Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Waclaw Orczyk and Version 2 by Peter Tang.
Chitosan (CS), a biopolymer derived from chitin, is known for strong antifungal activity while being biodegradable, biocompatible, and non-toxic. Because of its characteristic it has been widely used in control of fungal pathogens. Antifungal activity of chitosan can be further enhanced by obtaining chitosan nanoparticles (CSNPs).
- chitin
- deacetylation
- dispersity
- fungi
- pathogen
- phospholipids
1. Introduction
Chitin, the main component of arthropod and insect exoskeleton, present also in fungal cell walls, is one of the most abundant biopolymers on Earth. It consists of N-acetylglucosamine residues linked by a β 1–4 glycosidic bond. Chitosan (CS) is the product of partial deacetylation of chitin and is composed of acetylglucosamine monomers (GlcNAc) and glucosamine monomers (GlcN) (Figure 1) [1]. The deacetylation process, inevitably accompanied by partial hydrolysis, gives a wide variety of CS molecules. The combination of various molecules might be unique for each batch of CS. This great diversity, enlarged by various sources of chitin, is a challenge for CS standardization and comparison of results obtained by different research groups. Commercially available products are characterized by source of the chitin, which might be crustacean of fungal, the type of deacetylation and hydrolysis processes, the degree of deacetylation (DD) expressed as the percentage of GlcN residues, the pattern of acetylation (PA), and the molecular weight (MW).
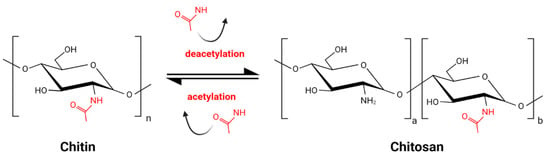
Figure 1.
Schematic reaction of partial chitin deacetylation and obtaining chitosan.
Chitosan has a strong antifungal effect due to its unique physicochemical properties, biodegradability, and biocompatibility [1][2][3][1,2,3]. Because of its biological activity, chitosan has been used to directly inhibit the growth of several fungal pathogens on crop plants [4][5][6][7][8][4,5,6,7,8] as well as indirectly stimulating plant defense mechanisms [9][10][9,10]. Furthermore, chitosan has been shown to inhibit the growth of disease-causing human fungal pathogens [11][12][13][11,12,13]. Additionally, good fiber and film formation properties of chitosan made it a suitable for food preservation, edible coating, and packaging [14]. Chitosan and chitosan-based particles can be also used as stabilizing component of Pickering emulsions with wide applications in medicine, cosmetics and food industry [15]. Chitosan was registered by the United States Environmental Protection Agency [16] as a sustainable and safe material for environmental applications [17][18][19][20][21][22][23][17,18,19,20,21,22,23].
Currently itwe is knownknow that the antifungal activity is shaped by the combination of several attributes such as the original source of chitin (crustaceans or fungal), an average DD and MW, as well as the diversity range of these parameters of a particular batch of CS (Figure 2) [24][25][26][27][24,25,26,27]. In certain cases, antifungal activity of CS might even exceed that of a commercial fungicide [28]. Diverse species of fungi respond differently to CS applications, and the sensitivity varies depending on their life cycle phase [29][30][29,30]. Several mechanisms have been proposed to explain the antifungal activity of CS. They include local disintegration of fungal cell membranes, leakage of cytoplasm, chelation of crucial nutrients, and binding of nucleic acids altering the flow of genetic information [4][31][4,31]. As stated above, the process of CS production determines the unique physicochemical properties of the CS batch, which have a huge impact on antifungal activity. The effect of pathogenic fungi by CS, although studied by a number of research groups, is not based on clear guidelines to standardize the experiments. Because of this, the biological material, experimental conditions, and characteristics of the CS samples varied significantly [13][32][33][13,32,33].
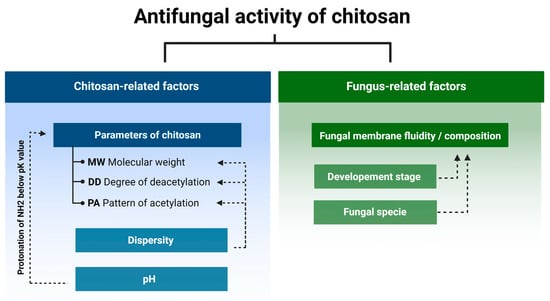
Figure 2.
