Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Cristina Russo and Version 2 by Dean Liu.
Increased levels of Chitinases, in particular Chitotriosidase (CHIT-1) and chitinase-3-like protein 1 (CHI3L1), have been found increased in several neurodegenerative disorders. Although having important biological roles in inflammation, to date, the molecular mechanisms of Chitinase involvement in the pathogenesis of neurodegenerative disorders is not well-elucidated.
- Alzheimer’s disease
- Parkinson’s disease
- chitinase-3-like 1
- Chitinases
- Neurodegenerative Diseases
1. Chitin and Chitinase Induction in Alzheimer’s Disease (AD)
AD is one of the most common neurodegenerative diseases worldwide [1][16]. It is a progressive neurodegenerative condition resulting in the regression of intellectual capabilities, memory loss, and spatial disorientation due to neuronal cell damage in higher brain centers [1][16]. The pathogenesis of AD mainly includes deposition of amyloid-beta (Aβ) protein and deposition of neurofibrillary tangles (NFTs) of tau proteins, which increase inflammatory response with accumulation of reactive oxygen species (ROS). Moreover, hormonal disorders, genetic predisposition, mitochondrial dysfunction, lack of neurotropic factors, metal ion dynamic equilibrium disorder, calcium toxicity, and acetylcholine (ACh) deficiency [2][17] contribute to the development of AD [1][16]. Originally, it was thought that chitin was absent in vertebrates, including humans. Only in the last decade has chitin been recognized as an element present also in humans. Basically, the increase of light chitin debris takes place in subjects with low Chitinase activity, as a result of peripheral fungal infections, or when rapid synthesis of hyaluronan occurs. Some studies have shown that chitin, being toxic for neurons, can also be involved in AD pathogenesis. Chitin is an insoluble molecule and a substrate for glycan–protein interactions. In the brain, chitin could facilitate nucleation of amyloid proteins resulting in AD onset [3][18]. In fact, elevated levels of chitin have been found in plasma, in CSF cells, and in CNS cells of AD patients [4][19]. When introduced, chitin has been found to exert both pro-inflammatory and anti-inflammatory effects, which are based on the size of the molecule [5][20]. Chitin molecules larger than 70 µm are named big chitin (BC) and are inert to the inflammatory response [6][21]. Nevertheless, if chitin molecules get degraded by mammalian Chitinase, they can generate small chitin (SC) and intermediate chitin (IC) molecules of a size minor than 40 µm and between 40 and 70 µm, respectively. SC induces an anti-inflammatory response via the production of interleukin 10 (IL-10) (Figure 1) [6][21]. IL-10, being an anti-inflammatory cytokine, inhibits the immune response. Its binding with its own receptors IL-10R1 and IL-10R2, triggering the JAK/STAT signaling pathway causes inhibition of pro-inflammatory cytokine production and phagocytosis inhibition [7][8][22,23]. Whereas IC stimulates a pro-inflammatory response via Toll–like receptor 2 (TLR2)- and nuclear factor κ-light chain of enhancer-activated B cell (NF-κB)-dependent pathways for tumor necrosis factor (TNF) production (Figure 1) [6][21]. A feature in the brain of AD patients is the presence of activated microglia and astroglia, releasing pro-inflammatory cytokines and chemokines [9][24]. The neuroinflammatory hypothesis of AD suggests that Aβ-induced microglia activation, and the consequent phagocytosis, lysosomal impairment, and NLR Family Pyrin Domain Containing 3 (NLRP3) inflammasome activation, affect microglia-induced neurotoxicity. It has been demonstrated that microglial cells are able to engulf chitin debris [9][24]. Next to their activation, both microglia and neurons produce GlcNAc polymers in the extracellular space that trigger neurotoxicity (Figure 1) [10][25]. It is also conceivable the amassing of IC adds a new element in microglia activation and neurotoxicity. Therefore, deposits of chitin in the brain of AD patients strongly indicate their contribution in eliciting the expression of Chitinases and the subsequent neuroinflammation in the pathogenesis of AD [11].
