Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Michał Zimecki.
To date, the best-studied active ingredient in bovine colostrum (BC) and milk is LFlactoferrin (LF). It is an evolutionarily old protein present in excretory fluids of mammals and secondary granules of neutrophils and is released from them at sites of inflammation. This 80 KDa, single polypeptide chain protein belongs to the transferring family and has a property to bind two Fe3+ ions. Lactoferrins from various species have similar amino acid sequences and similar tertiary structures. This high interspecies homology indicates a similar function for this protein in different mammalian species.
- lactoferrin
- bovine colostrum
- adverse side effects
- NSAIDs
- antibiotic therapy
1. Lactoferrin
Over the past 60 years of intensive research, as many as 20 different physiological activities of LF have been described [73,74,75,76,77,78][1][2][3][4][5][6]. In this researticle, wech, the researchers will mainly focus on LF, with particular attention being paid to its actions in the amelioration of side effects caused by antibiotic and NSAID therapies, as well as psychophysical stress, so LF activity is described below in some detail.
The ability to bind iron, fight microbes and regulate hematopoiesis are among the most important properties of LF and LF-derived peptides. Other properties of LF include regulating immune system function (immunosuppression or immunoactivation) and oxidoreduction (redox) processes (i.e., those during which oxygen free radicals are released), inhibiting cancer cell growth, promoting osteogenesis and wound healing, regulating glucose and lipid metabolism, supporting bowel functions and enriching intestinal microbiota. Other properties of LF, such as antistress, hypotensive and analgesic effects, are equally important. All of these effects of LF positively affect many aspects of our health, playing a particularly important role in the development of newborns and infants, as well as in older patients treated for a variety of infections (including bacteremia and sepsis) and during convalescence after serious diseases, surgery or chemotherapy [60,67,68,70,73,74,75,76,77,78][1][2][3][4][5][6][7][8][9][10]. LF may be regarded as a “biological drug” which is active by systemic (oral, intravenous, intramuscular and subcutaneous) and topical (on mucous membranes and skin) administration [60,61,62,64,65,66,67,68,70,73,74,75,76,77,78,79,80,81][1][2][3][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18]. An overview of LF activity is shown in Figure 3.
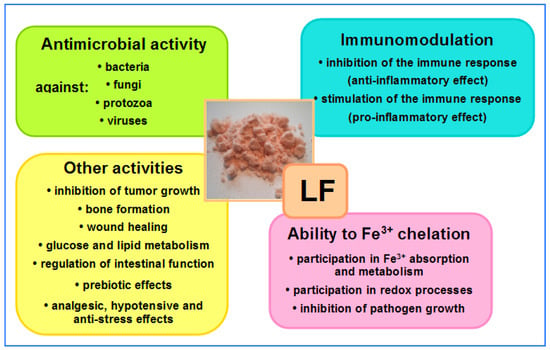
Figure 3. Overview of LF activities. In the central part, native LF isolated from bovine milk—salmon-colored powder (the intensity of the color depends on the degree of iron saturation—the more iron bound to the LF molecules, the darker the color of the powder); native LF is saturated at around 10% (photo by J. Artym).
LF inhibits the development of infections in both a direct and indirect manner. Direct effects of LF on microbes include: • binding iron ions and removing them from the microbe growth environment; • damage to cell structures (mainly cell membranes and walls and mitochondria), which leads to osmotic disruption and limited nutrition, resulting in microbe cells being weakened or killed; • induction of apoptotic death of infected cells, which restricts the spread of infection; • inhibiting pathogen adhesion to epithelial cells, which prevents the development of infection; • enzymatic destruction of virulence factors (e.g., enzymes and receptors) produced by pathogens; • inhibiting proton pumps (both H+/K+ ATPases and V-ATPases); • inhibiting the formation of biofilms on the surfaces of affected epithelia, implants, medical prostheses, etc.; • forming reactive oxygen species, which signal the inflammatory process and are toxic to microbes; • promoting growth of commensal physiological microflora on the skin and mucosa surfaces (these microbes compete with pathogenic or potentially pathogenic microbiota, thus restricting their growth) [58,60,67,68,70,76,79][4][7][8][9][10][16][19].
