to target analytes or to detect multiple analytes simultaneously using techniques such as microarray analysis, providing a more comprehensive view of the sample. Polymer dots, also known as fluorescent polymer nanoparticles, have emerged as a promising class of probes for liquid biopsy analysis. These nanoparticles can be functionalized with various chemical groups to enable specific binding to target analytes. The nanosized polymer dots can be detected using techniques such as fluorescence microscopy and flow cytometry. Additionally, the high fluorescence of these nanoparticles enables easy visualization and quantification [44,45][32][33]. For instance, Balzani et al. synthesized a poly(propylene amine) dendrimer compound with 32 dansyl moieties, highlighting the impact of these dansyl groups to produce strong fluorescence [46][34].
Fouz et al. prepared a bottlebrush polymer to strengthen the fluorescent signal obtained from a secondary antibody by a 1000-fold and prevent adverse self-quenching effects [47][35]. The polymer-DNA assembly, in this case, sequesters intercalated fluorophores, preventing their detachment, and can be tethered to the antibody via DNA hybridization. Thus, a fluorescent nanotag can detect target molecules with a bright signal in different techniques, such as confocal fluorescence microscopy, flow cytometry, and dot blots.
In another approach, fluorescently labeled DNA dendrimers were created to strengthen the exosomes’ detection signal [48][36]. When in contact with exosomes, the aptamers bind to them and release the DNA probe, initiating the hairpin DNA cascade reaction (HDCR) by opening hairpin DNA (HP1) bound to AuNPs. Subsequently, the probe DNA dendrimers link with HP1, leading to an enhanced signal-to-noise ratio. The highly effective method provided an excellent linear response for exosomes derived from HepG2 cells.
To develop a fluorescence liquid biopsy (FLB) analysis technique, Morcuende-Ventura et al. prepared fluorescent dendronized hyperbranched polymers (DHP) created from bis(hydroxymethyl)propionic acid [49][37]. The output of this study indicated that the inclusion of DHP-bMPA improved the classification performance of FLB in diagnosing pancreatic and ovarian cancers, with a performance reaching 85% (specificity and selectivity) for both pathologies.
Besides the application of polymer dots as imaging agents, several reports show these particles’ potential in different biosensors [50,51][38][39]. For example, gold or indium tin oxide (ITO) coated with ferrocene-functionalized PAMAM dendrimers were used in the structure of electrochemical sensors to detect DNA hybridization [52][40], avidin capture [53][41], and antibodies [54][42]. Furthermore, the capture of exosomes on the AuNP surface and the labeling with a polydopamine-coated liquid metal shell-core-core nanohybrid can also generate luminescent signals through electrochemical reactions [55][43]. In one study, an antifouling electrochemical biosensor based on the conducting polymer poly(3,4-ethylenedioxythiophene) (PEDOT) and a multifunctional peptide was manufactured for CTCs in blood [56][44]. The designed peptide with antifouling and targeting breast cancer cells capabilities, in addition to the electrodeposited PEDOT, could enhance electron transfer on the detection surface and amplify the signal-to-noise ratio, resulting in an increased sensitivity of the biosensor.
4. Microfluidic Technology
Microfluidic technology is a field that involves the manipulation and control of small volumes of fluids, typically in the microliter or nanoliter range, using microscale channels and devices. Microfluidic devices offer several advantages for liquid biopsy applications [63][45]. First, they can handle small volumes of samples, which is important for non-invasive tests that require only a small amount of fluid. Second, they can provide great sensitivity and specificity, allowing for the accurate detection and analysis of specific biomolecules. Third, microfluidic devices can be designed to perform multiplexed analyses to detect and analyze multiple biomolecules simultaneously. This can be especially useful for cancer diagnosis, providing a more complete picture of cancer characteristics and potential response to treatment.