Chitosan and fungus-related factors that affect the antifungal activity of chitosan.
2. Determinants of Chitosan Antifungal Activity
2.1. Fungi Cell Membrane Composition and Chitosan Susceptibility
Fungi species can be divided into two groups: chitosan susceptible or sensitive and chitosan resistant. Most species of plant pathogenic fungi are sensitive to chitosan, while nematophagous fungi and insect entomopathogens are chitosan resistant [6].
Cell membrane of sensitive species is permeabilized by chitosan [34][41] and in response to this treatment a set of genes encoding cell wall-related proteins, oxidoreductases, and transport-related proteins are up-regulated [35][36][42,43]. It was proposed that negatively charged phospholipids of the plasma membrane were the main target of positively charged chitosan molecules [37][44] and the notion was confirmed by high ratio of phospholipids in cell membranes of species sensitive to chitosan [38][45]. In contrary to the above, a detailed analysis of the lipid composition in four phylogenetically distant species showed that the relative content of phospholipids in chitosan susceptible (Neurospora crassa and Fusarium oxysporum) and chitosan tolerant species (Pochonia chlamydosporia and Beauveria bassiana) were found to be very similar within each group. The main difference between the two groups was much higher content of polyunsaturated fatty acids (mainly linoleic) and lower content of saturated palmitic, stearic, and monounsaturated oleic acids in sensitive species comparing to the tolerant ones [34][41].
In line with this was characteristics of N. crassa mutant with inactive fatty acid desaturase, and thus devoid of polyunsaturated fatty acids, which showed elevated chitosan tolerance compared to the wild type. The feature, observed in germinating conidia and growing mycelium, indicated that the fluidity of the fungal cell membranes determines the sensitivity to chitosan. The higher the content of polyunsaturated fatty acids and the greater fluidity of the membranes, the greater susceptibility to chitosan [34][41]. This notion was experimentally confirmed by Zakrzewska, et al. [28]. The authors elevated plasma membrane fluidity in yeast Saccharomyces cerevisiae cells by higher growth temperature or miconazole treatment (an inhibitor of ergosterol biosynthesis) and observed a significant increase of yeast sensitivity to chitosan [28]. There are clues that chitin content in fungal cell wall might be important. When it is bigger than 10%, as in chitosan tolerant Aspergillus niger, it may be associated with a bigger tolerance to this polymer [39][40][46,47].
2.2. Fungus Developmental Stages and Chitosan Susceptibility
Restriction of fungal growth by chitosan is not only species-specific but also depends on the fungus developmental stage. In vitro study of Penicillium expansum and Botrytis cinerea treated with chitosan (15 cps, DD 90%) showed that spore germination was restricted stronger in P. expansum compared to B. cinerea while mycelial growth of P. expansum was less restricted than that of B. cinerea [41][48]. The higher susceptibility of P. expansum spores was further analyzed by testing integrity of the spore cell membranes subjected to CS treatment. The authors found lower integrity of P. expansum spore cell membranes compared to those of B. cinerea, confirming spore germination results. At the same time, the mycelium of B. cinerea was more susceptible to CS compared to P. expansum. This directly indicates that the sensitivity to chitosan might change during development [41][48].
In line with the above, Palma-Guerrero, et al. [42][49] found that cells representing different developmental stages of Neurospora crassa varied in their sensitivity to chitosan. The conidia treated with chitosan sample (MW 70 kDa, DD 79.6%) 100 ppm concentration were killed within less than 4 min, the conidial germlings within 35–45 min, and the vegetative hyphae within 40 min. CS permeabilized the fungal plasma membrane and was detected inside the conidia five minutes after the treatment. The process, associated with rapid Ca2+ cellular uptake, destabilized the Ca2+ homeostasis and led to the cell death. The authors also found that the process was ATP-dependent, and it did not involve endocytosis [42][49].