Indirect effects occur through regulation of immune system activity by strengthening it at early stages of infection (to accelerate the elimination of infectious agents and heal damaged areas) and weakening it at later stages (to facilitate the restoration of homeostasis).
LF regulates the maturation of T and B lymphocytes, the activity of NK cells, granulocytes, macrophages, dendritic cells and other immune cells, and the production of cytokines, metalloproteinases, oxygen free radicals and other inflammatory agents. Moreover, it accelerates the repair of damaged tissues and healing wounds [75][3].
LF has an extremely important role in the gastrointestinal tract, which is particularly important in newborns as well as in the elderly and active athletes. It stimulates growth, accelerates maturation and has a protective effect on intestinal tissues—epithelial cells and immune cells of lymph nodules—increases the secretion of digestive enzymes, strengthens the physiological intestinal microbiota, inhibits toxin production and the proliferation of pathogenic microorganisms, and seals tight junctions between epithelial cells, resulting in reducing intestinal permeability. In addition, LF inhibits chronic inflammation and the development of polyps, which represent an early stage of intestinal cancer [80,81,82][17][18][20].
Evidence from preclinical and clinical studies indicates that bovine-milk-derived LF has good bioavailability and a good safety profile with no serious side effects, both after systemic and topical use in humans and animals [73,77,78,83,84][1][5][6][21][22]. The protein is widely available and relatively cheap because it is isolated from milk in a well-established, standardized, large-scale manufacturing process [73,85][1][23]. The application of bovine LF has been approved by various agencies, such as the European Food Safety Authority (EFSA) in Europe and the Food and Drug Administration (FDA) in the US [86,87][24][25]. Of importance, despite concerns regarding possible enzymatic degradation of LF in the gastrointestinal tract, oral administration of LF in rats elicits similar intracellular signaling to LF given intravenously [88][26]. More importantly, buccal administration of LF to healthy volunteers resulted in regulation of their immune status [89,90][27][28].
2. Lactoferrin as Supportive Therapy in Antibiotic Treatment
Adverse side effects may occur during antibiotic therapy or combinatorial therapy with antibiotics with other types of drugs. The most common side effects of antibiotics are diarrhea, nausea, headache, intestinal inflammation, thrush, aphthae, rashes and vaginal mycosis. The side effects of excessive or non-selective antibiotic use are caused by elimination of both physiological and pathogenic bacteria. As a consequence, disruption to the composition of the natural intestinal and urogenital microbiota occurs. A therapy with single classical antibiotics may not be sufficient to cure some infections, so protocols involving several antibiotics with a different killing spectrum must be used. Such an approach increases the undesirable effects of the therapy. BC, LF and other BC-derived biocomponents can alleviate the side effects of antibiotic therapy but also may enhance its efficacy by direct and indirect (via immune system stimulation) antimicrobial effects. These natural products have a multi-directional effect on pathogenic microorganisms, so antibiotic resistance does not develop.
2.1. Antibiotic Treatment in In Vitro and Animal Models
In a mouse model, inflammatory foci in lungs of Mycobacterium tuberculosis-infected animals hamper the penetration of therapeutics to infected sites and bacteria destruction. Oral recombinant human LF (rhLF) was used in C57BL mice as an adjuvant therapy with ofloxacin fluoroquinolone [91][29]. Histological analysis revealed that treatment with LF enabled penetration of the antibiotic to the pathologic sites populated with activated macrophages. The increased antibiotic penetration also preserved endothelial cell integrity. In addition, the phenotypes of macrophages showed an increased M-2-like pattern.
Entamoeba histolytica is a dangerous parasite that attacks the liver and intestine, often causing dysentery by perforating the large intestine. Metronidazole is applied for the treatment of amoebiosis but produces toxic effects (such as nausea and a bad taste in the mouth) in patients. Hamsters infected with amoeba that were given bLF intragastrically (2.5 mg/100 g b.w.) for 8 days showed no symptoms of the disease, and amoebic liver abscess was present in less than 1% of the animals, compared with 63% in controls. In addition, the liver function and blood cell parameters were restored to almost normal levels [92][30].