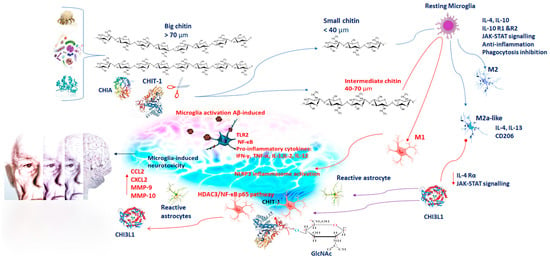
Figure 1. Chitin has both pro-inflammatory and anti-inflammatory effects on the basis of its molecular size: BC (>70 µm) is inert to the inflammatory response; SC (<40 µm) exerts an anti-inflammatory response via IL-10 production. IL-10, binding with its own receptors IL-10R1 and IL-10R2, triggers the JAK/STAT signaling pathway, resulting in pro-inflammatory cytokine and phagocytosis inhibition; IC (40–70 µm) induces pro-inflammatory responses via TLR2- and NF-κB-dependent pathways for IFN-γ, TNF-α, IL1, IL2, and IL12 production. Microglial cells engulfing chitin debris, GlcNAc polymers, and Aβ induce NLRP3 inflammasome activation and neurotoxicity. CHIT-1 and CHIA are the most important active enzymes able to degrade chitin polymers. CHIT-1 modulates neuroinflammation via the HDAC3/NF-κB p65 pathway contributing to AD progression. Microglia polarize in M1 and M2, displaying pro-inflammatory and anti-inflammatory phenotypes, respectively. The M1-polarized phenotype promotes neuronal damage, whereas the M2-polarized phenotype is immunosuppressive and neuroprotective. M2a-like microglia induce CHI3L1, IL13, and CD206. CHI3L1 modulates IL-4Rα expression and STAT6 phosphorylation, affecting M2 polarization. CHI3L1 is expressed in microglia, infiltrating macrophages and astrocytes. The expression of CHI3L1 is induced by pro-inflammatory cytokines, including IL-6, IFN-γ, IL-1β, and TNF-α. Its regulation by IL-6 and TNF-α needs NF-κB activation. CHI3L1 is induced by inflammatory proteins, such as MMP-10, CX3CL1, and 4E-BP1, and therefore, its activity facilitates AD neuroinflammation. Blue arrows represent induction; red arrows represent inhibition. Abbreviation= BC, IC, SC: big, intermediate, small, chitin; IL: interleukin; TLR2: Toll–like receptor 2; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; IFN-γ: interferon-gamma; TNF-α: tumor necrosis factor-α; GlcNAc: β-1,4-linked N-acetylglucosamine; Aβ: beta-amyloid protein; NLRP3: NLR Family Pyrin Domain Containing 3; CHIT-1: Chitotriosidase; CHIA: chitinase acid; AD: Alzheimer’s disease; CHI3L1: chitinase-3-like protein 1; MMP: marrow matrix metalloproteinase; CXCL: Chemokine family; 4E-BP1: eukaryotic translation initiation factor 4E-binding protein 1.
2. Chitinases and Activated Microglia in Neuroinflammation
Subsequent to the onset of AD, the massive formation of amyloid plaques and intracellular neurofibrillary tangles enclosing a misfolded phosphorylated tau protein induces synaptic dysfunction followed by axonal impairment and cognitive changes. While this protein-related process is ongoing, the immune system is highly responsive [12][26].
Misfolded and aggregated proteins, conceivably including IC, binding to pattern recognition receptors on microglia and astroglia [6][9][10][21,24,25], trigger neuroinflammatory response characterized by the release of inflammatory mediators, which contribute to the process of neurodegeneration and to disease progression [6][12][13][14][21,26,27,28].
Microglia are immunomodulatory cells playing a significant role in immune surveillance of the CNS. In the course of neuroinflammation, specific pro-inflammatory factors stimulate microglia. Microglia have phagocytic activity to remove infectious agents, neurofibrillary plaques, and damaged neurons. Microglia are generally considered the primary innate immune cells of the CNS. In response to many types of damage, microglia first change their morphology from ramified to amoeboid form, and then they migrate to the damaged cells and clear the debris of the dead cells by phagocytosis. Microglia recognize damaging stimuli and react by producing inflammatory cytokines and several chemokines [15][29]. By promoting the release of pro-inflammatory cytokines, microglia are involved in the innate response providing a rapid control of invading pathogens and influencing T and B cell activation. In the immune response, microglial cells act in different ways releasing pro-inflammatory molecules and acting as antigen-presenting cells.
Additionally, microglia control the interaction between neurons and astrocytes for the restoration and reorganization of damaged synapses. Microglia polarize in two different phenotypes: cytotoxic M1, pro-inflammatory, and cytoprotective M2 [16][30]. After the induction of TLR, microglia activation leads to proinflammatory mediator release such as TNF-α, interferon-gamma (IFN-γ), IL-1(IL-1β), IL-6, and IL-12. These cytokines increase oxidative stress and nitrogen free radicals [16][30], and they also promote neuroinflammation, which is the major pathological feature of neurodegenerative diseases [15][29]. In contrast, IL-4 and IL-10 promote the M2 phenotype (Figure 1). M2 microglial cells reduce brain inflammation, inhibiting M1 cytokines and other inflammatory mediators and exerting regenerative functions to restore homeostasis [17][31].
It has recently been reported that microglia display an overlapping functional state, shifting from one to the other depending on the activated pathways. Other phenotypes of microglia, such as M2a-like microglia, M2b and M2c, have been identified to be able to express both pro-inflammatory and anti-inflammatory markers [17][31]. In particular, the M2b phenotype, producing both inflammatory and restorative markers, would constitute an intermediate state. Whereas M2a-like microglia, stimulated with IL-4 and IL-13, induce arginase, chitinase 3-like 3 (or Ym1) gene, and CD206 [18][32]. These data suggest to deepen the role of CH3L3 in activated microglia during AD.
M2c suppresses the immune response upon IL-10, induces tissue remodeling, and restoration producing the transforming growth factor beta (TGF-β), CD206, and CD163 [19][33], followed by the resolution of the neuroinflammation. Thus, the M1-polarized phenotype promotes neuronal damage, whereas the M2-polarized phenotype is immunosuppressive and neuroprotective (Figure 1) [19][33].