2.3. Physiochemical Attributes of Chitosan and Its Antifungal Activity
The results of numerous reported experiments indicate that the CS antifungal activity depends on its physicochemical parameters, however, the lack of standardized experimental conditions does not allow for a direct comparison of different CS batches. Depending on the provider, CS batches are characterized by an average MW in kDa units, by degree of polymerization (DP) expressed as number of monomers in a single polymer molecule and are characterized by viscosity of the standardized water solutions expressed in cps units. Considering these parameters, the CS batches can be roughly divided into the four groups: (1) the batches of very low MW, sometimes designated as CS oligomers or CS oligosaccharides, those could be additionally characterized by the values of their DP, (2) the bathes of low MW (LMW) with an average molecular weight smaller than 100 kDa, (3) the batches with MW ranging from 100 to 1000 kDa and representing medium molecular weight (MMW) chitosan, and (4) the batches of high MW (HMW) with molecules of over 1000 kDa. The CSs, tested by different teams to assess their antifungal activity, represented a broad spectrum of batches with different values of MW, DP or viscosity. Since they represented independently designed experiments with diverse fungi species and a wide range of tested CS samples, the results could be used to draw only general conclusions on CS antifungal characteristics.
Park, et al. [33] determined the Minimal Inhibitory Concentration (MIC) for nine species of fungi (Candida albicans, Trichosporon beigelii, Saccharomyces cerevisiae, Aspergillus fumigatus, A. parasiticus, Botrytis cinerea, Fusarium solani, F. oxysporum, Penicillium verrucosum) using chitosan oligosaccharides 1, 3, 5 and 10 kDa. They found that MIC of CSs 5 kDa and 10 kDa did not exceed 0.04 mg/mL for all tested species while MIC of the remaining CSs was bigger. Thus, out of the tested four chitosan batches, the CS 5 and 10 kDa showed stronger antifungal activity than 1 and 3 kDa [33].
2.4. Chitosan Degree of Deacetylation and Antifungal Activity
The degree of deacetylation (DD) is another parameter of CS essential for its antifungal activity. Younes, et al. [13] investigated the antifungal activity of chitosan with varying MW from 42.5 to 135 kDa and DD from 39% to 98% testing three species: A. niger, F. oxysporum, and A. solani. All were affected by the tested chitosan samples, but the response pattern was strongly species dependent. Growth inhibition of A. solani and F. oxysporum clearly depended on chitosan DD, indicating that higher DD values had stronger antifungal activity. The inhibitory effect on F. oxysporum depended mainly on MW, but at the same time, DD greater than 59% was required to restrict growth. The growth of A. niger was restricted by chitosan; however, no effect of different DD and MW was observed [13].
Tsai, et al. [27] compared the antifungal effect of CS on two groups of species: susceptible (Candida albicans, F. oxysporum) and resistant (Aspergillus fumigatus, Aspergillus parasiticus). The tested CS samples had DD 56% to 98% and MW from 51 to 1080 kDa. The results showed that the strong antifungal effect, assessed by Minimum Lethal Concertation (MLC), depended on the DD of the chitosan sample. As expected, it was observed only for susceptible species while the growth of tolerant species was not restricted [27].
Diverse studies generally confirmed a positive correlation between antifungal activity and DD of the CS sample. It was explained by strong electrostatic interaction between fungal cell membranes and CS amine groups which were protonated in solutions with pH value lower than protonation constant pKa of the particular chitosan sample. The relative number of the groups was directly associated with the DD of the CS molecules. The effect caused by deacetylation may overlap, to some extent, with the effect of the MW (Figure 3). According to several groups, CS molecules of high MW may form internal hydrogen bonds, which lower the effective number of amine groups capable for interaction with the membranes [13][27][43][13,27,53].