As mentioned before, microfluidic devices can be used to isolate and purify specific types of biomolecules from complex mixtures. Polymeric materials are useful in developing and enhancing microfluidic technology due to the ease of being molded and patterned using various techniques, such as photolithography, soft lithography, and micro-injection molding. In addition, some polymers can be modified with functional groups or incorporated with nanoparticles to give them specific properties that can be useful in liquid biopsy applications. Therefore, polymeric microfluidic assays have advantages that make them more suitable than non-polymeric assays. These advantages include low cost, ease of fabrication, customizability, flexibility, biocompatibility, and functionality. Taking advantage of polymeric microfluidic assays, Nwankire et al. created an electrochemical lab-on-a-disc (eLoaD) instrument comprising two adhesive layers inserted between three PMMA layers and sputter-coated anti-EpCAM antibodies-functionalized gold electrodes onto the bottom layer [64][46]. This device allowed computerized quantification of CTCs in the whole blood, which, after applying impedance spectroscopy, can produce comprehensive outputs for the detection and quantification of CTCs in whole blood. In another study, a microfluidic device that utilizes immunoaffinity to separate tumor-derived exosomes directly from plasma was fabricated via soft lithography of polydimethylsiloxane (PDMS) [65][47]. This device consists of a number of herringbone micromixers, which generate an anisotropic flow to increase the likelihood of exosomes binding to specific antibodies that offer a potential mean of exosome isolation from plasma for further analysis.
A chip made of a thermal-sensitive polymer and graphene oxide (GO) has been created for use in a microfluidic device. It allows for efficient capture and release of CTCs from patients with breast and pancreatic cancer based on immunocapture [66][48]. A microfluidic chip employing a temperature-sensitive polymer-GO coating has been created to capture and release CTCs from individuals with breast and pancreatic cancer. This composite film allows for temperature-controlled capture and release for the analysis of cells. The film overcomes the limitations of technologies that rely on antibodies attached to the capture surface and can prevent the release of live cells.
Optimizing the integration of functional substrates with microfluidic technology is important for detecting cancer biomarkers. One approach to reducing non-specific adsorption and fluorescence background interference is to coat the substrate with a hydrophilic polymer. Wu et al. proposed a microfluidic device containing zinc oxide nanorods (ZnO NRs) constructed via layer-by-layer electrostatic self-assembly [67][49]. The introduced polyacrylic acid onto the ZnO NRs (as a hydrophilic layer) significantly suppressed non-specific attachment and provided outstanding antibody immobilization. Under ideal settings, the platform reached 100 fg/mL as LOD for CEA (carcinoembryonic antigen used as a cancer marker). This demonstrates the potential of using hydrophilic polymer coatings on ZnO NR-based microfluidic devices to detect cancer biomarkers.
References
- Maan, A.M.C.; Hofman, A.H.; Vos, W.M.; Kamperman, M. Recent Developments and Practical Feasibility of Polymer-Based Antifouling Coatings. Adv. Funct. Mater. 2020, 30, 2000936.
- Mauriz, E. Low-Fouling Substrates for Plasmonic Sensing of Circulating Biomarkers in Biological Fluids. Biosensors 2020, 10, 63.
- Wang, Y.S.; Yau, S.; Chau, L.K.; Mohamed, A.; Huang, C.J. Functional Biointerfaces Based on Mixed Zwitterionic Self-Assembled Monolayers for Biosensing Applications. Langmuir 2019, 35, 1652–1661.
- Zhang, K.; Huang, H.; Hung, H.C.; Leng, C.; Wei, S.; Crisci, R.; Jiang, S.; Chen, Z. Strong Hydration at the Poly(ethylene glycol) Brush/Albumin Solution Interface. Langmuir 2020, 36, 2030–2036.
- Zhang, Y.; Liu, Y.; Ren, B.; Zhang, D.; Xie, S.; Chang, Y.; Yang, J.; Wu, J.; Xu, L.; Zheng, J. Fundamentals and applications of zwitterionic antifouling polymers. J. Phys. D Appl. Phys. 2019, 52, 403001.
- Unsworth, L.D.; Tun, Z.; Sheardown, H.; Brash, J.L. Chemisorption of thiolated poly(ethylene oxide) to gold: Surface chain densities measured by ellipsometry and neutron reflectometry. J. Colloid. Interface. Sci. 2005, 281, 112–121.
- Liu, B.; Liu, X.; Shi, S.; Huang, R.; Su, R.; Qi, W.; He, Z. Design and mechanisms of antifouling materials for surface plasmon resonance sensors. Acta Biomater. 2016, 40, 100–118.
- Hucknall, A.; Simnick, A.J.; Hill, R.T.; Chilkoti, A.; Garcia, A.; Johannes, M.S.; Clark, R.L.; Zauscher, S.; Ratner, B.D. Versatile synthesis and micropatterning of nonfouling polymer brushes on the wafer scale. Biointerphases 2009, 4, FA50–FA57.