Cystic fibrosis (CF) is often accompanied with infections by the opportunistic pathogen Pseudomonas aeruginosa. Inhibition of its elastase enzymes and iron uptake by its siderophores—pyoverdins—could be valuable therapeutic targets [93][31]. The elastase produced by P. aeruginosa causes a rapid release of iron from transferrin and its uptake by pyoverdins, but LF is not a source of iron for these bacteria [94][32]. This suggests the possibility of using LF to treat CF patients. These facts prompted clinicians to use exogenous LF for treatment of CF patients, since endogenous LF in the airways of CF patients is proteolytically cleaved by cathepsin activity in P. aeruginosa [95][33]. In vitro experiments were performed to evaluate a potential, concerted effect of LF and antibiotics on reducing bacterial colony forming units (CFUs) numbers in an in vitro cell line model [96][34] and sputum from CF patients [97][35]. For these studies, ALX-109—a combination of apo-bLF with hypothiocyanite (OSCN-—a bactericidal agent)—an experimental drug developed by Alaxia (Lyon, France), was used. In a model applying airway epithelial cells from CF patients infected with P. aeruginosa, ALX-109 was used in combination with tobramycin or aztreonam [96][34]. It appeared that ALX-109 alone reduced bacterial biofilms and showed an additive effect with tobramycin in reducing bacterial CFUs and enhanced the ability of aztreonam to reduce P. aeruginosa biofilm. The authors concluded that a combination of ALX-109 with antibiotics, in the form of an inhalatory aerosol, could be beneficial in decreasing bacterial infection in the airways of CF patients. Such an assumption was supported by another study in which sputum from CF patients was treated with ALX-009 (another combination of apo-bLF with hypothiocyanite) alone or combined with tobramycin [97][35]. ALX-009 alone demonstrated a bactericidal action on the P. aeruginosa in the sputum samples more potent than that of tobramycin, but this effect was even stronger when combined with tobramycin.
Bovine LF saturated in 85% with Fe3+ (holo-bLF) prevented the vegetative cell growth and toxin production of a clinical strain of Clostridium difficile in triple-stage chemostat gut models [98][36]. The glass vessels were inoculated with human feces and spiked with C. difficile spores, holo-bLF or apo-bLF (saturated in 1% with Fe3+), and clindamycin for simulation of C. difficile infection (CDI). Apo-bLF was ineffective. The authors concluded that LF may be a proper addition to the standard prevention and treatment of CDI in patients in long-term care. CDI develops after treatment with antibiotics and is a major cause of chronic, debilitating diarrhea in hospitals and care facilities. Hospital strains of this bacterium are often antibiotic-resistant, making treatment much more difficult. The results are consistent with the observation in a clinical trial that bLF reduced the incidence of post-antibiotic diarrhea in a long-term-care patient [99][37].
In in vitro tests, bLF and other iron chelators were tested against various strains of Cryptococcus, an opportunistic yeast that causes dangerous infections in immunocompromised patients. LF showed a synergistic effect with amphotericin B against all yeast strains tested, but this effect was not mainly due to iron chelation, but to other LF activities that were enhanced in the presence of the antibiotic [100][38].
2.2. Antibiotic Treatment in Clinics
In a case report with 26-year-old women suffering from post-influenza otitis media infection, no therapeutic benefit was obtained after treatment with antibiotics and steroids (Atecortin and Dicortineff) [101][39]. The pathogens were Staphylococcus homis and Staphylococcus epidermidis. A specific bacteriophage therapy (3 weeks) was also not completely effective. However, oral application of bLF (50 mg daily for 7 days, with two-week intervals), led to a full recovery, associated with increased myelopoiesis and elevated serum LF concentration.
Tooth extraction is often associated with post-extraction complications, even with application of antibiotics. A total of 111 patients with local post-extraction complications treated with antibiotics were enrolled in a clinical study [102][40]. The patients were divided into three groups taking (A) amoxicillin and clavulanic acid (2 g/day for 6 days), (B) the antibiotics plus Bifidobacterium longum (a probiotic) plus bLF and (C) a placebo group. The degree of complications was recorded on days 7, 14 and 21 after the extractions. The results showed that pain was present for 48%, 30% and 71.4% of the respective groups, with similar differences in the mean numeric rating scores. Two patients from the placebo group experienced dry socket. Nine patients from the antibiotic only group and one patient from the antibiotic and probiotic/LF group had intestinal distention. Diarrhea was registered in three patients from the antibiotic alone group but was absent in the other groups.