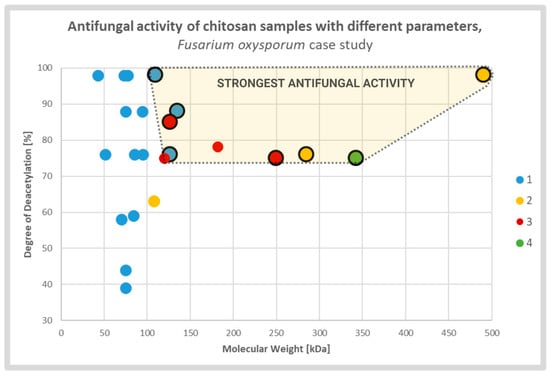
Figure 3. Scatterplot graph of antifungal activities of chitosan samples with different parameters tested on Fusarium oxysporum. Chitosan samples showing the strongest antifungal activities are encircled and highlighted. The colors and respective numbers indicate the cited articles: 1 [13] 2 [27] 3 [44][54] 4 [45][55].
2.5. Chitosan Pattern of Acetylation and Antifungal Activity
Although DD informs about the average rate of deacetylated monomers of the entire CS sample, the pattern of acetylation (PA) depicts the sequence of acetylated monomers on a single chitosan particle. PA is the parameter that has not been thoroughly investigated till now, but with recent advancements of analytical methods the gap is expected to be filled [46][47][48][56,57,58]. Chitosan samples with a specified PA is hardly commercially available. Most commercially available samples are obtained by a chemical process of heterogeneous or homogeneous deacetylation of chitin. CS samples obtained through either of these processes have random PA, which is different in different polymer molecules present in one sample. In other words, the methods lead to uncontrolled differences between different CS batches. Currently, it is not possible to test the effect of PA unless highly specialized methods of obtaining and characterization of CS with specific PA are employed. In consequence, there is still a small amount of data on how PA affects the biological activity of CS. Recently developed methods of enzymatic deacetylation of chitin open new possibilities to obtain chitosans with specified, nonrandom PA [49][59]. One of the first article in this field, by Sreekumar, et al. [49][59], shown that enzymatically obtained chitosan with block-PA (DD 67%, DP 800) had different physicochemical characteristics compared to chemically obtained chitosan with random-PA (DD 66%, DP 700). They found that, water solution of block-PA chitosan had lower viscosity than that of random-PA chitosan. Also, block-PA chitosan showed stringer hydrophobicity what, according to the authors, was the result of bigger proportion of hydrophobic block domains compared to the random-PA chitosan samples. Although they did not test directly antifungal activity of samples with different PA types, they found that block-PA chitosan samples exhibited stronger antimicrobial activity against Pseudomonas syringae pv. Tomato. Testing different CS samples with block-PA, the authors reported stronger antibacterial activity of samples with bigger DD values. As they discussed, this was the result of higher charge density of the molecules, stronger electrostatic interactions between GlcN-rich blocks with membrane components, and, in consequence, more evident disruption of cell integrity [49][59]. Similar effect concerning antifungal activities of CSs with random PA, reported in many articles, was discussed earlier.
Additionally, as it was shown by Basa, et al. [48][58], different PA of chitosan oligomers activated different levels of plant priming. This observation indicated that defined PA could be recognized by plant cells and could activate distinct plant response. This set of results might also indicate that the biological activity of chitosan with different Pas comes not only from the electrostatic interactions, but also from the specific recognition of chitosan molecules with defined PA by plant receptors which in turn would trigger specific signaling pathway. More tests are required, but this information could open a new chapter in designing CS with specific PA to maximize its antifungal activity.
2.6. Dispersity of the Chitosan Sample Promotes Antifungal Activity
Most of the CS samples are heterogeneous, meaning that they consist of various fractions with different molecular weight/degrees of polymerization (MW/DP), deacetylation degrees (DD), and different patterns of acetylation (PA). For each of the basic CS parameters (i.e., MW/DP, DD and PA) the dispersity parameter (Ð) was introduced. It provides farther information on the variety of fractions present in a defined CS sample. ÐMW/ÐDP indicates the variety of size fractions present in a single chitosan sample. ÐDD defines the dispersity of chitosan polymers with different DD. ÐPA describes how differently acetylated monomers are distributed in the polymer molecule (or how many molecules with a specific sequence are present in the specified chitosan sample) [48][58]. So far, only the antifungal effect of CS dispersity of MW/DP (ÐMW/ÐDP) was investigated.