- Su, X.; Hao, D.; Li, Z.; Guo, X.; Jiang, L. Design of hierarchical comb hydrophilic polymer brush (HCHPB) surfaces inspired by fish mucus for anti-biofouling. J. Mater. Chem. B 2019, 7, 1322–1332.
- Morgese, G.; Verbraeken, B.; Ramakrishna, S.N.; Gombert, Y.; Cavalli, E.; Rosenboom, J.G.; Zenobi-Wong, M.; Spencer, N.D.; Hoogenboom, R.; Benetti, E.M. Chemical Design of Non-Ionic Polymer Brushes as Biointerfaces: Poly(2-oxazine)s Outperform Both Poly(2-oxazoline)s and PEG. Angew. Chem. Int. Ed. Engl. 2018, 57, 11667–11672.
- Konradi, R.; Pidhatika, B.; Muhlebach, A.; Textor, M. Poly-2-methyl-2-oxazoline: A peptide-like polymer for protein-repellent surfaces. Langmuir 2008, 24, 613–616.
- Wang, P.; Dong, Y.; Zhang, S.; Liu, W.; Wu, Z.; Chen, H. Protein-resistant properties of poly(N-vinylpyrrolidone)-modified gold surfaces: The advantage of bottle-brushes over linear brushes. Colloids. Surf. B Biointerfaces 2019, 177, 448–453.
- Voit, B.I.; Lederer, A. Hyperbranched and highly branched polymer architectures--synthetic strategies and major characterization aspects. Chem. Rev. 2009, 109, 5924–5973.
- Esfand, R.; Tomalia, D.A. Poly(amidoamine) (PAMAM) dendrimers: From biomimicry to drug delivery and biomedical applications. Drug Discov. Today 2001, 6, 427–436.
- Bugno, J.; Poellmann, M.J.; Sokolowski, K.; Hsu, H.J.; Kim, D.H.; Hong, S. Tumor penetration of Sub-10 nm nanoparticles: Effect of dendrimer properties on their penetration in multicellular tumor spheroids. Nanomedicine 2019, 21, 102059.
- Schömer, M.; Schüll, C.; Frey, H. Hyperbranched aliphatic polyether polyols. J. Polym. Sci. A Polym. Chem. 2013, 51, 995–1019.
- Kurunczi, S.; Hainard, A.; Juhasz, K.; Patko, D.; Orgovan, N.; Turck, N.; Sanchez, J.C.; Horvath, R. Polyethylene imine-based receptor immobilization for label free bioassays. Sens. Actuators B Chem. 2013, 181, 71–76.
- Sun, C.; Han, Q.; Wang, D.; Xu, W.; Wang, W.; Zhao, W.; Zhou, M. A label-free and high sensitive aptamer biosensor based on hyperbranched polyester microspheres for thrombin detection. Anal. Chim. Acta 2014, 850, 33–40.
- Li, H.; Zhao, F.; Yue, L.; Li, S.; Xiao, F. Nonenzymatic Electrochemical Biosensor Based on Novel Hydrophilic Ferrocene-terminated Hyperbranched Polymer and its Application in Glucose Detection. Electroanalysis 2016, 28, 1003–1011.
- Bruce, T.F.; Slonecki, T.J.; Wang, L.; Huang, S.; Powell, R.R.; Marcus, R.K. Exosome isolation and purification via hydrophobic interaction chromatography using a polyester, capillary-channeled polymer fiber phase. Electrophoresis 2019, 40, 571–581.
- Dong, X.; Chi, J.; Zheng, L.; Ma, B.; Li, Z.; Wang, S.; Zhao, C.; Liu, H. Efficient isolation and sensitive quantification of extracellular vesicles based on an integrated ExoID-Chip using photonic crystals. Lab. Chip. 2019, 19, 2897–2904.
- Woo, H.K.; Sunkara, V.; Park, J.; Kim, T.H.; Han, J.R.; Kim, C.J.; Choi, H.I.; Kim, Y.K.; Cho, Y.K. Exodisc for Rapid, Size-Selective, and Efficient Isolation and Analysis of Nanoscale Extracellular Vesicles from Biological Samples. ACS Nano. 2017, 11, 1360–1370.