Antibiotic-associated diarrhea (AAD) can result from hospital-acquired infections. In a clinical, randomized, double-blind study involving tube-fed, long-term-care adult patients with antibiotic-associated diarrhea, rhLF was orally applied for 8 weeks [99][37]. It appeared that fewer patients in the LF-treated group suffered from diarrhea. However, in another prospective, randomized, double-blind, placebo-controlled, single-center study, no therapeutic benefit of bLF application in children (n = 156) with AAD was found [103][41].
In several clinical trials, LF was included in therapeutic protocols to prevent side effects of antibiotic therapy in Helicobacter pylori infection. In a clinical trial involving children, all patients were treated for 4 weeks with a combination of omeprazole, amoxicillin and clarithromycin (the therapeutic regimen also for group A) [104][42]. Some of the patients received, in addition, a probiotic Probinul-Cadigroup containing LF (group B). Health status was evaluated at the end of the treatment. The results of the treatments were as follows. Epigastric pain occurred in 17.6% of group A versus 5.8% of B group patients, nausea in 8.8% of group A versus 2.9% of group B, diarrhea and vomiting in 5.8% and 23.5%, respectively, of group A versus none in group B. A total of 2.3% of patients tested negative for H. pylori. In conclusion, the addition of LF to the therapeutic protocol decreased the occurrence of the described side effects.
In a similar study on adult patients (n = 206), bLF and probiotics were used to improve therapeutic efficacy and lower side effects of standard antibiotic therapy (esomeprazole, clarithromycin and amoxicillin) during an 8-week treatment [105][43]. At the end of the treatment, 84.95% of patients showed negative results for H. pylori in the group treated with antibiotics only and 92.0% in the group given, in addition, LF + probiotics. Moreover, more patients in the antibiotic group reported side effects. In conclusion, better eradication of infection and limitation of side effects were registered thanks to the inclusion of LF and probiotics in the triple antibiotic therapy for H. pylori.
A prospective, randomized study was also performed on 76 patients after failure of a first, conventional quadruple therapy [106][44]. All patients were treated with a combination of ranitidine bismuth citrate, esomeprazole, amoxicillin and tinidazole for 7 days. The therapeutic regimen was supplemented in one group with bLF (400 mg daily). One month after the therapy, an endoscopy and the 13C-urea breath test (UBT) were performed to evaluate the efficacy of the treatment. Eradication H. pylori in the bLF group was 94.3% (33/35) and in the control group was 88.6% (31/35), as assessed by UBTs and histological analysis. Side effects were recorded in 29.4% of patients in the antibiotic alone group versus 17.6% in the patients taking LF supplementation.
A recent parallel RCT included 400 adult patients with H. pylori infection [107][45]. They were randomized into four equal groups: (A) proton-pump-based triple therapy (PpTT) involving proton pump inhibitors: esomeprazole plus antibiotics: amoxicillin and clarithromycin for 2 weeks, (B) esomeprazole plus amoxicillin for 5 days and then esomeprazole plus metronidazole and clarithromycin 10 days, (C) PpTT as in group A plus bLF in 200 mg sachets (overall daily dose 400 mg) for 2 weeks and (D) sequential therapy as in group B plus bLF for 2 weeks, as above. Bacterial eradication was significantly better in both groups treated with LF (percentages of eradication for groups 1, 2, 3 and 4 were 70.3%, 82.8%, 85.6% and 94.5%, respectively). The authors made no mention of differences in the frequencies of adverse side effects of these therapies (dizziness and headache, fatigue, nausea, taste disturbance and colonic distension were observed).
LF, in addition to its antimicrobial effect, exhibits IPP-like activity, inhibiting both H+/K+-ATPase and V-ATPase. The protein was shown to be more active at acidic pH and had a good safety profile [79,108][16][46]. This LF activity may further enhance the efficacy of classical therapy used in H. pylori eradication.