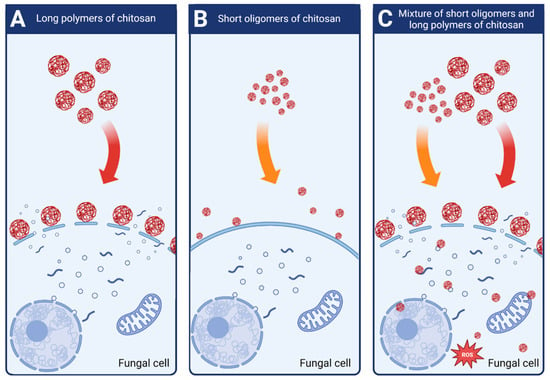
Attjioui, et al. [32] tested inhibition of F. graminearum growth using different CS samples; the sample with DP 300, ÐDP 1.24, the product of its hydrolysis with the average DP 70 and the two fractions of the hydrolysate: DP 90 and oligomers DP 2 to 17. All samples had the same DD 90%. Both, the initial chitosan sample (DP 300) and the product of its hydrolysis (DP 70) had a similar MIC at 100 µg/mL. Tested separately, the larger polymer fraction (DP 90) had similar antifungal activity as the parent chitosan, whereas the oligomer fraction (DP 2–17) had much weaker activity with MIC 200 µg/mL. The results implied a synergy of antifungal activities of both fractions when applied together (Figure 4). According to the authors, the synergy was the result of destabilization of the cell membranes by long CS polymers, which allowed the CS oligomers to penetrate the fungal cell and to interact with intracellular components. The authors concluded that oligomers showed very weak antifungal activity because molecules of this fraction could not disrupt fungal membrane. As reported previously, disruption of the membranes by fraction of longer polymers was required for efficient reduction of fungi growth [32].

Figure 4. Schematic mechanisms of antifungal activity of different weight fractions of chitosan. (A) High molecular weight chitosan destabilizes fungal cell membranes leading to the leakage of intracellular components. (B) Low molecular weight chitosan fractions do not destabilize fungal membranes and show very weak antifungal effect. (C) Chitosan mixture of high and low molecular weight fractions shows strong antifungal activity. High molecular weight fractions destabilize the membranes allowing the low molecular fractions to penetrate fungal cell and to disturb cell processes.
Lemke, et al. [50][60] confirmed observations that fractions of chitosan differing in size act in synergy in decreasing fungal growth. Antifungal activity was evaluated by measuring CS inhibitory concentration (IC50), which restricted F. graminearum growth by 50%. They found that the IC50 of the polymeric CS fraction (MW 43.3 kDa, DP 250) was 155 µg/mL, the IC50 of the oligomeric fraction (MW 2–4 kDa. DP 2–15) was 739 µg/mL while a mixture of the two had IC50 at 133 µg/mL. However, it should be noted that the antifungal activity of the original CS (MW 58.7 kDa, DP 347) was much greater with IC50 at 25 µg/mL [50][60].
2.7. Effect of pH on Chitosan Antifungal Activity
CS sample can be dissolved in an acidic solution when the pH value is lower than the protonation constants (pKa) of this sample. The pKa of the CS is narrowly increasing from 6.39 to 6.51 when the MW changes from 60 to 1370 kDa. A similar and narrow range of pKa changes from 6.51 to 6.17 is associated with DD from 73.3 to 94.6% [51][61]. The pKa is determined by the MW and DD of the CS sample, and these parameters, plus the solubility in water and the antifungal activity of the CS sample, are interconnected.
Alburquenque, et al. [52][62] treated 30 strains of Candida spp. With CS (70 kDa, DD 75%) and found that for 69% strains the minimal inhibitory concentration at pH 4.0 was 4.8 mg/L while at pH 7.0 it ranged depending on strain and the values of MIC were from 1 to 4-times bigger. At low pH, the CS amine groups are protonated, and the CS molecules become polycationic. Biological activity is the direct result of the positive charge of protonated amine groups which are responsible for interaction of protonated chitosan molecules with negatively charged proteins, fatty acids, lipids, and nucleic acids of the fungal cell. This, in turn, leads to disintegration of fungal cell membranes, sequestration of cell components, and observed antifungal activity [13][27][43][13,27,53].