- Myung, J.H.; Eblan, M.J.; Caster, J.M.; Park, S.J.; Poellmann, M.J.; Wang, K.; Tam, K.A.; Miller, S.M.; Shen, C.; Chen, R.C.; et al. Multivalent Binding and Biomimetic Cell Rolling Improves the Sensitivity and Specificity of Circulating Tumor Cell Capture. Clin. Cancer Res. 2018, 24, 2539–2547.
- Myung, J.H.; Gajjar, K.A.; Chen, J.; Molokie, R.E.; Hong, S. Differential detection of tumor cells using a combination of cell rolling, multivalent binding, and multiple antibodies. Anal. Chem. 2014, 86, 6088–6094.
- Myung, J.H.; Gajjar, K.A.; Saric, J.; Eddington, D.T.; Hong, S. Dendrimer-mediated multivalent binding for the enhanced capture of tumor cells. Angew. Chem. Int. Ed. Engl. 2011, 50, 11769–11772.
- Myung, J.H.; Roengvoraphoj, M.; Tam, K.A.; Ma, T.; Memoli, V.A.; Dmitrovsky, E.; Freemantle, S.J.; Hong, S. Effective capture of circulating tumor cells from a transgenic mouse lung cancer model using dendrimer surfaces immobilized with anti-EGFR. Anal. Chem. 2015, 87, 10096–10102.
- Bu, J.; Nair, A.; Kubiatowicz, L.J.; Poellmann, M.J.; Jeong, W.J.; Reyes-Martinez, M.; Armstrong, A.J.; George, D.J.; Wang, A.Z.; Zhang, T.; et al. Surface engineering for efficient capture of circulating tumor cells in renal cell carcinoma: From nanoscale analysis to clinical application. Biosens. Bioelectron. 2020, 162, 112250.
- Jeon, S.; Lee, H.; Bae, K.; Yoon, K.A.; Lee, E.S.; Cho, Y. Efficient Capture and Isolation of Tumor-Related Circulating Cell-Free DNA from Cancer Patients Using Electroactive Conducting Polymer Nanowire Platforms. Theranostics 2016, 6, 828–836.
- Takeuchi, T.; Mori, K.; Sunayama, H.; Takano, E.; Kitayama, Y.; Shimizu, T.; Hirose, Y.; Inubushi, S.; Sasaki, R.; Tanino, H. Antibody-Conjugated Signaling Nanocavities Fabricated by Dynamic Molding for Detecting Cancers Using Small Extracellular Vesicle Markers from Tears. J. Am. Chem. Soc. 2020, 142, 6617–6624.
- Riethdorf, S.; Fritsche, H.; Muller, V.; Rau, T.; Schindlbeck, C.; Rack, B.; Janni, W.; Coith, C.; Beck, K.; Janicke, F.; et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: A validation study of the CellSearch system. Clin. Cancer Res. 2007, 13, 920–928.
- Liu, H.; Li, Y.; Sun, K.; Fan, J.; Zhang, P.; Meng, J.; Wang, S.; Jiang, L. Dual-responsive surfaces modified with phenylboronic acid-containing polymer brush to reversibly capture and release cancer cells. J. Am. Chem. Soc. 2013, 135, 7603–7609.
- Ong, K.K.; Jenkins, A.L.; Cheng, R.; Tomalia, D.A.; Durst, H.D.; Jensen, J.L.; Emanuel, P.A.; Swim, C.R.; Yin, R. Dendrimer enhanced immunosensors for biological detection. Anal. Chim. Acta 2001, 444, 143–148.
- Zhao, W.; Hu, J.; Liu, J.; Li, X.; Sun, S.; Luan, X.; Zhao, Y.; Wei, S.; Li, M.; Zhang, Q.; et al. Si nanowire Bio-FET for electrical and label-free detection of cancer cell-derived exosomes. Microsyst. Nanoeng. 2022, 8, 57.
- Balzani, V.; Ceroni, P.; Gestermann, S.; Kauffmann, C.; Gorka, M.; Vögtle, F. Dendrimers as fluorescent sensors with signal amplification. Chem. Comm. 2000, 853–854.
- Fouz, M.F.; Mukumoto, K.; Averick, S.; Molinar, O.; McCartney, B.M.; Matyjaszewski, K.; Armitage, B.A.; Das, S.R. Bright Fluorescent Nanotags from Bottlebrush Polymers with DNA-Tipped Bristles. ACS Cent. Sci. 2015, 1, 431–438.