References
- Jańczuk, A.; Brodziak, A.; Czernecki, T.; Król, J. Lactoferrin-The Health-Promoting Properties and Contemporary Application with Genetic Aspects. Foods 2022, 12, 70.
- Takayama, Y.; Aoki, R. Roles of lactoferrin on skin wound healing. Biochem. Cell Biol. 2012, 90, 497–503.
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596.
- Drago-Serrano, M.E.; Rafael Campos-Rodriguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin and Peptide-derivatives: Antimicrobial Agents with Potential Use in Nonspecific Immunity Modulation. Curr. Pharm. Des. 2018, 24, 1067–1078.
- Ochoa, T.J.; Pezo, A.; Cruz, K.; Chea-Woo, E.; Cleary, T.G. Clinical studies of lactoferrin in children. Biochem. Cell Biol. 2012, 90, 457–467.
- Kaczyńska, K.; Jampolska, M.; Wojciechowski, P.; Sulejczak, D.; Andrzejewski, K.; Zając, D. Potential of Lactoferrin in the Treatment of Lung Diseases. Pharmaceuticals 2023, 16, 192.
- Bukowska-Osko, I.; Sulejczak, D.; Kaczynska, K.; Kleczkowska, P.; Kramkowski, K.; Popiel, M.; Wietrak, E.; Kowalczyk, P. Lactoferrin as a Human Genome “Guardian”—An Overall Point of View. Int. J. Mol. Sci. 2022, 23, 5248.
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438.
- Lepanto, M.S.; Rosa, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in Aseptic and Septic Inflammation. Molecules 2019, 24, 1323.
- Zarzosa-Moreno, D.; Avalos-Gómez, C.; Ramírez-Texcalco, L.S.; Torres-López, E.; Ramírez-Mondragón, R.; Hernández-Ramírez, J.O.; Serrano-Luna, J.; de la Garza, M. Lactoferrin and its derived peptides: An alternative for combating virulence mechanisms developed by pathogens. Molecules 2020, 25, 5763.
- Cutone, A.; Rosa, L.; Ianiro, G.; Lepanto, M.S.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G. Lactoferrin’s Anti-Cancer Properties: Safety, Selectivity, and Wide Range of Action. Biomolecules 2020, 10, 456.
- Drago-Serrano, M.E.; Campos-Rodríguez, R.; Carrero, J.C.; de la Garza, M. Lactoferrin: Balancing Ups and Downs of Inflammation Due to Microbial Infections. Int. J. Mol. Sci. 2017, 18, 501.
- Gruden, S.; Ulrih, N.P. Diverse mechanisms of antimicrobial activities of lactoferrins, lactoferricins, and other lactoferrin-derived peptides. Int. J. Mol. Sci. 2021, 22, 11264.
- Hao, L.; Shan, Q.; Wei, J.; Ma, F.; Sun, P. Lactoferrin: Major Physiological Functions and Applications. Curr. Protein Pept. Sci. 2019, 20, 139–144.
- Kowalczyk, P.; Kaczyńska, K.; Kleczkowska, P.; Bukowska-Ośko, I.; Kramkowski, K.; Sulejczak, D. The Lactoferrin Phenomenon—A Miracle Molecule. Molecules 2022, 27, 2941.
- Santos-Pereira, C.; Andrés, M.T.; Fierro, J.F.; Rodrigues, L.R.; Côrte-Real, M. A review on lactoferrin as a proton pump inhibitor. Int. J. Biol. Macromol. 2022, 202, 309–317.
- Liu, N.; Feng, G.; Zhang, X.; Hu, Q.; Sun, S.; Sun, J.; Sun, Y.; Wang, R.; Zhang, Y.; Wang, P.; et al. The Functional Role of Lactoferrin in Intestine Mucosal Immune System and Inflammatory Bowel Disease. Front. Nutr. 2021, 8, 759507.
- Liao, Y.; Jiang, R.; Lönnerdal, B. Biochemical and molecular impacts of lactoferrin on small intestinal growth and development during early life. Biochem. Cell Biol. 2012, 90, 476–484.
- Artym, J.; Zimecki, M. Beneficial effect of lactoferrin on the microbiota from gastrointestinal tract. Adv. Microbiol. 2020, 59, 277–290.
- Ramirez-Rico, G.; Drago-Serrano, M.E.; Leon-Sicairos, N.; de la Garza, M. Lactoferrin: A nutraceutical with activity against colorectal cancer. Front. Pharmacol. 2022, 13, 855852.
- Tamano, S.; Sekine, K.; Takase, M.; Yamauchi, K.; Iigo, M.; Tsuda, H. Lack of chronic oral toxicity of chemopreventive bovine lactoferrin in F344/DuCrj rats. Asian Pac. J. Cancer Prev. 2008, 9, 313–316.
- Yamauchi, K.; Toida, T.; Nishimura, S.; Nagano, E.; Kusuoka, O.; Teraguchi, S.; Hayasawa, H.; Shimamura, S.; Tomita, M. 13-Week oral repeated administration toxicity study of bovine lactoferrin in rats. Food Chem. Toxicol. 2000, 38, 503–512.
- Wakabayashi, H.; Yamauchi, K.; Abe, F. Quality control of commercial bovine lactoferrin. Biometals 2018, 31, 313–319.
- European Food Safety Authority, EFSA Panel on Dietetic Products, Nutrition and Allergies, Scientific opinion on bovine lactoferrin. EFSA J. 2012, 10, 2811–2825.
- US Food and Drug Administration. GRAS Notice No. GRN 669. 2016. Available online: https://www.fda.gov/media/124472/download (accessed on 20 February 2023).
- Kruzel, M.L.; Olszewska, P.; Pazdrak, B.; Krupinska, A.M.; Actor, J.A. New insights into the systemic effects of oral lactoferrin: Transcriptome profiling. Biochem. Cell Biol. 2021, 99, 47–53.
- Kruzel, M.L.; Actor, J.K.; Boldogh, I.; Zimecki, M. Lactoferrin in health and disease. Postepy Hig. Med. Dosw. 2007, 61, 261–267.
- Mulder, A.M.; Connellan, P.A.; Oliver, C.J.; Morris, C.A.; Stevenson, L.M. Bovine lactoferrin supplementation supports immune and antioxidant status in healthy human males. Nutr. Res. 2008, 28, 583–589.
- Nguyen, T.K.T.; Niaz, Z.; Kruzel, M.L.; Actor, J.K. Recombinant Human Lactoferrin Reduces Inflammation and Increases Fluoroquinolone Penetration to Primary Granulomas During Mycobacterial Infection of C57Bl/6 Mice. Arch. Immunol. Ther. Exp. 2022, 70, 9.
- Ordaz-Pichardo, C.; León-Sicairos, N.; Hernández-Ramírez, V.I.; Talamás-Rohana, P.; de la Garza, M. Effect of bovine lactoferrin in a therapeutic hamster model of hepatic amoebiasis. Biochem. Cell Biol. 2012, 90, 425–434.
- Jeong, G.-J.; Khan, F.; Khan, S.; Tabassum, N.; Mehta, S.; Kim, Y.-M. Pseudomonas aeruginosa virulence attenuation by inhibiting siderophore functions. Appl. Microbiol. Biotechnol. 2023, 107, 1019–1038.
- Döring, G.; Pfestorf, M.; Botzenhart, K.; Abdallah, M.A. Impact of proteases on iron uptake of Pseudomonas aeruginosa pyoverdin from transferrin and lactoferrin. Infect. Immun. 1988, 56, 291–293.
- Britigan, B.E.; Hayek, M.B.; Doebbeling, B.N.; Fick, R.B., Jr. Transferrin and lactoferrin undergo proteolytic cleavage in the Pseudomonas aeruginosa-infected lungs of patients with cystic fibrosis. Infect. Immun. 1993, 61, 5049–5055.