- Gao, M.L.; He, F.; Yin, B.C.; Ye, B.C. A dual signal amplification method for exosome detection based on DNA dendrimer self-assembly. Analyst 2019, 144, 1995–2002.
- Morcuende-Ventura, V.; Hermoso-Duran, S.; Abian-Franco, N.; Pazo-Cid, R.; Ojeda, J.L.; Vega, S.; Sanchez-Gracia, O.; Velazquez-Campoy, A.; Sierra, T.; Abian, O. Fluorescence Liquid Biopsy for Cancer Detection Is Improved by Using Cationic Dendronized Hyperbranched Polymer. Int. J. Mol. Sci. 2021, 22, 6501.
- Hasanzadeh, M.; Shadjou, N.; Eskandani, M.; Soleymani, J.; Jafari, F.; de la Guardia, M. Dendrimer-encapsulated and cored metal nanoparticles for electrochemical nanobiosensing. TrAC-Trends Analyt. Chem. 2014, 53, 137–149.
- Park, J.Y.; Park, S.M. DNA hybridization sensors based on electrochemical impedance spectroscopy as a detection tool. Sensors 2009, 9, 9513–9532.
- Kim, E.; Kim, K.; Yang, H.; Kim, Y.T.; Kwak, J. Enzyme-amplified electrochemical detection of DNA using electrocatalysis of ferrocenyl-tethered dendrimer. Anal. Chem. 2003, 75, 5665–5672.
- Yoon, H.C.; Hong, M.Y.; Kim, H.S. Affinity biosensor for avidin using a double functionalized dendrimer monolayer on a gold electrode. Anal. Biochem. 2000, 282, 121–128.
- Kwon, S.J.; Kim, E.; Yang, H.; Kwak, J. An electrochemical immunosensor using ferrocenyl-tethered dendrimer. Analyst 2006, 131, 402–406.
- Zhang, Y.; Wang, F.; Zhang, H.; Wang, H.; Liu, Y. Multivalency Interface and g-C(3)N(4) Coated Liquid Metal Nanoprobe Signal Amplification for Sensitive Electrogenerated Chemiluminescence Detection of Exosomes and Their Surface Proteins. Anal. Chem. 2019, 91, 12100–12107.
- Han, R.; Li, Y.; Chen, M.; Li, W.; Ding, C.; Luo, X. Antifouling Electrochemical Biosensor Based on the Designed Functional Peptide and the Electrodeposited Conducting Polymer for CTC Analysis in Human Blood. Anal. Chem. 2022, 94, 2204–2211.
- Kulasinghe, A.; Wu, H.; Punyadeera, C.; Warkiani, M.E. The Use of Microfluidic Technology for Cancer Applications and Liquid Biopsy. Micromachines 2018, 9, 397.
- Nwankire, C.E.; Venkatanarayanan, A.; Glennon, T.; Keyes, T.E.; Forster, R.J.; Ducree, J. Label-free impedance detection of cancer cells from whole blood on an integrated centrifugal microfluidic platform. Biosens. Bioelectron. 2015, 68, 382–389.
- Zhang, Y.; Tong, X.; Yang, L.; Yin, R.; Li, Y.; Zeng, D.; Wang, X.; Deng, K. A herringbone mixer based microfluidic device HBEXO-chip for purifying tumor-derived exosomes and establishing miRNA signature in pancreatic cancer. Sens. Actuators B Chem. 2021, 332, 129511.
- Yoon, H.J.; Shanker, A.; Wang, Y.; Kozminsky, M.; Jin, Q.; Palanisamy, N.; Burness, M.L.; Azizi, E.; Simeone, D.M.; Wicha, M.S.; et al. Tunable Thermal-Sensitive Polymer-Graphene Oxide Composite for Efficient Capture and Release of Viable Circulating Tumor Cells. Adv. Mater. 2016, 28, 4891–4897.
- Wu, Z.; Zhao, D.; Hou, C.; Liu, L.; Chen, J.; Huang, H.; Zhang, Q.; Duan, Y.; Li, Y.; Wang, H. Enhanced immunofluorescence detection of a protein marker using a PAA modified ZnO nanorod array-based microfluidic device. Nanoscale 2018, 10, 17663–17670.
More