- Moreau-Marquis, S.; Coutermarsh, B.; Stanton, B.A. Combination of Hypothiocyanite and Lactoferrin (ALX-109) Enhances the Ability of Tobramycin and Aztreonam to Eliminate Pseudomonas Aeruginosa Biofilms Growing on Cystic Fibrosis Airway Epithelial Cells. J. Antimicrob. Chemother. 2015, 70, 160–166.
- Tunney, M.M.; Payne, J.E.; McGrath, S.J.; Einarsson, G.G.; Ingram, R.J.; Gilpin, D.F.; Juarez-Perez, V.; Elborn, J.S. Activity of Hypothiocyanite and Lactoferrin (ALX-009) against Respiratory Cystic Fibrosis Pathogens in Sputum. J. Antimicrob. Chemother. 2018, 73, 3391–3397.
- Chilton, C.H.; Crowther, G.S.; Śpiewak, K.; Brindell, M.; Singh, G.; Wilcox, M.H.; Monaghan, T.M. Potential of lactoferrin to prevent antibiotic-induced Clostridium difficile infection. J. Antimicrob. Chemother. 2016, 71, 975–985.
- Laffan, A.M.; McKenzie, R.; Forti, J.; Conklin, D.; Marcinko, R.; Shrestha, R.; Bellantoni, M.; Greenough, W.B. 3rd Lactoferrin for the prevention of post-antibiotic diarrhea. J. Health Popul. Nutr. 2011, 29, 547–551.
- Lai, Y.-W.; Campbell, L.T.; Wilkins, M.R.; Pang, C.N.I.; Chen, S.; Carter, D.A. Synergy and antagonism between iron chelators and antifungal drugs in Cryptococcus. Int. J. Antimicrob. Agents 2016, 48, 388–394.
- Weber-Dabrowska, B.; Zimecki, M.; Kruzel, M.; Kochanowska, I.; Lusiak-Szelachowska, M. Alternative therapies in antibiotic-resistant infection. Adv. Med. Sci. 2006, 51, 242–244.
- Barone, A.; Marchionni, F.S.; Cinquini, C.; Panattoni, A.C.; Toti, P.; Marconcini, S.; Covani, U.; Gabriele, M. Antibiotic treatment to prevent postextraction complications: A monocentric, randomized clinical trial. Preliminary outcomes. Minerva Stomatol. 2017, 66, 148–156.
- Wronowski, M.F.; Kotowska, M.; Banasiuk, M.; Kotowski, A.; Kuzmicka, W.; Albrecht, P. Bovine Lactoferrin in the Prevention of Antibiotic-Associated Diarrhea in Children: A Randomized Clinical Trial. Front. Pediatr. 2021, 9, 675606.
- Tolone, S.; Pellino, V.; Vitaliti, G.; Lanzafame, A.; Tolone, C. Evaluation of Helicobacter pylori eradication in pediatric patients by triple therapy plus lactoferrin and probiotics compared to triple therapy alone. Ital. J. Pediatr. 2012, 38, 63.
- de Bortoli, N.; Leonardi, G.; Ciancia, E.; Merlo, A.; Bellini, M.; Costa, F.; Mumolo, M.G.; Ricchiuti, A.; Cristiani, F.; Santi, S.; et al. Helicobacter pylori eradication: A randomized prospective study of triple therapy versus triple therapy plus lactoferrin and probiotics. Am. J. Gastroenterol. 2007, 102, 951–956.
- Tursi, A.; Elisei, W.; Brandimarte, G.; Giorgetti, G.M.; Modeo, M.E.; Aiello, F. Effect of lactoferrin supplementation on the effectiveness and tolerability of a 7-day quadruple therapy after failure of a first attempt to cure Helicobacter pylori infection. Med. Sci. Monit. 2007, 13, CR187–CR190.
- Hablass, F.H.; Lashen, S.A.; Alsayed, E.A. Efficacy of lactoferrin with standard triple therapy or sequential therapy for Helicobacter pylori eradication: A randomized controlled trial. Turk. J. Gastroenterol. 2021, 32, 742–749.
- Andres, M.T.; Fierro, J.F. Antimicrobial mechanism of action of transferrins: Selective inhibition of H+-ATPase. Antimicrob. Agents Chemother. 2010, 54, 4335–4342.
More
